Evidence for Cardiorenal Protection with SGLT-2 Inhibitors and GLP-1 Receptor Agonists in Patients with Diabetic Kidney Disease
Abstract
:1. Introduction
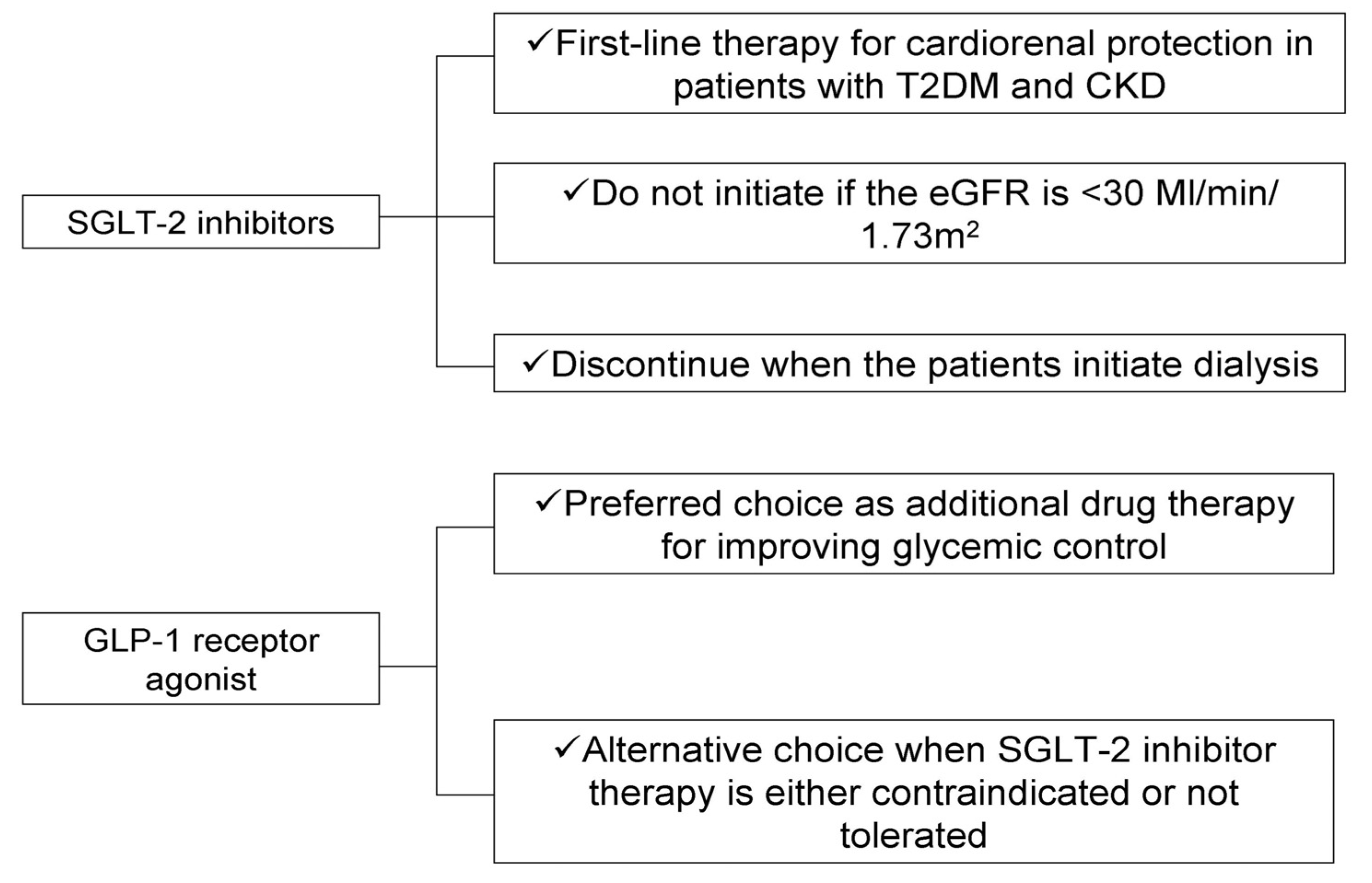
2. SGLT-2 Inhibitors
3. GLP-1 Receptor Agonists
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thomas, M.; Cooper, M.E.; Zimmet, P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat. Rev. Nephrol. 2016, 12, 73–81. [Google Scholar] [CrossRef]
- de Boer, I.H.; Rue, T.C.; Hall, Y.N.; Heagerty, P.J.; Weiss, N.S.; Himmelfarb, J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 2011, 305, 2532–2539. [Google Scholar] [CrossRef] [PubMed]
- Retnakaran, R.; Cull, C.A.; Thorne, K.I.; Adler, A.I.; Holman, R.R. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes 2006, 55, 1832–1839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeBella, Y.T.; Giduma, H.D.; Light, R.P.; Agarwal, R. Chronic Kidney Disease as a Coronary Disease Equivalent—A Comparison with Diabetes over a Decade. Clin. J. Am. Soc. Nephrol. 2011, 6, 1385–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, C.P.; Chang, C.H.; Tsai, M.K.; Lee, J.H.; Lu, P.J.; Tsai, S.P.; Wen, C.; Chen, C.H.; Kao, C.W.; Tsao, C.K.; et al. Diabetes with early kidney involvement may shorten life expectancy by 16 years. Kidney Int. 2017, 92, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.K.; Sperati, C.J.; Thavarajah, S.; Grams, M.E. Reducing Kidney Function Decline in Patients With CKD: Core Curriculum 2021. Am. J. Kidney Dis. 2021, 77, 969–983. [Google Scholar] [CrossRef]
- Perkovic, V.; Agarwal, R.; Fioretto, P.; Hemmelgarn, B.R.; Levin, A.; Thomas, M.C.; Wanner, C.; Kasiske, B.L.; Wheeler, D.C.; Groop, P.-H.; et al. Management of patients with diabetes and CKD: Conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 2016, 90, 1175–1183. [Google Scholar] [CrossRef] [Green Version]
- Brenner, B.M.; Cooper, M.E.; De Zeeuw, D.; Keane, W.F.; Mitch, W.E.; Parving, H.-H.; Remuzzi, G.; Snapinn, S.M.; Zhang, Z.; Shahinfar, S. Effects of Losartan on Renal and Cardiovascular Outcomes in Patients with Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2001, 345, 861–869. [Google Scholar] [CrossRef] [Green Version]
- Lewis, E.J.; Hunsicker, L.G.; Clarke, W.R.; Berl, T.; Pohl, M.A.; Lewis, J.B.; Ritz, E.; Atkins, R.C.; Rohde, R.; Raz, I.; et al. Renoprotective Effect of the Angiotensin-Receptor Antagonist Irbesartan in Patients with Nephropathy Due to Type 2 Diabetes. N. Engl. J. Med. 2001, 345, 851–860. [Google Scholar] [CrossRef] [Green Version]
- Afkarian, M.; Sachs, M.C.; Kestenbaum, B.; Hirsch, I.B.; Tuttle, K.R.; Himmelfarb, J.; de Boer, I.H. Kidney disease and increased mortality risk in type 2 diabetes. J. Am. Soc. Nephrol. 2013, 24, 302–308. [Google Scholar] [CrossRef]
- Georgianos, P.I.; Agarwal, R. Revisiting RAAS blockade in CKD with newer potassium-binding drugs. Kidney Int. 2017, 93, 325–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Albajrami, O.; Zhuo, M.; Hawley, C.E.; Paik, J.M. Decision Algorithm for Prescribing SGLT2 Inhibitors and GLP-1 Receptor Agonists for Diabetic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2020, 15, 1678–1688. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, K.R.; Brosius, F.C.; Cavender, M.A.; Fioretto, P.; Fowler, K.J.; Heerspink, H.J.; Manley, T.; McGuire, D.K.; Molitch, M.E.; Mottl, A.K.; et al. SGLT2 Inhibition for CKD and Cardiovascular Disease in Type 2 Diabetes: Report of a Scientific Workshop Sponsored by the National Kidney Foundation. Am. J. Kidney Dis. 2021, 77, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Pratley, R.; Dagogo-Jack, S.; Mancuso, J.; Huyck, S.; Masiukiewicz, U.; Charbonnel, B.; Frederich, R.; Gallo, S.; Cosentino, F.; et al. Cardiovascular Outcomes with Ertugliflozin in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 1425–1435. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [Green Version]
- Wanner, C.; Inzucchi, S.E.; Lachin, J.M.; Fitchett, D.; Von Eynatten, M.; Mattheus, M.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Zinman, B. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 323–334. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [Green Version]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- McGuire, D.K.; Shih, W.J.; Cosentino, F.; Charbonnel, B.; Cherney, D.Z.; Dagogo-Jack, S.; Pratley, R.; Greenberg, M.; Wang, S.; Huyck, S.; et al. Association of SGLT2 Inhibitors with Cardiovascular and Kidney Outcomes in Patients with Type 2 Diabetes: A Meta-analysis. JAMA Cardiol. 2021, 6, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Georgianos, P.I.; Vaios, V.; Dounousi, E.; Salmas, M.; Eleftheriadis, T.; Liakopoulos, V. Mechanisms for Cardiorenal Protection of SGLT-2 Inhibitors. Curr. Pharm. Des. 2021, 27, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Cherney, D.Z.; Perkins, B.A.; Soleymanlou, N.; Maione, M.; Lai, V.; Lee, A.; Fagan, N.M.; Woerle, H.J.; Johansen, O.E.; Broedl, U.C.; et al. Renal Hemodynamic Effect of Sodium-Glucose Cotransporter 2 Inhibition in Patients with Type 1 Diabetes Mellitus. Circ. 2014, 129, 587–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgianos, P.I.; Divani, M.; Eleftheriadis, T.; Mertens, P.R.; Liakopoulos, V. SGLT-2 inhibitors in Diabetic Kidney Disease: What Lies Behind their Renoprotective Properties? Curr. Med. Chem. 2019, 26, 5564–5578. [Google Scholar] [CrossRef] [PubMed]
- Oshima, M.; Jardine, M.J.; Agarwal, R.; Bakris, G.; Cannon, C.P.; Charytan, D.M.; de Zeeuw, D.; Edwards, R.; Greene, T.; Levin, A.; et al. Insights from CREDENCE trial indicate an acute drop in estimated glomerular filtration rate during treatment with canagliflozin with implications for clinical practice. Kidney Int. 2021, 99, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Bakris, G.; Oshima, M.; Mahaffey, K.W.; Agarwal, R.; Cannon, C.P.; Capuano, G.; Charytan, D.M.; De Zeeuw, D.; Edwards, R.; Greene, T.; et al. Effects of Canagliflozin in Patients with Baseline eGFR <30 mL/min per 1.73 m2. Clin. J. Am. Soc. Nephrol. 2020, 15, 1705–1714. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, C.C.J.; Wheeler, D.C.; Sjöström, C.D.; Stefansson, B.V.; Cain, V.; Heerspink, H.J.L. Effects of the sodium–glucose co-transporter 2 inhibitor dapagliflozin in patients with type 2 diabetes and Stages 3b–4 chronic kidney disease. Nephrol. Dial. Transplant. 2018, 33, 2005–2011. [Google Scholar] [CrossRef]
- Kumar, S.; Costello, A.J.; Colman, P.G. Fournier’s gangrene in a man on empagliflozin for treatment of Type 2 diabetes. Diabet Med. 2017, 34, 1646–1648. [Google Scholar] [CrossRef]
- Cohen, C.M.; Cahn, A.; Pollack, R.; Shalev, V.; Chodick, G. PDB1—Acute Renal Outcomes with Sodium Glucose Co-Transporter 2 Inhibitors- Real World Data Analysis. Value Health 2018, 21, S119. [Google Scholar] [CrossRef] [Green Version]
- KDIGO. 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020, 98, S1–S115. [Google Scholar] [CrossRef]
- Alicic, R.Z.; Cox, E.J.; Neumiller, J.J.; Tuttle, K.R. Incretin drugs in diabetic kidney disease: Biological mechanisms and clinical evidence. Nat. Rev. Nephrol. 2021, 17, 227–244. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Botros, F.T.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and renal outcomes in type 2 diabetes: An exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet 2019, 394, 131–138. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Sattar, N.; Rosenstock, J.; Ramasundarahettige, C.; Pratley, R.; Lopes, R.D.; Lam, C.S.; Khurmi, N.S.; Heenan, L.; Del Prato, S.; et al. Cardiovascular and Renal Outcomes with Efpeglenatide in Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 896–907. [Google Scholar] [CrossRef]
- Hernandez, A.F.; Green, J.B.; Janmohamed, S.; D’Agostino, R.B.; Granger, C.B.; Jones, N.P.; Leiter, L.A.; Rosenberg, A.E.; Sigmon, K.N.; Somerville, M.C.; et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): A double-blind, randomised placebo-controlled trial. Lancet 2018, 392, 1519–1529. [Google Scholar] [CrossRef] [Green Version]
- Holman, R.R.; Bethel, M.A.; Mentz, R.J.; Thompson, V.P.; Lokhnygina, Y.; Buse, J.; Chan, J.; Choi, J.; Gustavson, S.M.; Iqbal, N.; et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 1228–1239. [Google Scholar] [CrossRef]
- Husain, M.; Birkenfeld, A.L.; Donsmark, M.; Dungan, K.; Eliaschewitz, F.G.; Franco, D.R.; Jeppesen, O.K.; Lingvay, I.; Mosenzon, O.; Pedersen, S.D.; et al. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 841–851. [Google Scholar] [CrossRef] [Green Version]
- Mann, J.F.; Ørsted, D.D.; Brown-Frandsen, K.; Marso, S.P.; Poulter, N.R.; Rasmussen, S.; Tornøe, K.; Zinman, B.; Buse, J.B. Liraglutide and Renal Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 839–848. [Google Scholar] [CrossRef] [Green Version]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef] [Green Version]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.E.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef] [Green Version]
- Pfeffer, M.A.; Claggett, B.; Diaz, R.; Dickstein, K.; Gerstein, H.C.; Køber, L.V.; Lawson, F.C.; Ping, L.; Wei, X.; Lewis, E.F.; et al. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N. Engl. J. Med. 2015, 373, 2247–2257. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; Lee, M.M.Y.; Kristensen, S.L.; Branch, K.R.H.; Del Prato, S.; Khurmi, N.S.; Lam, C.S.P.; Lopes, R.D.; McMurray, J.J.V.; Pratley, R.E.; et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021, 9, 653–662. [Google Scholar] [CrossRef]
- Shaman, A.M.; Bain, S.C.; Bakris, G.L.; Buse, J.B.; Idorn, T.; Mahaffey, K.W.; Mann, J.F.; Nauck, M.A.; Rasmussen, S.; Rossing, P.; et al. Effect of the Glucagon-like Peptide-1 Receptor Agonists Semaglutide and Liraglutide on Kidney Outcomes in Patients with Type 2 Diabetes: A Pooled Analysis of SUSTAIN 6 and LEADER Trials. Circulation 2021. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, K.R.; Lakshmanan, M.C.; Rayner, B.; Busch, R.S.; Zimmermann, A.G.; Woodward, D.B.; Botros, F.T. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): A multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018, 6, 605–617. [Google Scholar] [CrossRef]
- Williams, D.M.; Evans, M. Semaglutide: Charting New Horizons in GLP-1 Analogue Outcome Studies. Diabetes Ther. 2020, 11, 2221–2235. [Google Scholar] [CrossRef]
- Komala, M.G.; Gross, S.; Zaky, A.; Pollock, C.; Panchapakesan, U. Saxagliptin reduces renal tubulointerstitial inflammation, hypertrophy and fibrosis in diabetes. Nephrology 2016, 21, 423–431. [Google Scholar] [CrossRef]
- Kodera, R.; Shikata, K.; Kataoka, H.U.; Takatsuka, T.; Miyamoto, S.; Sasaki, M.; Kajitani, N.; Nishishita, S.; Sarai, K.; Hirota, D.; et al. Glucagon-like peptide-1 receptor agonist ameliorates renal injury through its anti-inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetology 2011, 54, 965–978. [Google Scholar] [CrossRef] [Green Version]
- Park, C.W.; Kim, H.W.; Ko, S.H.; Lim, J.H.; Ryu, G.R.; Chung, H.W.; Han, S.W.; Shin, S.J.; Bang, B.K.; Breyer, M.D.; et al. Long-Term Treatment of Glucagon-Like Peptide-1 Analog Exendin-4 Ameliorates Diabetic Nephropathy through Improving Metabolic Anomalies indb/dbMice. J. Am. Soc. Nephrol. 2007, 18, 1227–1238. [Google Scholar] [CrossRef] [Green Version]
- Sancar-Bas, S.; Gezginci-Oktayoglu, S.; Bolkent, S. Exendin-4 attenuates renal tubular injury by decreasing oxidative stress and inflammation in streptozotocin-induced diabetic mice. Growth Factors 2015, 33, 419–429. [Google Scholar] [CrossRef]
- Nauck, M.A.; Muus Ghorbani, M.L.; Kreiner, E.; Saevereid, H.A.; Buse, J.B. Effects of Liraglutide Compared with Placebo on Events of Acute Gallbladder or Biliary Disease in Patients with Type 2 Diabetes at High Risk for Cardiovascular Events in the LEADER Randomized Trial. Diabetes Care 2019, 42, 1912–1920. [Google Scholar] [CrossRef] [Green Version]
- Palmer, S.C.; Tendal, B.; Mustafa, R.A.; Vandvik, P.O.; Li, S.; Hao, Q.; Tunnicliffe, D.; Ruospo, M.; Natale, P.; Saglimbene, V.; et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: Systematic review and network meta-analysis of randomised controlled trials. BMJ 2021, 372, m4573. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Clinical Trial | |||||
|---|---|---|---|---|---|---|
| CV Outcome Trials | Kidney Failure Outcome Trials | |||||
| EMPA-REG OUTCOME | CANVAS | DECLARE-TIMI 58 | VERTIS | CREDENCE | DAPA-CKD | |
| Year | 2015 | 2017 | 2018 | 2020 | 2019 | 2020 |
| Patient characteristics | T2DM at high CV risk | T2DM at high CV risk | T2DM who had or were at high risk for atherosclerotic CV disease | T2DM and atherosclerotic CV disease | T2DM and albuminuric CKD | CKD with or without T2DM |
| N | 7020 | 10,142 | 17,160 | 8246 | 4401 | 4304 |
| SGLT-2 inhibitor | Empagliflozin | Canagliflozin | Dapagliflozin | Ertugliflozin | Canagliflozin | Dapagliflozin |
| Median follow-up (years) | 3.1 | 2.4 | 4.2 | 3.5 | 2.6 | 2.4 |
| eGFR (mL/min/1.73 m2) | 74 | 76 | 85 | 76 | 56 | 43 |
| eGFR < 60 mL/min/1.73 m2 (%) | 26 | 20 | 7 | 22 | 59 | 89 |
| UACR < 30 mg/g (%) | 60 | 70 | 69 | 58 | 0 | 0 |
| UACR 30–300 mg/g (%) | 29 | 22 | 24 | 30 | 0 | 10 |
| UACR > 300 mg/g (%) | 11 | 8 | 7 | 9 | 100 | 90 |
| Baseline ACEI or ARB use (%) | 81 | 80 | 81 | 81 | 99.9 | 98 |
| Primary outcome | CV death, non-fatal MI, or non-fatal stroke | CV death, non-fatal MI, or non-fatal stroke | CV death, non-fatal MI, or non-fatal stroke | CV death, non-fatal MI, or non-fatal stroke | Doubling of serum creatinine, ESKD or death from CV and renal causes | Sustained eGFR decline ≥50%, ESKD or death from CV and renal causes |
| HR * (95% CI) | 0.86 (0.74–0.99) | 0.86 (0.75–0.97) | 0.93 (0.84–1.03) | 0.97 (0.85–1.11) | 0.70(0.59–0.82) | 0.61 (0.51–0.72) |
| Parameter | CV Outcome Trials | |||||||
|---|---|---|---|---|---|---|---|---|
| ELIXA | LEADER | SUSTAIN-6 | EXSCEL | HARMONY | PIONEER 6 | REWIND | AMPLITUDE-0 | |
| Year | 2015 | 2016 | 2016 | 2017 | 2018 | 2019 | 2019 | 2021 |
| Patient characteristics | T2DM with a recent acute coronary event | T2DM at high CV risk | T2DM at high CV risk | T2DM with and without established CV disease | T2DM and CV disease | T2DM at high CV risk | T2DM with and without established CV disease | T2DM and either history of CV disease or current CKD |
| N | 6068 | 9340 | 3297 | 14,752 | 9463 | 3183 | 9901 | 4076 |
| GLP1-RA | Lixisenatide | Liraglutide | Semaglutide | Exenatide | Albiglutide | Semaglutide | Dulaglutide | Efpeglenatide |
| Median follow-up (years) | 2.1 | 3.8 | 2.1 | 3.2 | 1.6 | 1.3 | 5.4 | 1.8 |
| eGFR (mL/min/1.73 m2) | 76 | 80 | 80 | 76 | 79 | 74 | 75 | 72 |
| eGFR < 60 mL/min/1.73 m2 (%) | 25 | 25 | 29 | 22 | 23 | 27 | 22 | 32 |
| UACR < 30 mg/g (%) | 74 | 64 | NA | 79 | NA | 67 | 65 | 54 |
| UACR 30–300 mg/g (%) | 19 | 26 | NA | 17 | NA | 33 * | 27 | 46 * |
| UACR > 300 mg/g (%) | 7 | 10 | NA | 4 | NA | - | 8 | NA |
| Baseline ACEI or ARB use (%) | 85 | 83 | 84 | 80 | 82 | NA | 81 | 79 |
| Primary outcome | CV death, MI, stroke or hospitalization for unstable angina | CV death, non-fatal MI, non-fatal stroke | CV death, non-fatal MI, non-fatal stroke | CV death, non-fatal MI, non-fatal stroke | CV death, non-fatal MI, non-fatal stroke | CV death, non-fatal MI, non-fatal stroke | CV death, non-fatal MI, non-fatal stroke | CV death, non-fatal MI, non-fatal stroke |
| HR (95% CI) | 1.02 (0.89–1.17) | 0.87 (0.78–0.97) | 0.74 (0.58–0.95) | 0.91 (0.83–1.00) | 0.78 (0.68–0.90) | 0.79 (0.57–1.11) | 0.88 (0.79–0.99) | 0.73 (0.58–0.92) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgianos, P.I.; Vaios, V.; Roumeliotis, S.; Leivaditis, K.; Eleftheriadis, T.; Liakopoulos, V. Evidence for Cardiorenal Protection with SGLT-2 Inhibitors and GLP-1 Receptor Agonists in Patients with Diabetic Kidney Disease. J. Pers. Med. 2022, 12, 223. https://doi.org/10.3390/jpm12020223
Georgianos PI, Vaios V, Roumeliotis S, Leivaditis K, Eleftheriadis T, Liakopoulos V. Evidence for Cardiorenal Protection with SGLT-2 Inhibitors and GLP-1 Receptor Agonists in Patients with Diabetic Kidney Disease. Journal of Personalized Medicine. 2022; 12(2):223. https://doi.org/10.3390/jpm12020223
Chicago/Turabian StyleGeorgianos, Panagiotis I., Vasilios Vaios, Stefanos Roumeliotis, Konstantinos Leivaditis, Theodoros Eleftheriadis, and Vassilios Liakopoulos. 2022. "Evidence for Cardiorenal Protection with SGLT-2 Inhibitors and GLP-1 Receptor Agonists in Patients with Diabetic Kidney Disease" Journal of Personalized Medicine 12, no. 2: 223. https://doi.org/10.3390/jpm12020223
APA StyleGeorgianos, P. I., Vaios, V., Roumeliotis, S., Leivaditis, K., Eleftheriadis, T., & Liakopoulos, V. (2022). Evidence for Cardiorenal Protection with SGLT-2 Inhibitors and GLP-1 Receptor Agonists in Patients with Diabetic Kidney Disease. Journal of Personalized Medicine, 12(2), 223. https://doi.org/10.3390/jpm12020223










