Increased Complement Activation in Systemic Sclerosis Patients with Skin and Lung Fibrosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Clinical Correlates of SSc Patients
2.3. Evaluation of Complement Cascade
2.4. Statystical Analysis
3. Results
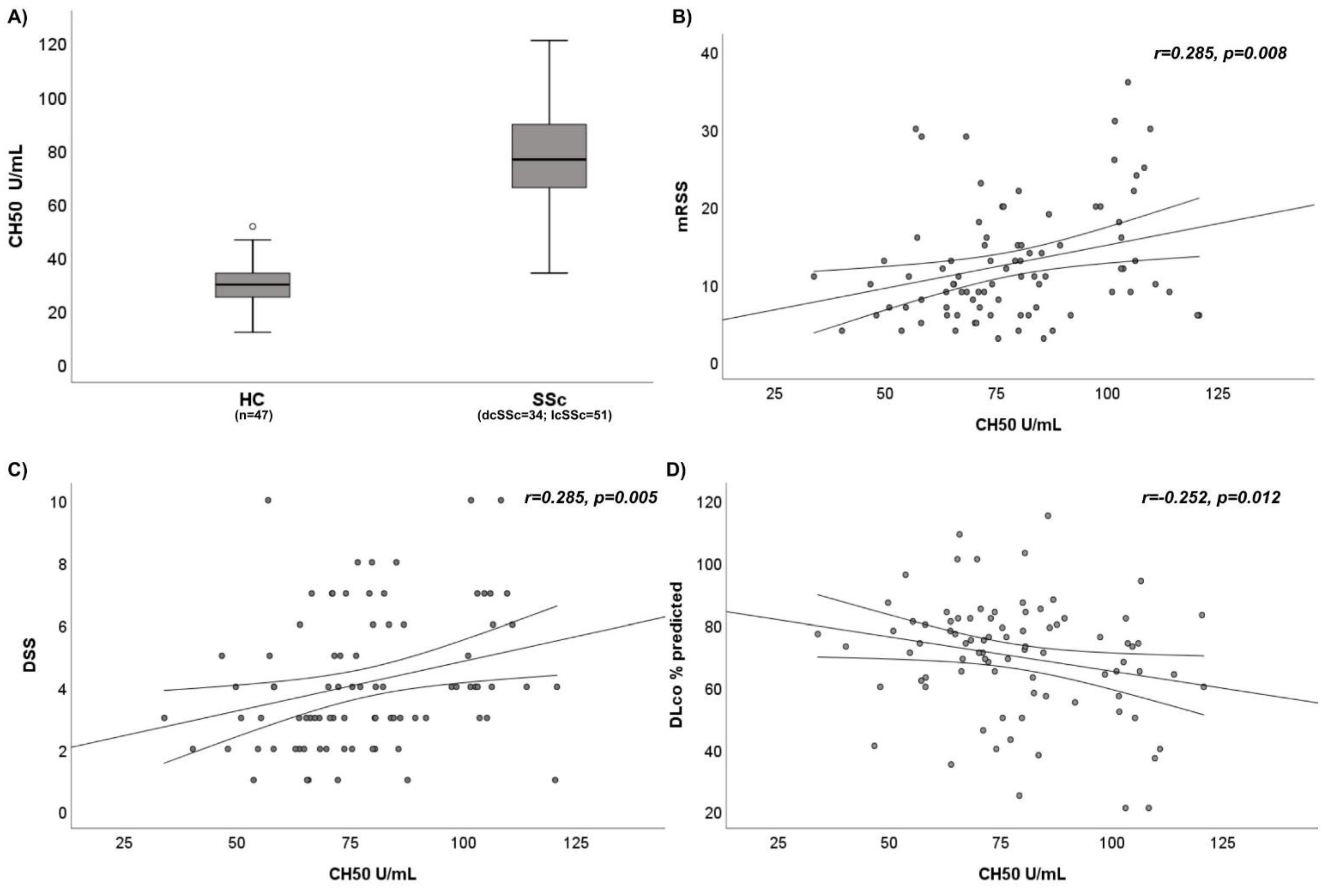
3.1. Evaluation of CH50 and Complement Fractions in SSc Patients
3.2. Analysis of Disease Variables and Complement Assessment in SSc Patients
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kowal-Bielecka, O.; Fransen, J.; Avouac, J.; Becker, M.; Kulak, A.; Allanore, Y.; Distler, O.; Clements, P.; Cutolo, M.; Czirjak, L.; et al. EUSTAR Coauthors. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann. Rheum. Dis. 2017, 76, 1327–1339. [Google Scholar] [CrossRef] [Green Version]
- Matucci-Cerinic, M.; Kahaleh, B.; Wigley, F.M. Review: Evidence that systemic sclerosis is a vascular disease. Arthritis Rheum. 2013, 65, 1953–1962. [Google Scholar] [CrossRef]
- Chizzolini, C.; Brembilla, N.C.; Montanari, E.; Truchetet, M.E. Fibrosis and immune dysregulation in systemic sclerosis. Autoimmun. Rev. 2011, 10, 276–281. [Google Scholar] [CrossRef]
- Sakkas, L.I.; Chikanza, I.C.; Platsoucas, C.D. Mechanisms of Disease: The role of immune cells in the pathogenesis of systemic sclerosis. Nat. Clin. Pract. Rheumatol. 2006, 2, 679–685. [Google Scholar] [CrossRef]
- Mihai, C.; Tervaert, J.W. Anti-endothelial cell antibodies in systemic sclerosis. Ann. Rheum. Dis. 2010, 69, 319–324. [Google Scholar] [CrossRef]
- Magro, C.M.; Marsh, C.B.; Allen, J.N.; Ross, P.; Liff, D.; Knight, D.A.; Waldman, W.J.; Nadasdy, T.; Cowden, D.J. The role of anti-endothelial cell antibody-mediated microvascular injury in the evolution of pulmonary fibrosis in the setting of collagen vascular disease. Am. J. Clin. Pathol. 2007, 127, 237–247. [Google Scholar] [CrossRef]
- Gabrielli, A.; Avvedimento, E.V.; Krieg, T. Scleroderma. N. Engl. J. Med. 2009, 360, 1989–2003. [Google Scholar] [CrossRef]
- Osthoff, M.; Jaeger, V.K.; Heijnen, I.A.F.M.; Trendelenburg, M.; Jordan, S.; Distler, O.; Walker, U.A. Role of lectin pathway complement proteins and genetic variants in organ damage and disease severity of systemic sclerosis: A cross-sectional study. Arthritis Res. Ther. 2019, 21, 76. [Google Scholar] [CrossRef] [Green Version]
- Ricklin, D.; Hajishengallis, G.; Yang, K.; Lambris, J.D. Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 2011, 11, 785–797. [Google Scholar] [CrossRef] [Green Version]
- Dempsey, P.W.; Allison, M.E.; Akkaraju, S.; Goodnow, C.C.; Fearon, D.T. C3d of complement as a molecular adjuvant: Bridging innate and acquired immunity. Science 1996, 271, 348–350. [Google Scholar] [CrossRef] [Green Version]
- Trouw, L.A.; Pickering, M.C.; Blom, A.M. The complement system as a potential therapeutic target in rheumatic disease. Nat. Rev. Rheumatol. 2017, 13, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Leffler, J.; Bengtsson, A.A.; Blom, A.M. The complement system in systemic lupus erythematosus: An update. Ann. Rheum. Dis. 2014, 73, 1601–1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, J.; Brown, Z.; Stevens, W.; Sahhar, J.; Rabusa, C.; Zochling, J.; Roddy, J.; Walker, J.; Proudman, S.M.; Nikpour, M. The association of low complement with disease activity in systemic sclerosis: A prospective cohort study. Arthritis Res. Ther. 2016, 18, 246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, M.; Murali, M. Analysis of the Complement System in the Clinical Immunology Laboratory. Clin. Lab. Med. 2019, 39, 579–590. [Google Scholar] [CrossRef]
- LeRoy, E.C.; Black, C.M.; Fleischmajer, R.; Jablonska, S.; Krieg, T.; Medsger, T.A., Jr.; Rowell, N.; Wollheim, F. Scleroderma (systemic sclerosis): Classification, subsets and pathogenesis. J. Rheumatol. 1988, 15, 202–205. [Google Scholar]
- Cutolo, M.; Sulli, A.; Secchi, M.E.; Paolino, S.; Pizzorni, C. Nailfold capillaroscopy is useful for the diagnosis and follow-up of autoimmune rheumatic diseases. A future tool for the analysis of microvascular heart involvement? Rheumatology 2006, 45 (Suppl. 4), iv43–iv46. [Google Scholar] [CrossRef] [Green Version]
- Khanna, D.; Furst, D.E.; Clements, P.J.; Allanore, Y.; Baron, M.; Czirjak, L.; Distler, O.; Foeldvari, I.; Kuwana, M.; Matucci-Cerinic, M.; et al. Standardization of the modified Rodnan skin score for use in clinical trials of systemic sclerosis. J. Scleroderma Relat. Disord. 2017, 2, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Amanzi, L.; Braschi, F.; Fiori, G.; Galluccio, F.; Miniati, I.; Guiducci, S.; Conforti, M.-L.; Kaloudi, O.; Nacci, F.; Sacu, O.; et al. Digital ulcers in scleroderma: Staging, characteristics, and sub-setting through observation of 1614 digital lesions. Rheumatology 2010, 49, 1374–1382. [Google Scholar] [CrossRef] [Green Version]
- Valentini, G.; Iudici, M.; Walker, U.A.; Jaeger, V.; Baron, M.; Carreira, P.E.; Czirják, L.; Denton, C.P.; Distler, O.; Hachulla, E.; et al. The European Scleroderma Trials and Research group (EUSTAR) task force for the development of revised activity criteria for systemic sclerosis: Derivation and validation of a preliminarily revised EUSTAR activity index. Ann. Rheum. Dis. 2017, 76, 270–276. [Google Scholar] [CrossRef]
- Medsger, T.A., Jr.; Silman, A.J.; Steen, V.D.; Black, C.M.; Akesson, A.; Bacon, P.A.; Harris, C.A.; Jablonska, S.; Jayson, M.I.; Jimenez, S.A.; et al. A disease severity scale for systemic sclerosis: Development and testing. J. Rheumatol. 1999, 26, 2159–2167. [Google Scholar]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of Echocardiography Endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Crapo, R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Enright, P.; van der Grinten, C.P.M.; Gustafsson, P.; et al. ATS/ERS Task Force. General considerations for lung function testing. Eur. Respir. J. 2005, 26, 153–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goh, N.S.L.; Desai, S.R.; Veeraraghavan, S.; Hansell, D.M.; Copley, S.J.; Maher, T.M.; Corte, T.J.; Sander, C.R.; Ratoff, J.; Devaraj, A.; et al. Interstitial lung disease in systemic sclerosis: A simple staging system. Am. J. Respir. Crit. Care Med. 2008, 177, 1248–1254. [Google Scholar] [CrossRef]
- CLSI. Procedures for the Handling and Processing of Blood Specimens for Common Laboratory Tests. In Approved Guideline, 4th ed.; CLSI Document GP44-A4; Clinical and Laboratory Standard Institute: Wayne, PA, USA, 2010. [Google Scholar]
- Valentini, G.; Della Rossa, A.; Bombardieri, S.; Bencivelli, W.; Silman, A.J.; D’Angelo, S.; Cerinic, M.M.; Belch, J.J.; Black, C.M.; Bruhlmann, P.; et al. European multicentre study to define disease activity criteria for systemic sclerosis. II. Identification of disease activity variables and development of preliminary activity indexes. Ann. Rheum. Dis. 2001, 60, 592–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foocharoen, C.; Distler, O.; Becker, M.; Müller-Ladner, U.; von Mühlen, C.; Leuchten, N.; Walker, U. Clinical correlations of hypocomplementaemia in systemic sclerosis: An analysis of the EULAR Scleroderma Trial and Research group (EUSTAR) database. Scand. J. Rheumatol. 2012, 41, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Senaldi, G.; Lupoli, S.; Vergani, D.; Black, C.M. Activation of the complement system in systemic sclerosis: Relationship to clinical severity. Arthritis Rheum. 1989, 32, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Benbassat, C.; Schlesinger, M.; Luderschmidt, C.; Valentini, G.; Tirri, G.; Shoenfeld, Y. The complement system and systemic sclerosis. Immunol. Res. 1993, 12, 312–316. [Google Scholar] [CrossRef]
- Venneker, G.T.; van der Hoogen, F.H.; Boerbooms, A.M.; Bos, J.D.; Asghar, S.S. Aberrant expression of membrane cofactor protein and decay-accelerating factor in the endothelium of patients with systemic sclerosis. A possible mechanism of vascular damage. Lab. Investig. 1994, 70, 830–835. [Google Scholar]
- Venneker, G.T. Morphea lesions are associated with aberrant expression of membrane cofactor protein and decay accelerating factor in vascular endothelium. Br. J. Dermatol. 1994, 131, 237–242. [Google Scholar] [CrossRef]
- Scambi, C.; Ugolini, S.; Jokiranta, T.S.; De Franceschi, L.; Bortolami, O.; La Verde, V.; Guarini, P.; Caramaschi, P.; Ravagnani, V.; Martignoni, G.; et al. The local complement activation on vascular bed of patients with systemic sclerosis: A hypothesis-generating study. PLoS ONE 2015, 10, e0114856. [Google Scholar] [CrossRef]
| Age, years, median and IQR | 56 (48–64) |
| Female, n (%) | 76 (89.4) |
| dcSSc, n (%) | 34 (40) |
| Disease duration, years, median and IQR | 12 (7–16) |
| mRSS, median and IQR | 11 (7–16) |
| SSc-specific autoantibodies: | |
| Anti-topoisomerase I, n (%) | 29 (34.1) |
| Anti-centromere, n (%) | 22 (25.9) |
| None, n (%) | 34 (60) |
| Nailfold capillaroscopic pattern | |
| Early, n (%) | 15 (17.6) |
| Active, n (%) | 27 (31.8) |
| Late, n (%) | 43 (50.6) |
| DAI, median and IQR | 1.5 (0.8–2.8) |
| DSS, median and IQR | 4 (3–6) |
| sPAP, mmHg, median and IQR | 27 (25–31) |
| DLco, % of predicted, median and IQR | 73 (60–81) |
| ILD, n (%) | 62 (72.9) |
| Active DUs, n (%) | 7 (8.2) |
| DUs history, n (%) | 47 (55.3) |
| ESR, mm/h, median and IQR | 22 (12–29) |
| CRP, mcg/L, median and IQR | 1850 (1000–4600) |
| SSc | HC | p | |
|---|---|---|---|
| CH50, U/mL, median and IQR | 76.3 (65.8–89.4) | 29.6 (24.7–34) | p < 0.0001 * |
| C2, mg/L, median and IQR | 26.1 (24.1–32.1) | 22.7 (20.6–24.4) | p < 0.0001 * |
| C3, g/L, median and IQR | 1.07 (0.99–1.12) | 0.84 (0.66–0.98) | p > 0.05 |
| C4, g/L, median and IQR | 0.2 (0.16–0.23) | 0.36 (0.27–0.41) | p > 0.05 |
| IgG, g/L, median and IQR | 10.4 (9.4–12.3) | 10.6 (9.2–14.2) | p > 0.05 |
| IgA, g/L, median and IQR | 2.37 (1.87–3.21) | 2.52 (1.93–3.44) | p > 0.05 |
| IgM, g/L, median and IQR | 1.27 (0.85–1.78) | 1.02 (0.78–1.88) | p > 0.05 |
| Age | Disease Duration | mRSS | DAI | DSS | sPAP | DLco | FVC | ESR | CRP | |
|---|---|---|---|---|---|---|---|---|---|---|
| CH50 | r = 0.062, p = 0.286 | r = 0.025, p = 0.411 | r = 0.285, p = 0.008 * | r = 0.261, p = 0.009 * | r = 0.285, p = 0.005 * | r = 0.227, p = 0.021 * | r = −0.252, p = 0.012 * | r = −0.113, p = 0.151 | r = 0.249, p = 0.012 * | r = 0.260, p = 0.01 * |
| C2 | r = 0.130, p = 0.118 | r = 0.099, p = 0.184 | r = −0.079, p = 0.237 | r = −0.160, p = 0.072 | r = −0.116, p = 0.146 | r = −0.053, p = 0.317 | r = 0.139, p = 0.102 | r = 0.042, p = 0.350 | r = 0.001, p = 0.496 | r = −0.096, p = 0.196 |
| C3 | r = −0.060, p = 0.293 | r = 0.035, p = 0.374 | r = 0.080, p = 0.232 | r = 0.107, p = 0.165 | r = 0.137, p = 0.106 | r = 0.078, p = 0.240 | r = −0.129, p = 0.119 | r = −0.128, p = 0.122 | r = 0.267, p = 0.007 * | r = 0.149, p = 0.090 |
| C4 | r = 0.058, p = 0.300 | r = −0.160, p = 0.073 | r = 0.179, p = 0.052 | r = 0.057, p = 0.304 | r = 0.071, p = 0.261 | r = 0.011, p = 0.459 | r = −0.090, p = 0.208 | r = −0.013, p = 0.455 | r = 0.041, p = 0.355 | r = 0.059, p = 0.299 |
| Variables | Beta Coefficient | Standard Error | p |
|---|---|---|---|
| mRSS | 0.162 | 1.189 | p = 0.238 |
| DAI | 0.101 | 0.589 | p = 0.558 |
| DSS | 2.446 | 0.926 | p = 0.01 * |
| ESR | 0.170 | 1.491 | p = 0.140 |
| CRP | 0.199 | 1.809 | p = 0.074 |
| sPAP | 0.136 | 0.861 | p = 0.228 |
| DLco | −0.117 | 0.645 | p = 0.346 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellicano, C.; Miglionico, M.; Romaggioli, L.; Colalillo, A.; Vantaggio, L.; Napodano, C.; Callà, C.; Gulli, F.; Marino, M.; Basile, U.; et al. Increased Complement Activation in Systemic Sclerosis Patients with Skin and Lung Fibrosis. J. Pers. Med. 2022, 12, 284. https://doi.org/10.3390/jpm12020284
Pellicano C, Miglionico M, Romaggioli L, Colalillo A, Vantaggio L, Napodano C, Callà C, Gulli F, Marino M, Basile U, et al. Increased Complement Activation in Systemic Sclerosis Patients with Skin and Lung Fibrosis. Journal of Personalized Medicine. 2022; 12(2):284. https://doi.org/10.3390/jpm12020284
Chicago/Turabian StylePellicano, Chiara, Marzia Miglionico, Laura Romaggioli, Amalia Colalillo, Lorenzo Vantaggio, Cecilia Napodano, Cinzia Callà, Francesca Gulli, Mariapaola Marino, Umberto Basile, and et al. 2022. "Increased Complement Activation in Systemic Sclerosis Patients with Skin and Lung Fibrosis" Journal of Personalized Medicine 12, no. 2: 284. https://doi.org/10.3390/jpm12020284
APA StylePellicano, C., Miglionico, M., Romaggioli, L., Colalillo, A., Vantaggio, L., Napodano, C., Callà, C., Gulli, F., Marino, M., Basile, U., & Rosato, E. (2022). Increased Complement Activation in Systemic Sclerosis Patients with Skin and Lung Fibrosis. Journal of Personalized Medicine, 12(2), 284. https://doi.org/10.3390/jpm12020284









