A Systematic Review and Meta-Analysis of the Direct Comparison of Second-Generation Cryoballoon Ablation and Contact Force-Sensing Radiofrequency Ablation in Patients with Paroxysmal Atrial Fibrillation
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Eligibility Criteria
2.3. Exposure and Outcomes
2.4. Quality Assessment and Data Extraction
2.5. Statistical Analysis
3. Results
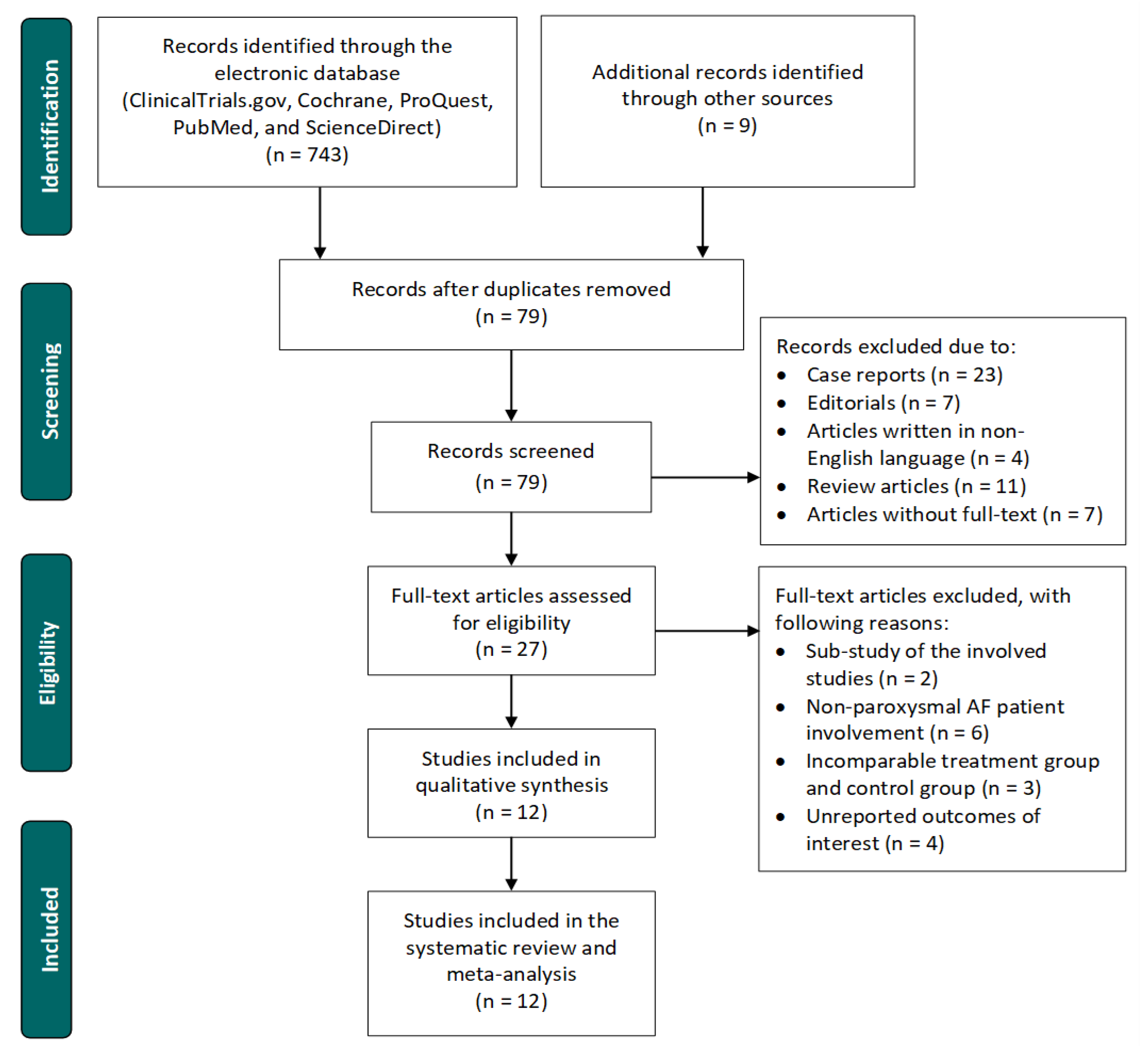
3.1. Study Selection Process
3.2. Baseline Characteristics
3.3. Heterogeneity and Publication Bias
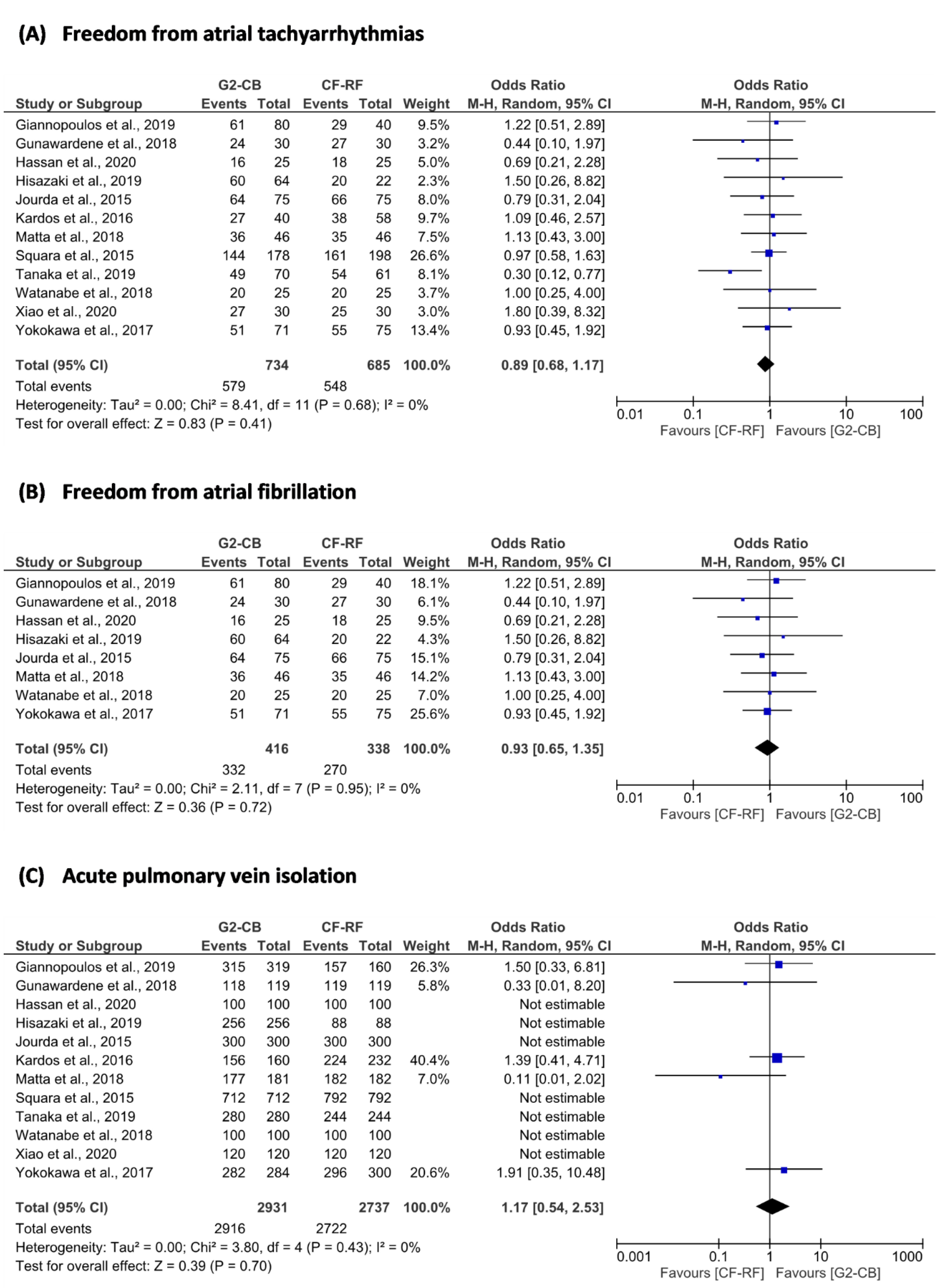
3.4. Primary Outcome
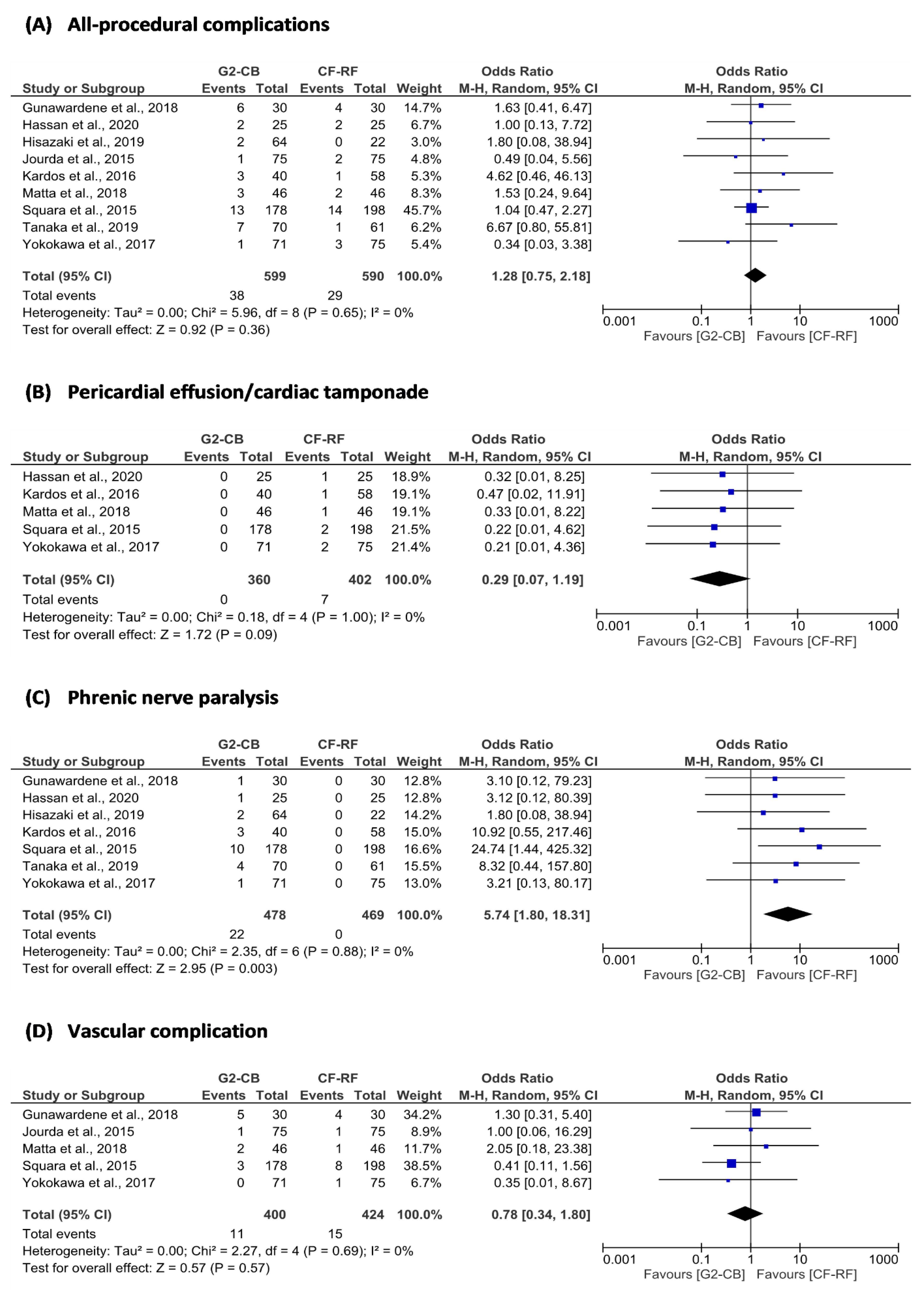
3.5. Secondary Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zulkifly, H.; Lip, G.Y.H.; Lane, D.A. Epidemiology of atrial fibrillation. Int. J. Clin. Pract. 2018, 72, e13070. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart disease and stroke statistics—2020 update: A report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Sanchis-Gomar, F.; Cervellin, G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int. J. Stroke 2021, 16, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.V.; Simon, D.N.; Go, A.S.; Spertus, J.; Fonarow, G.; Gersh, B.J.; Hylek, E.M.; Kowey, P.R.; Mahaffey, K.W.; Thomas, L.E.; et al. Association between atrial fibrillation symptoms, quality of life, and patient outcomes: Results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Circ. Cardiovasc. Qual. Outcomes 2015, 8, 393–402. [Google Scholar] [CrossRef]
- Pistoia, F.; Sacco, S.; Tiseo, C.; Degan, D.; Ornello, R.; Carolei, A. The epidemiology of atrial fibrillation and stroke. Cardiol. Clin. 2016, 34, 255–268. [Google Scholar] [CrossRef]
- Magnussen, C.; Niiranen, T.J.; Ojeda, F.M.; Gianfagna, F.; Blankenberg, S.; Njølstad, I.; Vartiainen, E.; Sans, S.; Pasterkamp, G.; Hughes, M.; et al. Sex differences and similarities in atrial fibrillation epidemiology, risk factors, and mortality in community cohorts: Results from the BiomarCaRE Consortium (Biomarker for Cardiovascular Risk Assessment in Europe). Circulation 2017, 136, 1588–1597. [Google Scholar] [CrossRef]
- Ziff, O.; Carter, P.R.; McGowan, J.; Uppal, H.; Chandran, S.; Russell, S.; Bainey, K.R.; Potluri, R. The interplay between atrial fibrillation and heart failure on long-term mortality and length of stay: Insights from the, United Kingdom ACALM registry. Int. J. Cardiol. 2018, 252, 117–121. [Google Scholar] [CrossRef]
- Haïssaguerre, M.; Jaïs, P.; Shah, D.C.; Takahashi, A.; Hocini, M.; Quiniou, G.; Garrigue, S.; Le Mouroux, A.; Le Métayer, P.; Clémenty, J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 1998, 339, 659–666. [Google Scholar] [CrossRef]
- Mahida, S.; Sacher, F.; Derval, N.; Berte, B.; Yamashita, S.; Hooks, D.; Denis, A.; Amraoui, S.; Hocini, M.; Haissaguerre, M.; et al. Science linking pulmonary veins and atrial fibrillation. Arrhythmia Electrophysiol. Rev. 2015, 4, 40–43. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Santangeli, P.; Lin, D. Catheter ablation of paroxysmal atrial fibrillation: Have we achieved cure with pulmonary vein isolation? Methodist DeBakey Cardiovasc. J. 2015, 11, 71–75. [Google Scholar] [CrossRef]
- Pérez-Castellano, N.; Fernández-Cavazos, R.; Moreno, J.; Cañadas, V.; Conde, A.; González-Ferrer, J.J.; Macaya, C.; Pérez-Villacastín, J. The COR trial: A randomized study with continuous rhythm monitoring to compare the efficacy of cryoenergy and radiofrequency for pulmonary vein isolation. Hear. Rhythm 2014, 11, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.J.; Baker, V.; Finlay, M.; Duncan, E.R.; Lovell, M.J.; Tayebjee, M.H.; Ullah, W.; Siddiqui, M.S.; McLean, A.; Richmond, L.; et al. Point-by-point radiofrequency ablation versus the cryoballoon or a novel combined approach: A randomized trial comparing 3 methods of pulmonary vein isolation for paroxysmal atrial fibrillation (the Cryo versus RF trial). J. Cardiovasc. Electrophysiol. 2015, 26, 1307–1314. [Google Scholar] [CrossRef]
- Luik, A.; Radzewitz, A.; Kieser, M.; Walter, M.; Bramlage, P.; Hörmann, P.; Schmidt, K.; Horn, N.; Brinkmeier-Theofanopoulou, M.; Kunzmann, K.; et al. Cryoballoon versus open irrigated radiofrequency ablation in patients with paroxysmal atrial fibrillation: The prospective, randomized, controlled, noninferiority FreezeAF study. Circulation 2015, 132, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Kuck, K.-H.; Brugada, J.; Fürnkranz, A.; Metzner, A.; Ouyang, F.; Chun, K.J.; Elvan, A.; Arentz, T.; Bestehorn, K.; Pocock, S.J.; et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N. Engl. J. Med. 2016, 374, 2235–2245. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.-I.; Arnold, A.; Younis, M.; Varghese, S.; Zeiher, A.M. Cryoballoon versus radiofrequency ablation for paroxysmal atrial fibrillation: A meta-analysis of randomized controlled trials. Clin. Res. Cardiol. 2018, 107, 658–669. [Google Scholar] [CrossRef]
- Georgiopoulos, G.; Tsiachris, D.; Manolis, A.S. Cryoballoon ablation of atrial fibrillation: A practical and effective approach: Cryoablation of atrial fibrillation. Clin. Cardiol. 2016, 40, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.G. Cryoballoon ablation for pulmonary vein isolation. J. Cardiovasc. Electrophysiol. 2020, 31, 2128–2135. [Google Scholar] [CrossRef]
- Di Giovanni, G.; Wauters, K.; Chierchia, G.-B.; Sieira, J.; Levinstein, M.; Conte, G.; de Asmundis, C.; Baltogiannis, G.; Saitoh, Y.; Ciconte, G.; et al. One-year follow-up after single procedure cryoballoon ablation: A comparison between the first- and second-generation balloon. J. Cardiovasc. Electrophysiol. 2014, 25, 834–839. [Google Scholar] [CrossRef]
- Liu, J.; Kaufmann, J.; Kriatselis, C.; Fleck, E.; Gerds-Li, J.H. Second generation of cryoballoons can improve efficiency of cryoablation for atrial fibrillation. Pacing Clin. Electrophysiol. 2014, 38, 129–135. [Google Scholar] [CrossRef]
- Ariyarathna, N.; Kumar, S.; Thomas, S.P.; Stevenson, W.G.; Michaud, G.F. Role of contact force sensing in catheter ablation of cardiac arrhythmias. JACC Clin. Electrophysiol. 2018, 4, 707–723. [Google Scholar] [CrossRef] [PubMed]
- Frisch, D.R.; Dikdan, S.J. Clinical and procedural effects of transitioning to contact force guided ablation for atrial fibrillation. J. Atr. Fibrillation 2019, 11, 2081. [Google Scholar] [CrossRef]
- Ullah, W.; McLean, A.; Tayebjee, M.H.; Gupta, D.; Ginks, M.R.; Haywood, G.A.; O’Neill, M.; Lambiase, P.; Earley, M.J.; Schilling, R.J. Randomized trial comparing pulmonary vein isolation using the SmartTouch catheter with or without real-time contact force data. Heart Rhythm 2016, 13, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Marijon, E.; Fazaa, S.; Narayanan, K.; Guy-Moyat, B.; Bouzeman, A.; Providência, R.; Treguer, F.; Combes, N.; Bortone, A.; Boveda, S.; et al. Real-time contact force sensing for pulmonary vein isolation in the setting of paroxysmal atrial fibrillation: Procedural and 1-year results: Real-time contact force sensing for pulmonary vein isolation. J. Cardiovasc. Electrophysiol. 2013, 25, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Oremus, M.; Wolfson, C.; Perrault, A.; Demers, L.; Momoli, F.; Moride, Y. interrater reliability of the modified Jadad quality scale for systematic reviews of Alzheimer’s disease drug trials. Dement. Geriatr. Cogn. Disord. 2001, 12, 232–236. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, L.; Liu, X.; Liu, L.; Wu, Y.; Zhao, Z.; Yi, D.; Yi, D. The effectiveness of the problem-based learning teaching model for use in introductory Chinese undergraduate medical courses: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0120884. [Google Scholar] [CrossRef] [PubMed]
- Waranugraha, Y.; Rizal, A.; Setiawan, D.; Aziz, I.J. Additional complex fractionated atrial electrogram ablation does not improve the outcomes of non-paroxysmal atrial fibrillation: A systematic review and meta-analysis of randomized controlled trials. Indian Heart J. 2020, 73, 63–73. [Google Scholar] [CrossRef]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (MINORS): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef]
- Öhlin, A.; Karlsson, L.; Senorski, E.H.; Jónasson, P.; Ahldén, M.; Baranto, A.; Ayeni, O.R.; Sansone, M. Quality assessment of prospective cohort studies evaluating arthroscopic treatment for femoroacetabular impingement syndrome: A systematic review. Orthop. J. Sports Med. 2019, 7, 232596711983853. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 1–13. [Google Scholar] [CrossRef]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef] [PubMed]
- Cleophas, T.J.; Zwinderman, A.H. Modern Meta-Analysis: Review and Update of Methodologies; Springer: Berlin, Germany, 2017. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J. What is heterogeneity and is it important? BMJ 2007, 334, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Waranugraha, Y.; Rizal, A.; Setiawan, D.; Aziz, I.J. The benefit of atrioventricular junction ablation for permanent atrial fibrillation and heart failure patients receiving cardiac resynchronization therapy: An updated systematic review and meta-analysis. Indian Pacing Electrophysiol. J. 2021, 21, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Waranugraha, Y.; Rizal, A.; Syaban, M.F.R.; Faratisha, I.F.D.; Erwan, N.E.; Yunita, K.C. Direct comparison of non-vitamin K antagonist oral anticoagulant versus warfarin for stroke prevention in non-valvular atrial fibrillation: A systematic review and meta-analysis of real-world evidence. Egypt. Heart J. 2021, 73, 70. [Google Scholar] [CrossRef]
- Syaban, M.F.R.; Yunita, K.C.; Faratisha, I.F.D.; Erwan, N.E.; Waranugraha, Y.; Rizal, A. Efficacy and safety of apixaban vs. warfarin in atrial fibrillation patients: Systematical review and meta-analysis. Heart Sci. J. 2022, 3, 28–36. [Google Scholar] [CrossRef]
- Giannopoulos, G.; Kossyvakis, C.; Vrachatis, D.; Aggeli, C.; Tsitsinakis, G.; Letsas, K.; Tsiachris, D.; Tsoukala, S.; Efremidis, M.; Katritsis, D.; et al. Effect of cryoballoon and radiofrequency ablation for pulmonary vein isolation on left atrial function in patients with nonvalvular paroxysmal atrial fibrillation: A prospective randomized study (Cryo-LAEF study). J. Cardiovasc. Electrophysiol. 2019, 30, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Gunawardene, M.A.; Hoffmann, B.A.; Schaeffer, B.; Chung, D.-U.; Moser, J.; Akbulak, R.O.; Jularic, M.; Eickholt, C.; Nuehrich, J.; Meyer, C.; et al. Influence of energy source on early atrial fibrillation recurrences: A comparison of cryoballoon vs. radiofrequency current energy ablation with the endpoint of unexcitability in pulmonary vein isolation. EP Eur. 2018, 20, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Hassan, W.S.; Zaky, S.H.; Mohamed, K.H.; Ibrahim, M.M. Smart touch radiofrequency catheter ablation versus cryoballoon ablation of pulmonary veins in patients with paroxysmal atrial fibrillation. Open Access Maced. J. Med. Sci. 2020, 8, 563–568. [Google Scholar] [CrossRef]
- Hisazaki, K.; Hasegawa, K.; Kaseno, K.; Miyazaki, S.; Amaya, N.; Shiomi, Y.; Tama, N.; Ikeda, H.; Fukuoka, Y.; Morishita, T.; et al. Endothelial damage and thromboembolic risk after pulmonary vein isolation using the latest ablation technologies: A comparison of the second-generation cryoballoon vs. contact force-sensing radiofrequency ablation. Heart Vessel. 2018, 34, 509–516. [Google Scholar] [CrossRef]
- Jourda, F.; Providencia, R.; Marijon, E.; Bouzeman, A.; Hireche, H.; Khoueiry, Z.; Cardin, C.; Combes, N.; Combes, S.; Boveda, S.; et al. Contact-force guided radiofrequency vs. second-generation balloon cryotherapy for pulmonary vein isolation in patients with paroxysmal atrial fibrillation—A prospective evaluation. EP Eur. 2015, 17, 225–231. [Google Scholar] [CrossRef]
- Kardos, A.; Kis, Z.; Som, Z.; Nagy, Z.; Foldesi, C. Two-year follow-up after contact force sensing radiofrequency catheter and second-generation cryoballoon ablation for paroxysmal atrial fibrillation: A comparative single centre study. BioMed Res. Int. 2016, 2016, 1–6. [Google Scholar] [CrossRef][Green Version]
- Matta, M.; Anselmino, M.; Ferraris, F.; Scaglione, M.; Gaita, F. Cryoballoon vs. radiofrequency contact force ablation for paroxysmal atrial fibrillation: A propensity score analysis. J. Cardiovasc. Med. 2018, 19, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Squara, F.; Zhao, A.; Marijon, E.; Latcu, D.G.; Providência, R.; Di Giovanni, G.; Jauvert, G.; Jourda, F.; Chierchia, G.-B.; de Asmundis, C.; et al. Comparison between radiofrequency with contact force-sensing and second-generation cryoballoon for paroxysmal atrial fibrillation catheter ablation: A multicentre European evaluation. EP Eur. 2015, 17, 718–724. [Google Scholar] [CrossRef]
- Tanaka, N.; Tanaka, K.; Ninomiya, Y.; Hirao, Y.; Oka, T.; Okada, M.; Inoue, H.; Nakamaru, R.; Takayasu, K.; Kitagaki, R.; et al. Comparison of the safety and efficacy of automated annotation-guided radiofrequency ablation and 2nd-generation cryoballoon ablation in paroxysmal atrial fibrillation. Circ. J. 2019, 83, 548–555. [Google Scholar] [CrossRef]
- Watanabe, R.; Sairaku, A.; Yoshida, Y.; Nanasato, M.; Kamiya, H.; Suzuki, H.; Ogura, Y.; Aoyama, Y.; Maeda, M.; Ando, M.; et al. Head-to-head comparison of acute and chronic pulmonary vein stenosis for cryoballoon versus radiofrequency ablation. Pacing Clin. Electrophysiol. 2018, 41, 376–382. [Google Scholar] [CrossRef]
- Xiao, F.-Y.; Ju, W.-Z.; Chen, H.-W.; Huang, W.-J.; Chen, M.-L. A comparative study of pericardial effusion and pleural effusion after cryoballoon ablation or radiofrequency catheter ablation of atrial fibrillation. J. Cardiovasc. Electrophysiol. 2020, 31, 1062–1067. [Google Scholar] [CrossRef] [PubMed]
- Yokokawa, M.; Chugh, A.; Latchamsetty, R.; Ghanbari, H.; Crawford, T.; Jongnarangsin, K.; Cunnane, R.; Saeed, M.; Hornsby, K.; Krishnasamy, K.; et al. Ablation of paroxysmal atrial fibrillation using a second-generation cryoballoon catheter or contact-force sensing radiofrequency ablation catheter: A comparison of costs and long-term clinical outcomes. J. Cardiovasc. Electrophysiol. 2018, 29, 284–290. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cigarroa, J.E.; Cleveland, J.C.; Conti, J.B.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. J. Am. Coll. Cardiol. 2014, 64, e1–e76. [Google Scholar] [CrossRef] [PubMed]
- Brieger, D.; Amerena, J.; Attia, J.; Bajorek, B.; Chan, K.H.; Connell, C.; Freedman, B.; Ferguson, C.; Hall, T.; Haqqani, H.; et al. National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand: Australian clinical guidelines for the diagnosis and management of atrial fibrillation 2018. Heart Lung Circ. 2018, 27, 1209–1266. [Google Scholar] [CrossRef] [PubMed]
- Michaud, G.F.; Kumar, S. Pulmonary vein isolation in the treatment of atrial fibrillation. Res. Rep. Clin. Cardiol. 2016, 7, 47–60. [Google Scholar] [CrossRef]
- Kaszala, K.; Ellenbogen, K.A. Biophysics of the second-generation cryoballoon: Cryobiology of the big freeze. Circ. Arrhythmia Electrophysiol. 2015, 8, 15–17. [Google Scholar] [CrossRef]
- Fürnkranz, A.; Bordignon, S.; Schmidt, B.; Gunawardene, M.; Schulte-Hahn, B.; Urban, V.; Bode, F.; Nowak, B.; Chun, J.K.R. Improved procedural efficacy of pulmonary vein isolation using the novel second-generation cryoballoon: Efficacy of the novel ccryoballoon. J. Cardiovasc. Electrophysiol. 2012, 24, 492–497. [Google Scholar] [CrossRef]
- Conti, S.; Moltrasio, M.; Fassini, G.; Tundo, F.; Riva, S.; Russo, A.D.; Casella, M.; Majocchi, B.; Marino, V.; De Iuliis, P.; et al. Comparison between first- and second-generation cryoballoon for paroxysmal atrial fibrillation ablation. Cardiol. Res. Pract. 2016, 2016, 5106127. [Google Scholar] [CrossRef][Green Version]
- Reddy, V.Y.; Shah, D.; Kautzner, J.; Schmidt, B.; Saoudi, N.; Herrera, C.; Jaïs, P.; Hindricks, G.; Peichl, P.; Yulzari, A.; et al. The relationship between contact force and clinical outcome during radiofrequency catheter ablation of atrial fibrillation in the TOCCATA study. Heart Rhythm 2012, 9, 1789–1795. [Google Scholar] [CrossRef]
- Jiang, J.; Li, J.; Zhong, G.; Jiang, J. Efficacy and safety of the second-generation cryoballoons versus radiofrequency ablation for the treatment of paroxysmal atrial fibrillation: A systematic review and meta-analysis. J. Interv. Card. Electrophysiol. 2016, 48, 69–79. [Google Scholar] [CrossRef]
- Buist, T.J.; Adiyaman, A.; Smit, J.J.J.; Misier, A.R.R.; Elvan, A. Arrhythmia-free survival and pulmonary vein reconnection patterns after second-generation cryoballoon and contact-force radiofrequency pulmonary vein isolation. Clin. Res. Cardiol. 2018, 107, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.G.; Champagne, J.; Dubuc, M.; Deyell, M.W.; Verma, A.; Macle, L.; Leong-Sit, P.; Novak, P.; Badra-Verdu, M.; Sapp, J.; et al. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: A randomized clinical trial. Circulation 2019, 140, 1779–1788. [Google Scholar] [CrossRef]
- Ravi, V.; Poudyal, A.; Pulipati, P.; Do, T.L.; Krishnan, K.; Trohman, R.G.; Sharma, P.S.; Huang, H.D. A systematic review and meta-analysis comparing second-generation cryoballoon and contact force radiofrequency ablation for initial ablation of paroxysmal and persistent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2020, 31, 2559–2571. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, W.; Yao, J.; Chen, L.; Yi, S. Second-generation cryoballoon vs. contact-force sensing radiofrequency catheter ablation in atrial fibrillation: A meta-analysis of randomized controlled trials. J. Interv. Card. Electrophysiol. 2021, 60, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Waranugraha, Y.; Rizal, A.; Firdaus, A.J.; Sihotang, F.A.; Akbar, A.R.; Lestari, D.D.; Firdaus, M.; Nurudinulloh, A.I. The superiority of high-power short-duration radiofrequency catheter ablation strategy for atrial fibrillation treatment: A systematic review and meta-analysis study. J. Arrhythmia 2021, 37, 975–989. [Google Scholar] [CrossRef] [PubMed]
- Baher, A.; Kheirkhahan, M.; Rechenmacher, S.J.; Marashly, Q.; Kholmovski, E.G.; Siebermair, J.; Acharya, M.; Aljuaid, M.; Morris, A.K.; Kaur, G.; et al. High-power radiofrequency catheter ablation of atrial fibrillation. JACC Clin. Electrophysiol. 2018, 4, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Kautzner, J.; Natale, A.; Peichl, P.; Cihak, R.; Wichterle, D.; Ikeda, A.; Santangeli, P.; Di Biase, L.; Jackman, W.M. Locations of high contact force during left atrial mapping in atrial fibrillation patients. Circ. Arrhythmia Electrophysiol. 2013, 6, 746–753. [Google Scholar] [CrossRef]

| Author | Study Design | Mapping System | CBA Strategy | RFA Strategy | Blanking Period | Follow-Up Period | AADs Treatment during Follow-Up Period | Arrhythmia Detection Methods |
|---|---|---|---|---|---|---|---|---|
| Giannopoulos et al., 2019 [42] | RCT–MC | CARTO 3 | 28 mm 2G-CB 240 → 180 s/vein | CF-RF | 2 months | 6 months | No | 12-lead ECG 24 h Holter monitor |
| Gunawardene et al., 2018 [43] | RCT–SC | CARTO 3 | 28 mm 2G-CB 1 × 240 s/vein | CF-RF FR 17–30 mL/min Power ≤ 30 W Duration 30–60 s Temperature ≤ 45 °C CF ≥ 10 g | 3 months | 10.3 ± 2.1 months | No | 12-lead ECG 24 h Holter monitor |
| Hassan et al., 2020 [44] | Cohort–SC | CARTO 3 | 28 mm 2G-CB 2 × 240 s/vein | CF-RF FR 17–20 mL/min Power 30–35 W Duration 20–40 s FTI > 400 gs | 3 months | 12 months | No | 12-lead ECG 24 h Holter monitor |
| Hisazaki et al., 2019 [45] | Cohort–SC | CARTO 3 | 28 mm 2G-CB 2 × 180 s/vein | CF-RF Power ≤ 35 W CF ≥ 10 g | 3 months | 20 ± 6 months | No/Yes | 12-lead ECG 24 h Holter monitor |
| Jourda et al., 2015 [46] | Cohort–SC | CARTO 3 | 28 mm 2G-CB 2 × 240 s/vein | CF-RF FR 17–20 mL/min Power ≤ 30 W Temperature ≤ 48 °C | 3 months | 12 months | No | 12-lead ECG 24 h Holter monitor |
| Kardos et al., 2016 [47] | Cohort–SC | CARTO 3 | 28 mm 2G-CB ≥1 × 240 s/vein | CF-RF Power ≤ 35 W Duration 20–40 s Temperature ≤ 48 °C | 3 months | 24 months | No | 12-lead ECG 24 h Holter monitor |
| Matta et al., 2018 [48] | Cohort–MC | CARTO 3 | 28 mm 2G-CB 180 → 240 s/vein | CF-RF CF 5–15 g | 3 months | 12 ± 5 months | No/Yes | 12-lead ECG 24 to 48 h Holter monitor |
| Squara et al., 2015 [49] | Cohort–MC | CARTO 3 EnSite | 23 or 28 mm 2G-CB 2 × 240 s/vein | CF-RF Power 30–35 W Duration 20–40 FTI > 400 gs | 1 months | 12 (10–18) months | No | 12-lead ECG 24 h Holter monitor |
| Tanaka et al., 2019 [50] | Cohort–SC | CARTO 3 EnSite | 28 mm 2G-CB 2 × 180 s/vein | CF-RF Duration ≥ 20 s CF ≥ 5 g FTI ≥ 150 gs | 3 months | 2.98 years (median) | No | 12-lead ECG Holter monitor External loop recorder |
| Watanabe et al., 2018 [51] | RCT–SC | CARTO 3 | 28 mm 2G-CB 2 × 180 s/vein | CF-RF FR 17 mL/min Power ≤ 30 W CF ≥ 10 g | NA | 12 months | No/Yes | 12-lead ECG 24 to 48 h Holter monitor |
| Xiao et al., 2020 [52] | Cohort–MC | CARTO 3 | 28 mm 2G-CB 1 × ≥ 180 s/vein | CF-RF FR 17–25 mL/min Power 25 to 35 W Temperature ≤ 43 °C CF 10–30 g | 3 months | 12 months | No | 12-lead ECG 24 h Holter monitor 7 d Holter monitor |
| Yokokawa et al., 2017 [53] | Cohort–SC | CARTO 3 EnSite | 28 mm 2G-CB 1 × 180 or 240 s/vein | CF-RF FR 30 mL/min Power ≤ 35 W Temperature ≤ 48 °C | 3 months | 25 ± 5 months | No | Auto-triggered event monitor |
| Author | Group | Patients | Age, Years | Male | Hypertension | CAD | Heart Failure | Sleep Apnea | DM | Stroke or TIA | LVEF, % | LAVI, mL/m2 | LAD, mm |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Giannopoulos, 2019 [42] | 2G-CB | 80 | 61.0 ± 2.5 | NA | 41 (51.3) | 6 (7.5) | 2 (2.5) | NA | 9 (11.3) | NA | 59.9 ± 2.3 | NA | 41.4 ± 4.3 |
| CF-RF | 40 | 58.3 ± 3.0 | NA | 18 (45.0) | 2 (5.0) | 2 (5.0) | NA | 6 (15.0) | NA | 60.0 ± 2.3 | NA | 39.9 ± 1.4 | |
| Gunawardene, 2018 [43] | 2G-CB | 30 | 62.0 ± 9.5 | 18 (60.0) | 16 (53.0) | NA | NA | NA | NA | NA | 59.8 ± 4.5 | NA | NA |
| CF-RF | 30 | 57.4 ± 10.5 | 24 (80.0) | 17 (56.0) | NA | NA | NA | NA | NA | 59.2 ± 5.0 | NA | NA | |
| Hassan et al., 2020 [44] | 2G-CB | 25 | 47.9 ± 11.6 | 15 (60.0) | 6 (24.0) | 2 (8.0) | 1 (4.0) | NA | 7 (28.0) | NA | 61.2 ± 5.7 | NA | 41.0 ± 3.8 |
| CF-RF | 25 | 45.9 ± 12.4 | 17 (68.0) | 5 (20.0) | 1 (4.0) | 2(8.0) | NA | 5 (20.0) | NA | 62.1 ± 7.8 | NA | 40.9 ± 5.7 | |
| Hisazaki et al., 2019 [45] | 2G-CB | 64 | 64.0 ± 12.0 | 40 (63.0) | 32 (50.0) | NA | NA | NA | NA | NA | 68.0 ± 8.0 | NA | 35.0 ± 5.0 |
| CF-RF | 22 | 67.0 ± 12.0 | 15 (68.0) | 10 (45.0) | NA | NA | NA | NA | NA | 67.0 ± 8.0 | NA | 36.0 ± 5.0 | |
| Jourda, et al., 2015 [46] | 2G-CB | 75 | 59.9 ± 10.6 | 20 (26.7) | 26 (34.7) | NA | 5 (6.7) | 9 (12.0) | 6 (8.0) | 3 (4.0) | 64.4 ± 7.4 | 42.8 ± 15.2 | NA |
| CF-RF | 75 | 62.5 ± 8.9 | 18 (24.0) | 36 (48.0) | NA | 2 (2.7) | 4 (5.3) | 3 (4.0) | 8 (10.7) | 65.5 ± 5.6 | 39.5 ± 11.3 | NA | |
| Kardos, et al., 2016 [47] | 2G-CB | 40 | 59.0 ± 10.0 | 27 (67.5) | 17 (42.5) | 5 (12.5) | NA | NA | 2 (5.0) | NA | NA | NA | 41.3 ± 4.0 |
| CF-RF | 58 | 61.0 ± 9.0 | 38 (66.0) | 30 (51.0) | 7 (12.0) | NA | NA | 3 (5.1) | NA | NA | NA | 42.1 ± 4.6 | |
| Matta, et al., 2018 [48] | 2G-CB | 46 | 59.0 ± 9.0 | 36 (78.0) | 21 (46.0) | 3 (7.0) | 1 (2.0) | 2 (4.0) | 3 (7.0) | 0 (0.0) | 61.0 ± 5.0 | NA | NA |
| CF-RF | 46 | 59.0 ± 9.0 | 38 (82.0) | 21 (46.0) | 3 (7.0) | 2 (4.0) | 3 (7.0) | 3 (7.0) | 1 (2.0) | 61.0 ± 6.0 | NA | NA | |
| Squara, et al., 2015 [49] | 2G-CB | 178 | 58.4 ± 11.5 | 128 (71.9) | 55 (30.1) | NA | NA | NA | 14 (7.9) | NA | 56.6 ± 7.7 | NA | NA |
| CF-RF | 198 | 61.0 ± 9.0 | 153 (77.3) | 74 (37.4) | NA | NA | NA | 13 (6.6) | NA | 55.8 ± 9.2 | NA | NA | |
| Tanaka, et al., 2019 [50] | 2G-CB | 70 | 64.1 ± 10.1 | 52 (74.0) | 40 (57.0) | NA | 1 (1.0) | NA | 7 (10.0) | 9 (13.0) | 68.0 ± 9.1 | NA | 37.1 ± 5.7 |
| CF-RF | 61 | 63.4 ± 10.5 | 42 (69.0) | 38 (62.0) | NA | 2 (3.0) | NA | 8 (13.0) | 4 (7.0) | 67.1 ± 6.6 | NA | 36.9 ± 4.7 | |
| Watanabe, et al., 2018 [41] | 2G-CB | 25 | 62.0 ± 12.0 | 17 (68.0) | 16 (64.0) | NA | 2 (8.0) | NA | 3 (12.0) | 1 (4.0) | 63.0 ± 5.0 | NA | 39.0 ± 6.0 |
| CF-RF | 25 | 68.0 ± 9.0 | 19 (76.0) | 14 (56.0) | NA | 2 (8.0) | NA | 5 (20.0) | 2 (8.0) | 58.0 ± 8.0 | NA | 42.0 ± 5.0 | |
| Xiao, et al., 2020 [52] | 2G-CB | 30 | 64.5 ± 12.1 | 17 (56.7) | NA | 7 (23.3) | NA | NA | NA | NA | 63.1 ± 9.6 | NA | 41.9 ± 5.2 |
| CF-RF | 30 | 64.1 ± 8.3 | 19 (63.3) | NA | 5 (16.7) | NA | NA | NA | NA | 66.4 ± 7.9 | NA | 40.8 ± 4.9 | |
| Yokokawa et al., 2017 [53] | 2G-CB | 71 | 63.0 ± 10.0 | 53 (75.0) | 40 (56.0) | 10 (14.0) | NA | NA | NA | NA | 59.0 ± 6.0 | NA | 42.5 ± 6.0 |
| CF-RF | 75 | 62.0 ± 9.0 | 42 (56.0) | 47 (63.0) | 5 (6.0) | NA | NA | NA | NA | 60.0 ± 5.0 | NA | 42.5 ± 6.0 | |
| Overall | 1419 | 60.8 ± 1.1 | 65.3 | 45.6 | 9.9 | 4.0 | 7.4 | 9.1 | 6.6 | 62.0 ± 1.3 | 40.7 ± 2.1 | 40.0 ± 1.1 |
| Parameters | Number of Studies | 2G-CB | CF-RF | Model | OR | 95% CI | p-Value of Heterogeneity | I2 (%) | p-Value of Begg’s Test | p-Value of Egger’s Test | p | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Event, n (%) | Total, n | Event, n (%) | Total, n | ||||||||||
| Primary outcomes | |||||||||||||
| Freedom from ATAs | 12 | 579 (78.9) | 734 | 548 (80.0) | 685 | Random | 0.89 | 0.68 to 1.17 | 0.68 | 0 | 0.73 | 0.89 | 0.41 |
| Secondary outcomes | |||||||||||||
| Freedom from AF | 8 | 332 (79.8) | 416 | 270 (79.9) | 338 | Random | 0.93 | 0.65 to 1.35 | 0.95 | 0 | 0.71 | 0.63 | 0.72 |
| Acute PVI | 12 | 2916 (99.5) | 2931 | 2722 (99.5) | 2737 | Random | 1.17 | 0.54 to 2.53 | 0.43 | 0 | 0.81 | 0.08 | 0.70 |
| All-procedural complications | 9 | 38 (6.3) | 599 | 29 (4.9) | 590 | Random | 1.28 | 0.75 to 2.18 | 0.65 | 0 | 1.00 | 0.57 | 0.36 |
| Pericardial effusion/cardiac tamponade | 5 | 0 (0.0) | 360 | 7 (1.7) | 402 | Random | 0.29 | 0.07 to 1.19 | 1.00 | 0 | 0.81 | 0.06 | 0.09 |
| Phrenic nerve paralysis | 7 | 22 (4.6) | 478 | 0 (0.0) | 469 | Random | 5.74 | 1.80 to 18.31 | 0.88 | 0 | 0.13 | 0.07 | <0.01 |
| Vascular complications | 5 | 11 (2.8) | 400 | 15 (3.5) | 424 | Random | 0.78 | 0.34 to 1.80 | 0.69 | 0 | 0.81 | 0.79 | 0.57 |
| Parameters | Number of Studies | 2G-CB, n | CF-RF, n | Model | MD, Minutes | 95% CI, Minutes | p-Value of Heterogeneity | I2 (%) | p-Value of Begg’s Test | p-Value of Egger’s Test | p |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Procedure time | 11 | 709 | 660 | Random | −18.78 | −27.72 to −9.85 | <0.01 | 90 | 0.44 | 0.89 | <0.01 |
| Fluoroscopy time | 11 | 709 | 660 | Random | 2.66 | −0.52 to 5.83 | <0.01 | 95 | 0.44 | 0.19 | 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waranugraha, Y.; Rizal, A.; Yuniadi, Y. A Systematic Review and Meta-Analysis of the Direct Comparison of Second-Generation Cryoballoon Ablation and Contact Force-Sensing Radiofrequency Ablation in Patients with Paroxysmal Atrial Fibrillation. J. Pers. Med. 2022, 12, 298. https://doi.org/10.3390/jpm12020298
Waranugraha Y, Rizal A, Yuniadi Y. A Systematic Review and Meta-Analysis of the Direct Comparison of Second-Generation Cryoballoon Ablation and Contact Force-Sensing Radiofrequency Ablation in Patients with Paroxysmal Atrial Fibrillation. Journal of Personalized Medicine. 2022; 12(2):298. https://doi.org/10.3390/jpm12020298
Chicago/Turabian StyleWaranugraha, Yoga, Ardian Rizal, and Yoga Yuniadi. 2022. "A Systematic Review and Meta-Analysis of the Direct Comparison of Second-Generation Cryoballoon Ablation and Contact Force-Sensing Radiofrequency Ablation in Patients with Paroxysmal Atrial Fibrillation" Journal of Personalized Medicine 12, no. 2: 298. https://doi.org/10.3390/jpm12020298
APA StyleWaranugraha, Y., Rizal, A., & Yuniadi, Y. (2022). A Systematic Review and Meta-Analysis of the Direct Comparison of Second-Generation Cryoballoon Ablation and Contact Force-Sensing Radiofrequency Ablation in Patients with Paroxysmal Atrial Fibrillation. Journal of Personalized Medicine, 12(2), 298. https://doi.org/10.3390/jpm12020298






