Finger Tapping as a Biomarker to Classify Cognitive Status in 80+-Year-Olds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Neuropsychological Testing and MCI Classification
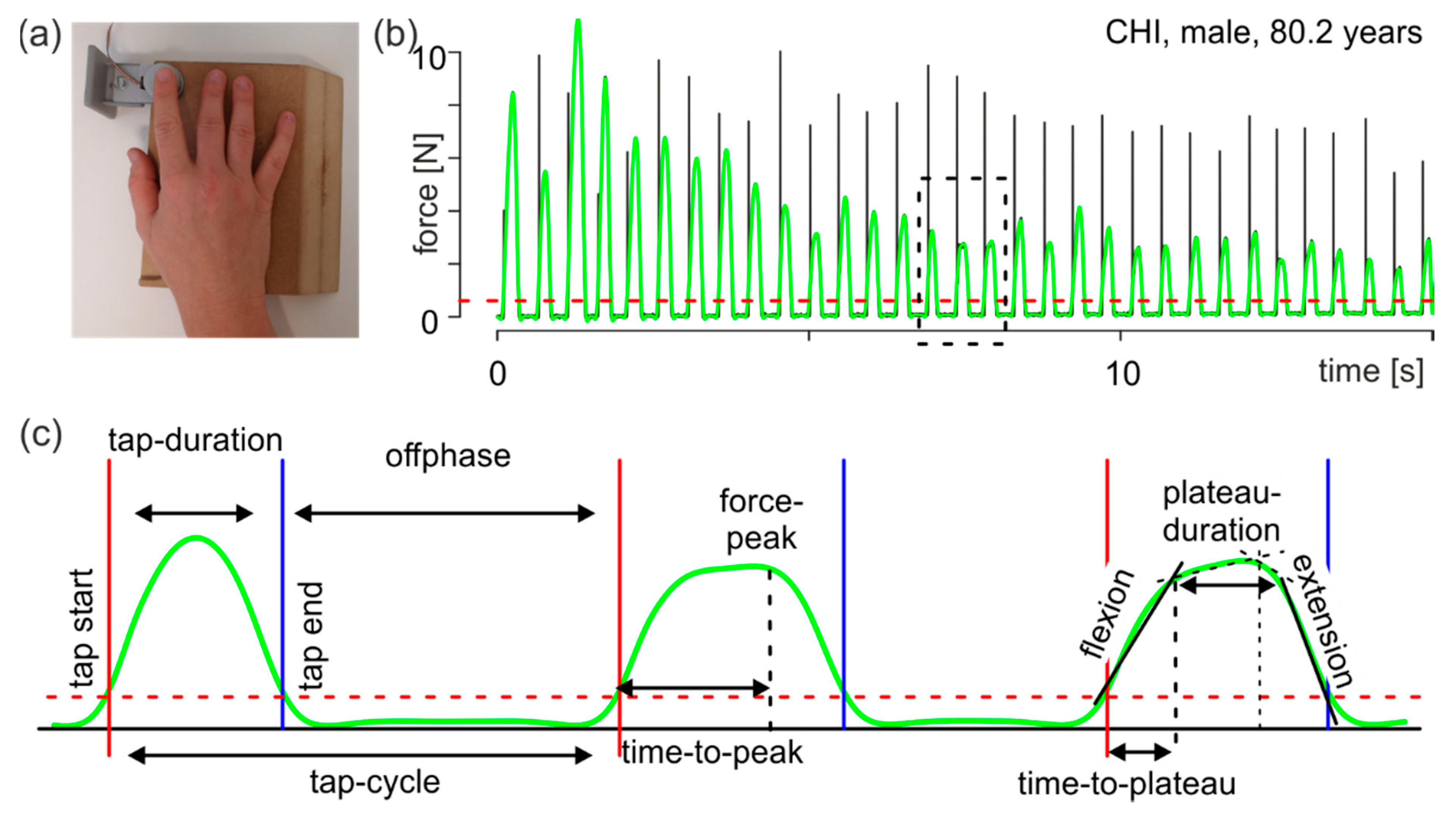
2.3. Tasks and Recording
2.4. Data Processing, Parameter Extraction and Statistical Analyses
- tap duration: interval from tap start to tap end;
- tap cycle: interval from a tap start to the following tap start;
- offphase: interval from a tap end to the next tap start, namely the time when the finger is not in contact with the force transducer;
- force peak: force maximum of an individual tap; and
- time to peak: time from tap start to the moment of the force peak.
- flexion: first force slope in the first interval describing the flexion performance during tapping (Figure 1c, flexion);
- extension: second force slope of the second interval describing the extension performance during tapping (Figure 1c, extension);
- time to plateau: duration from tap start to the break point of the first interval (Figure 1c, right tap). This time describes the duration of the execution of flexion after contact of the finger with the force sensor; and
- plateau duration: duration from the first break point to the second break point (Figure 1c, right tap).
3. Results
3.1. Statistical Analyses
3.1.1. ANOVA
3.1.2. Linear Discriminant Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Müller, K.; Fröhlich, S.; Germano, A.M.C.; Kondragunta, J.; Agoitia Hurtado, M.F.d.C.; Rudisch, J.; Schmidt, D.; Hirtz, G.; Stollmann, P.; Voelcker-Rehage, C. Sensor-based systems for early detection of dementia (SENDA): A study protocol for a prospective cohort sequential study. BMC Neurol. 2020, 20, 84. [Google Scholar] [CrossRef] [PubMed]
- Deutsche Alzheimer Gesellschaft e.V. Informationsblatt 1 Die Häufigkeit von Demenzerkrankungen. 2018. Available online: https://www.deutsche-alzheimer.de/fileadmin/alz/pdf/factsheets/infoblatt1_haeufigkeit_demenzerkrankungen_dalzg.pdf (accessed on 25 October 2021).
- Lin, P.-J.; Neumann, P.J. The economics of mild cognitive impairment. Alzheimers Dement. 2013, 9, 58–62. [Google Scholar] [CrossRef]
- Frank, L.; Lenderking, W.R.; Howard, K.; Cantillon, M. Patient self-report for evaluating mild cognitive impairment and prodromal Alzheimer’s disease. Alzheimers Res. Ther. 2011, 3, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, A.; Stokholm, J.; Gade, A.; Andersen, B.B.; Hejl, A.M.; Waldemar, G. Awareness of Deficits in Mild Cognitive Impairment and Alzheimer’s Disease: Do MCI Patients Have Impaired Insight? Dement. Geriatr. Cogn. Disord. 2004, 17, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Farias, S.T.; Mungas, D.; Jagust, W. Degree of discrepancy between self and other-reported everyday functioning by cognitive status: Dementia, mild cognitive impairment, and healthy elders. Int. J. Geriatr. Psychiatry 2005, 20, 827–834. [Google Scholar] [CrossRef] [Green Version]
- Argüelles, S.; Loewenstein, D.A.; Eisdorfer, C.; Argüelles, T. Caregivers’ Judgments of the Functional Abilities of the Alzheimer’s Disease Patient: Impact of Caregivers’ Depression and Perceived Burden. J. Geriatr. Psychiatry Neurol. 2001, 14, 91–98. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Moms, J.C.; Heyman, A.; Mohs, R.C.; Hughes, J.P.; van Belle, G.; Fillenbaum, G.; Mellits, E.D.; Clark, C. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assesment of Alzheimer’s disease. Neurology 1989, 39, 1159. [Google Scholar] [CrossRef]
- Fröhlich, S.; Kutz, D.F.; Müller, K.; Voelcker-Rehage, C. Characteristics of Resting State EEG Power in 80+-Year-Olds of Different Cognitive Status. Front. Aging Neurosci. 2021, 13, 469. [Google Scholar] [CrossRef]
- Fröhlich, S.; Müller, K.; Voelcker-Rehage, C. Normative Data for the CERAD-NP for Healthy High-Agers (80–84 years) and Effects of Age-Typical Visual Impairment and Hearing Loss. J. Int. Neuropsychol. Soc. 2021, in press. [Google Scholar] [CrossRef]
- Rabinowitz, I.; Lavner, Y. Association between Finger Tapping, Attention, Memory, and Cognitive Diagnosis in Elderly Patients. Percept. Mot. Ski. 2014, 119, 259–278. [Google Scholar] [CrossRef] [PubMed]
- Bangert, A.S.; Balota, D.A. Keep Up the Pace: Declines in Simple Repetitive Timing Differentiate Healthy Aging from the Earliest Stages of Alzheimer’s Disease. J. Int. Neuropsychol. Soc. 2012, 18, 1052–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roalf, D.R.; Rupert, P.; Mechanic-Hamilton, D.; Brennan, L.; Duda, J.E.; Weintraub, D.; Trojanowski, J.Q.; Wolk, D.; Moberg, P.J. Quantitative assessment of finger tapping characteristics in mild cognitive impairment, Alzheimer’s disease, and Parkinson’s disease. J. Neurol. 2018, 265, 1365–1375. [Google Scholar] [CrossRef]
- Cole, K.J.; Rotella, D.L.; Harper, J.G. Mechanisms for Age-Related Changes of Fingertip Forces during Precision Gripping and Lifting in Adults. J. Neurosci. 1999, 19, 3238–3247. [Google Scholar] [CrossRef] [PubMed]
- Johansson, R.S.; Cole, K.J. Sensory-motor coordination during grasping and manipulative actions. Curr. Opin. Neurobiol. 1992, 2, 815–823. [Google Scholar] [CrossRef]
- Johansson, R.S.; Cole, K.J. Grasp stability during manipulative actions. Can. J. Physiol. Pharmacol. 1994, 72, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Rudisch, J.; Müller, K.; Kutz, D.F.; Brich, L.; Sleimen-Malkoun, R.; Voelcker-Rehage, C. How Age, Cognitive Function and Gender Affect Bimanual Force Control. Front. Physiol. 2020, 11, 245. [Google Scholar] [CrossRef]
- Breton, A.; Casey, D.; Arnaoutoglou, N.A. Cognitive tests for the detection of mild cognitive impairment (MCI), the prodromal stage of dementia: Meta-analysis of diagnostic accuracy studies. Int. J. Geriatr. Psychiatry 2019, 34, 233–242. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef] [Green Version]
- Petersen, R.C.; Caracciolo, B.; Brayne, C.; Gauthier, S.; Jelic, V.; Fratiglioni, L. Mild cognitive impairment: A concept in evolution. J. Intern. Med. 2014, 275, 214–228. [Google Scholar] [CrossRef]
- Petersen, R.C.; Lopez, O.; Armstrong, M.J.; Getchius, T.S.D.; Ganguli, M.; Gloss, D.; Gronseth, G.S.; Marson, D.; Pringsheim, T.; Day, G.S.; et al. Practice guideline update summary: Mild cognitive impairment. Rep. Guidel. Dev. Dissem. Implement. Subcomm. Am. Acad. Neurol. 2018, 90, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.H. Effects of Aging on Linear and Curvilinear Aiming Arm Movements. Exp. Aging Res. 2000, 26, 393–407. [Google Scholar] [CrossRef] [PubMed]
- R-CoreTeam. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Meindl, T.; Schmid, B.C.; Timmann, D.; Kolb, F.P.; Kutz, D.F. Contribution of the Cerebellum to the Coupling of Grip Force and Pull Force During an Isometric Precision Grip Task. Cerebellum 2012, 11, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.A. ez: Easy Analysis and Visualization of Factorial Experiments, R package version 4.4-0; R Core Team: Vienna, Austria, 2016. [Google Scholar]
- Bakeman, R. Recommended effect size statistics for repeated measures designs. Behav. Res. Methods 2005, 37, 379–384. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, BY, USA, 2002; p. 498. [Google Scholar]
- Diermayr, G.; McIsaac, T.L.; Gordon, A.M. Finger Force Coordination Underlying Object Manipulation in the Elderly—A Mini-Review. Gerontology 2011, 57, 217–227. [Google Scholar] [CrossRef]
- Cave, A.E.; Barry, R.J. Sex differences in resting EEG in healthy young adults. Int. J. Psychophysiol. 2021, 161, 35–43. [Google Scholar] [CrossRef]
- Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef]
- Shulman, R.G.; Rothman, D.L.; Behar, K.L.; Hyder, F. Energetic basis of brain activity: Implications for neuroimaging. Trends Neurosci. 2004, 27, 489–495. [Google Scholar] [CrossRef]
- Manto, M.; Bower, J.M.; Conforto, A.B.; Delgado-Garcia, J.M.; da Guarda, S.N.; Gerwig, M.; Habas, C.; Hagura, N.; Ivry, R.B.; Marien, P.; et al. Consensus paper: Roles of the cerebellum in motor control--the diversity of ideas on cerebellar involvement in movement. Cerebellum 2012, 11, 457–487. [Google Scholar] [CrossRef]
- Kutz, D.F.; Schmid, B.C.; Meindl, T.; Timmann, D.; Kolb, F.P. Contribution of the Cerebellum in Cue-Dependent Force Changes During an Isometric Precision Grip Task. Cerebellum 2016, 15, 439–450. [Google Scholar] [CrossRef] [Green Version]
- Schmid, B.C.; Meindl, T.; Timmann, D.; Kolb, F.P.; Kutz, D.F. Motor learning of cue-dependent pull-force changes during an isometric precision grip task. Hum. Mov. Sci. 2015, 39, 138–153. [Google Scholar] [CrossRef] [PubMed]
- Kutz, D.F.; Wölfel, A.; Meindl, T.; Timmann, D.; Kolb, F.P. Spatio-temporal human grip force analysis via sensor arrays. Sensors 2009, 9, 6330–6345. [Google Scholar] [CrossRef] [PubMed]
- Kutz, D.F.; Wölfel, A.; Timmann, D.; Kolb, F.P. Dynamic torque during a precision grip task comparable to picking a raspberry. J. Neurosci. Methods 2009, 177, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Hulst, T.; van der Geest, J.N.; Thürling, M.; Goericke, S.; Frens, M.A.; Timmann, D.; Donchin, O. Ageing shows a pattern of cerebellar degeneration analogous, but not equal, to that in patients suffering from cerebellar degenerative disease. NeuroImage 2015, 116, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Koppelmans, V.; Hoogendam, Y.Y.; Hirsiger, S.; Mérillat, S.; Jäncke, L.; Seidler, R.D. Regional cerebellar volumetric correlates of manual motor and cognitive function. Brain Struct. Funct. 2017, 222, 1929–1944. [Google Scholar] [CrossRef] [PubMed]
- Koziol, L.F.; Budding, D.; Andreasen, N.; D’Arrigo, S.; Bulgheroni, S.; Imamizu, H.; Ito, M.; Manto, M.; Marvel, C.; Parker, K.; et al. Consensus Paper: The Cerebellum’s Role in Movement and Cognition. Cerebellum 2014, 13, 151–177. [Google Scholar] [CrossRef]
- Gellersen, H.M.; Guell, X.; Sami, S. Differential vulnerability of the cerebellum in healthy ageing and Alzheimer’s disease. NeuroImage Clin. 2021, 30, 102605. [Google Scholar] [CrossRef]
- Liang, K.J.; Carlson, E.S. Resistance, vulnerability and resilience: A review of the cognitive cerebellum in aging and neurodegenerative diseases. Neurobiol. Learn. Mem. 2020, 170, 15. [Google Scholar] [CrossRef]


| CHI | pMCI | MCI | |
|---|---|---|---|
| n (in %) | 79 (35) | 80 (36) | 66 (29) |
| m/f | 35/44 | 43/37 | 38/28 |
| Age in years | 82.0 ± 0.3 | 82.5 ± 0.2 | 82.9 ± 0.3 |
| Education in years | 14.3 ± 0.4 | 13.9 ± 0.4 | 13.7 ± 0.4 |
| MMSE (0–30) | 28.3 ± 0.2 | 27.8 ± 0.2 | 27.3 ± 0.2 |
| MoCA (0–30) | 27.7 ± 0.1 | 25.8 ± 0.2 | 22.8 ± 0.2 |
| Self-Selected Pace | Fast Pace | |||||
|---|---|---|---|---|---|---|
| CHI (n = 79) | pMCI (n = 80) | MCI (n = 66) | CHI (n = 79) | pMCI (n = 80) | MCI (n = 65) 1 | |
| tap-cycle_median | −0.593 ± 0.047 | −0.433 ± 0.045 | −0.440 ± 0.057 | −1.442 ± 0.020 | −1.380 ± 0.026 | −1.365 ± 0.029 |
| tap-cycle_iqr | −2.885 ± 0.081 | −2.792 ± 0.080 | −2.703 ± 0.099 | −3.657 ± 0.066 | −3.594 ± 0.050 | −3.449 ± 0.080 |
| tap-duration_median | −1.681 ± 0.050 | −1.510 ± 0.054 | −1.482 ± 0.066 | −2.239 ± 0.020 | −2.203 ± 0.026 | −2.144 ± 0.029 |
| tap-duration_iqr | −3.364 ± 0.074 | −3.173 ± 0.081 | −3.138 ± 0.089 | −3.939 ± 0.046 | −3.914 ± 0.037 | −3.869 ± 0.053 |
| offphase_median | −1.037 ± 0.051 | −0.890 ± 0.049 | −0.915 ± 0.060 | −2.046 ± 0.025 | −1.971 ± 0.032 | −1.983 ± 0.035 |
| offphase_iqr | −2.934 ± 0.067 | −2.836 ± 0.070 | −2.789 ± 0.083 | −3.744 ± 0.059 | −3.751 ± 0.053 | −3.653 ± 0.075 |
| force-peak_median | 0.558 ± 0.109 | 0.527 ± 0.104 | 0.7901 ± 0.142 | 0.327 ± 0.089 | 0.403 ± 0.090 | 0.725 ± 0.112 |
| force-peak_iqr | −0.449 ± 0.126 | −0.594 ± 0.121 | −0.328 ± 0.123 | −0.454 ± 0.093 | −0.420 ± 0.087 | −0.189 ± 0.119 |
| time-to-peak_median | −2.397 ± 0.054 | −2.246 ± 0.057 | −2.195 ± 0.072 | −2.979 ± 0.019 | −2.940 ± 0.028 | −2.884 ± 0.030 |
| time-to-peak_iqr | −3.934 ± 0.095 | −3.710 ± 0.111 | −3.619 ± 0.112 | −4.677 ± 0.045 | −4.631 ± 0.039 | −4.644 ± 0.057 |
| flexion_median | −3.743 ± 0.082 | −3.852 ± 0.081 | −3.676 ± 0.107 | −3.551 ± 0.080 | −3.508 ± 0.074 | −3.228 ± 0.096 |
| flexion_iqr | −4.853 ± 0.101 | −4.935 ± 0.094 | −4.764 ± 0.104 | −4.497 ± 0.086 | −4.497 ± 0.085 | −4.306 ± 0.104 |
| extension_median | −3.735 ± 0.089 | −3.839 ± 0.086 | −3.628 ± 0.114 | −3.650 ± 0.078 | −3.601 ± 0.074 | −3.324 ± 0.094 |
| extension_iqr | −4.766 ± 0.109 | −4.896 ± 0.090 | −4.763 ± 0.101 | −4.624 ± 0.083 | −4.604 ± 0.076 | −4.443 ± 0.103 |
| time-to-plateau_median | −2.722 ± 0.038 | −2.682 ± 0.040 | −2.617 ± 0.050 | −3.053 ± 0.015 | −3.030 ± 0.021 | −2.985 ± 0.021 |
| time-to-plateau_iqr | −4.446 ± 0.100 | −4.448 ± 0.089 | −4.235 ± 0.108 | −4.871 ± 0.041 | −4.855 ± 0.042 | −4.888 ± 0.057 |
| plateau-duration_median | −2.732 ± 0.076 | −2.406 ± 0.084 | −2.408 ± 0.097 | −3.547 ± 0.025 | −3.492 ± 0.032 | -3.446 ± 0.040 |
| plateau-duration_iqr | −3.778 ± 0.120 | −3.457 ± 0.121 | −3.434 ± 0.133 | −5.347 ± 0.063 | −5.278 ± 0.067 | −5.207 ± 0.083 |
| Parameter | Effect | DFn | DFd | F | p | η2G |
|---|---|---|---|---|---|---|
| force-peak_median | group | 2 | 219 | 1.22 | 0.30 | 0.01 |
| sex | 1 | 219 | 6.32 | 0.03 | 0.03 | |
| group × sex | 2 | 219 | 3.85 | 0.06 | 0.03 | |
| FLSD (sex) | 0.263 | |||||
| flexion_median | group | 2 | 219 | 1.04 | 0.35 | 0.01 |
| sex | 1 | 219 | 7.43 | 0.03 | 0.03 | |
| group × sex | 2 | 219 | 3.23 | 0.06 | 0.03 | |
| FLSD (sex) | 0.200 | |||||
| extension_median | group | 2 | 219 | 1.18 | 0.31 | 0.01 |
| sex | 1 | 219 | 6.81 | 0.03 | 0.03 | |
| group × sex | 2 | 219 | 3.36 | 0.06 | 0.03 | |
| FLSD (sex) | 0.214 | |||||
| plateau-duration_median | group | 2 | 219 | 4.49 | 0.03 | 0.04 |
| sex | 1 | 219 | 1.68 | 0.30 | 0.01 | |
| group × sex | 2 | 219 | 0.99 | 0.37 | 0.01 | |
| FLSD (group) | 0.238 |
| Parameter | Effect | DFn | DFd | F | p | η2G |
|---|---|---|---|---|---|---|
| tap-cycle_median | group | 2 | 218 | 3.41 | 0.03 | 0.03 |
| sex | 1 | 218 | 6.62 | 0.015 | 0.03 | |
| group × sex | 2 | 218 | 5.24 | 0.015 | 0.05 | |
| FLSD (group) | 0.070 | |||||
| FLSD (sex) | 0.057 | |||||
| FLSD (group × sex) | 0.097 | |||||
| tap-cycle_iqr | group | 2 | 218 | 3.24 | 0.06 | 0.03 |
| sex | 1 | 218 | 7.11 | 0.03 | 0.03 | |
| group × sex | 2 | 218 | 2.11 | 0.12 | 0.02 | |
| FLSD (sex) | 0.147 | |||||
| tap-duration_median | group | 2 | 218 | 3.63 | 0.045 | 0.03 |
| sex | 1 | 218 | 0.10 | 0.75 | 0.00 | |
| group × sex | 2 | 218 | 4.04 | 0.02 | 0.04 | |
| FLSD (group) | 0.070 | |||||
| FLSD (group × sex) | 0.098 | |||||
| offphase_median | group | 2 | 218 | 2.69 | 0.07 | 0.02 |
| sex | 1 | 218 | 13.4 | <0.001 | 0.06 | |
| group × sex | 2 | 218 | 4.51 | 0.015 | 0.04 | |
| FLSD (sex) | 0.069 | |||||
| FLSD (group × sex) | 0.117 | |||||
| offphase_iqr | group | 2 | 218 | 1.09 | 0.34 | 0.01 |
| sex | 1 | 218 | 10.8 | 0.003 | 0.05 | |
| group × sex | 2 | 218 | 1.25 | 0.34 | 0.01 | |
| FLSD (sex) | 0.137 | |||||
| force-peak_median | group | 2 | 218 | 4.04 | 0.02 | 0.04 |
| sex | 1 | 218 | 4.83 | 0.03 | 0.02 | |
| group × sex | 2 | 218 | 1.60 | 0.20 | 0.01 | |
| FLSD (group) | 0.267 | |||||
| FLSD (sex) | 0.219 | |||||
| flexion_median | group | 2 | 218 | 3.72 | 0.045 | 0.03 |
| sex | 1 | 218 | 6.34 | 0.03 | 0.03 | |
| group × sex | 2 | 218 | 1.06 | 0.35 | 0.01 | |
| FLSD (group) | 0.230 | |||||
| FLSD (sex) | 0.188 | |||||
| extension_median | group | 2 | 218 | 3.78 | 0.03 | 0.03 |
| sex | 1 | 218 | 8.10 | 0.015 | 0.04 | |
| group × sex | 2 | 218 | 1.03 | 0.36 | 0.01 | |
| FLSD (group) | 0.227 | |||||
| FLSD (sex) | 0.185 |
| Parameter | LDA1 | LDA2 |
|---|---|---|
| force-peak_median_self | 0.38 | 0.93 |
| flexion_median_self | −0.61 | −0.97 |
| extension_median_self | −0.69 | 0.21 |
| plateau-duration_median_self | 0.50 | −0.69 |
| tap-cycle_median_fast | −0.04 | −4.50 |
| tap-cycle_iqr_fast | 1.01 | 0.31 |
| tap-duration_median_fast | 0.31 | 2.98 |
| offphase_median_fast | 0.17 | 2.45 |
| offphase_iqr_fast | −1.02 | 0.09 |
| force-peak_median_fast | −1.42 | −3.17 |
| flexion_median_fast | 0.91 | 3.27 |
| extension-median_fast | 1.26 | −0.15 |
| Classification Based on Cognitive Assessments | ||||
|---|---|---|---|---|
| CHI | pMCI | MCI | ||
| LDA classification | CHI | 49 | 26 | 18 |
| pMCI | 20 | 41 | 24 | |
| MCI | 10 | 13 | 23 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kutz, D.F.; Fröhlich, S.; Rudisch, J.; Müller, K.; Voelcker-Rehage, C. Finger Tapping as a Biomarker to Classify Cognitive Status in 80+-Year-Olds. J. Pers. Med. 2022, 12, 286. https://doi.org/10.3390/jpm12020286
Kutz DF, Fröhlich S, Rudisch J, Müller K, Voelcker-Rehage C. Finger Tapping as a Biomarker to Classify Cognitive Status in 80+-Year-Olds. Journal of Personalized Medicine. 2022; 12(2):286. https://doi.org/10.3390/jpm12020286
Chicago/Turabian StyleKutz, Dieter F., Stephanie Fröhlich, Julian Rudisch, Katrin Müller, and Claudia Voelcker-Rehage. 2022. "Finger Tapping as a Biomarker to Classify Cognitive Status in 80+-Year-Olds" Journal of Personalized Medicine 12, no. 2: 286. https://doi.org/10.3390/jpm12020286
APA StyleKutz, D. F., Fröhlich, S., Rudisch, J., Müller, K., & Voelcker-Rehage, C. (2022). Finger Tapping as a Biomarker to Classify Cognitive Status in 80+-Year-Olds. Journal of Personalized Medicine, 12(2), 286. https://doi.org/10.3390/jpm12020286







