Neuro-Oncology Multidisciplinary Tumor Board: The Point of View of the Neuroradiologist
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Data
2.2. Statistical Analyses
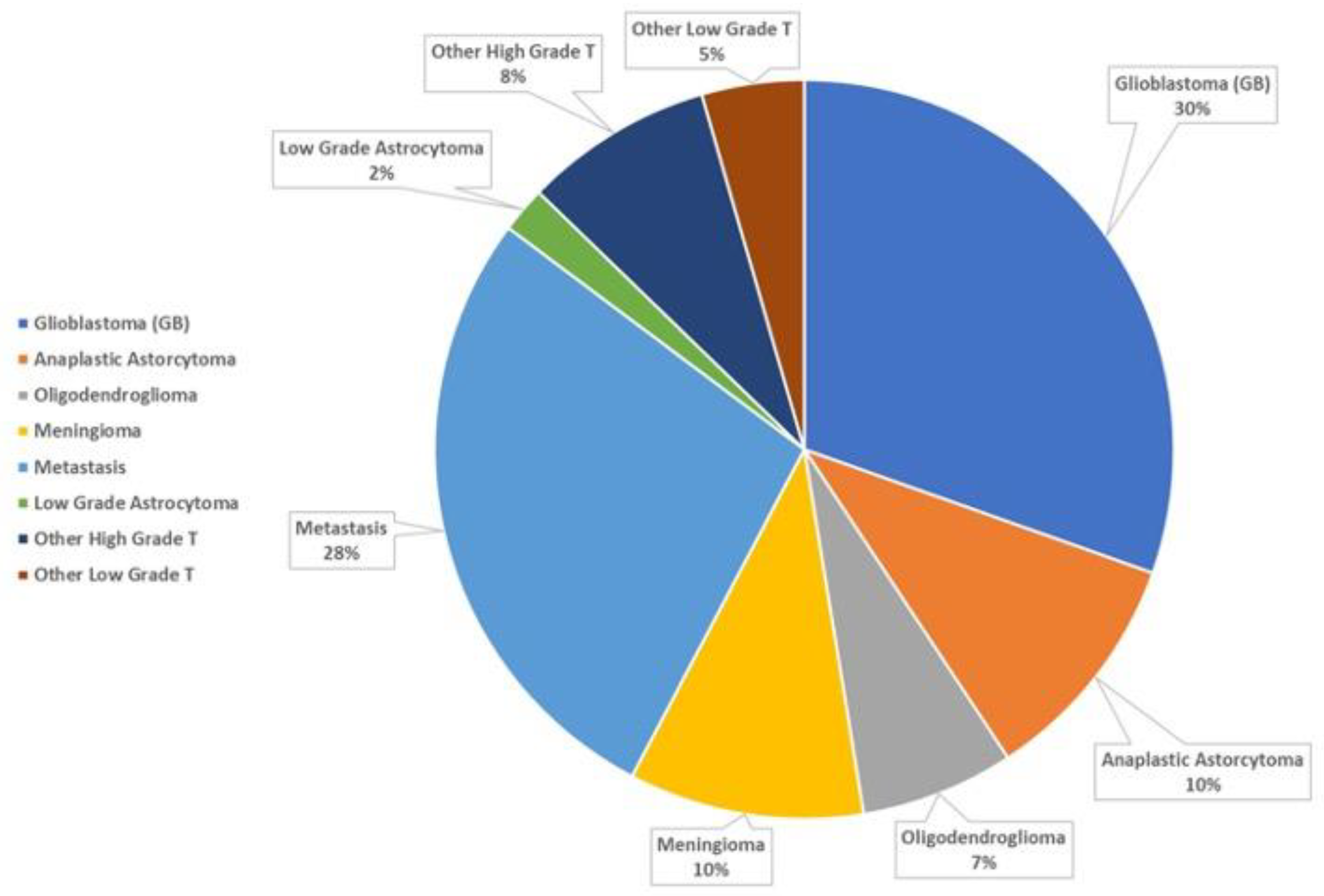
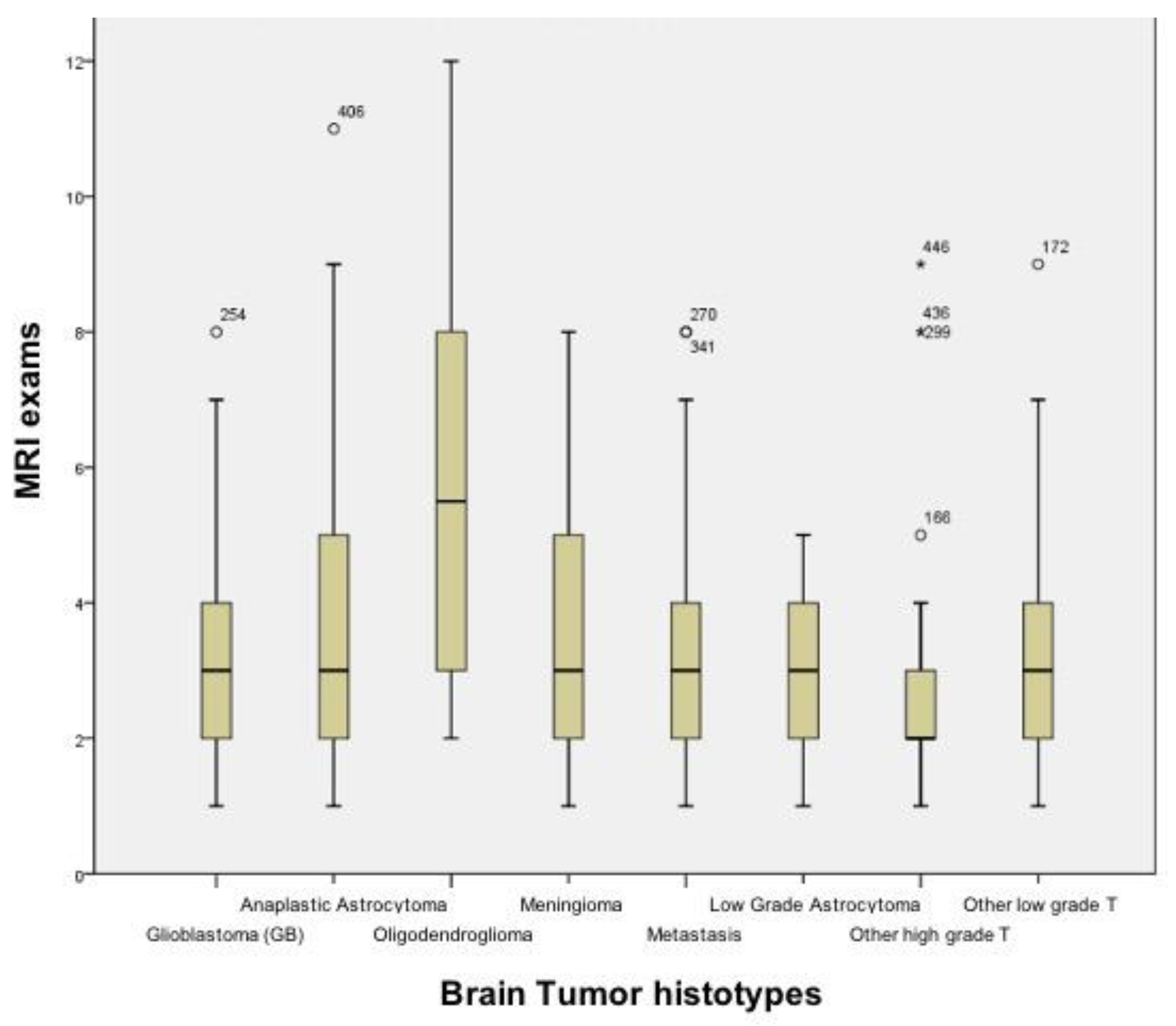
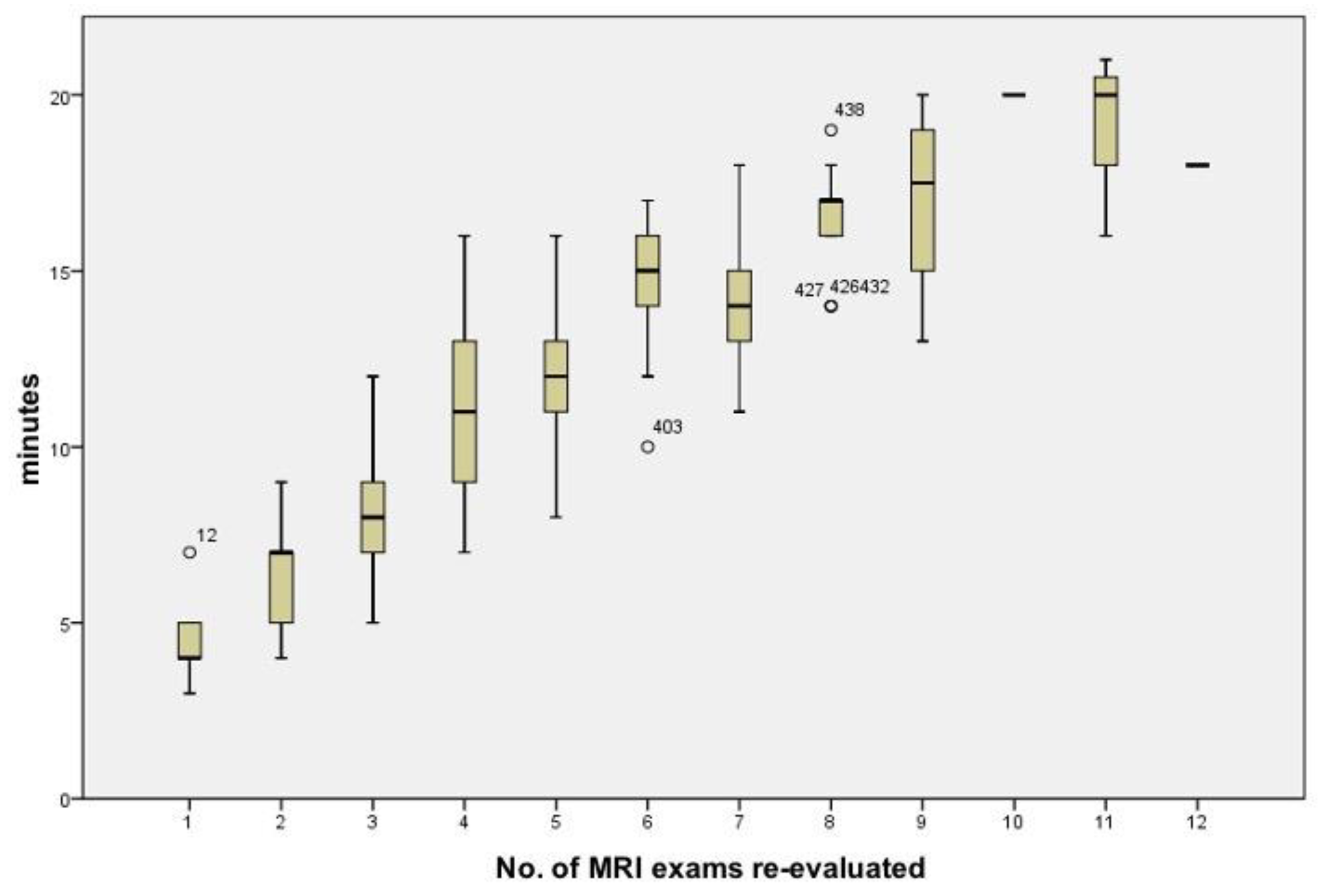
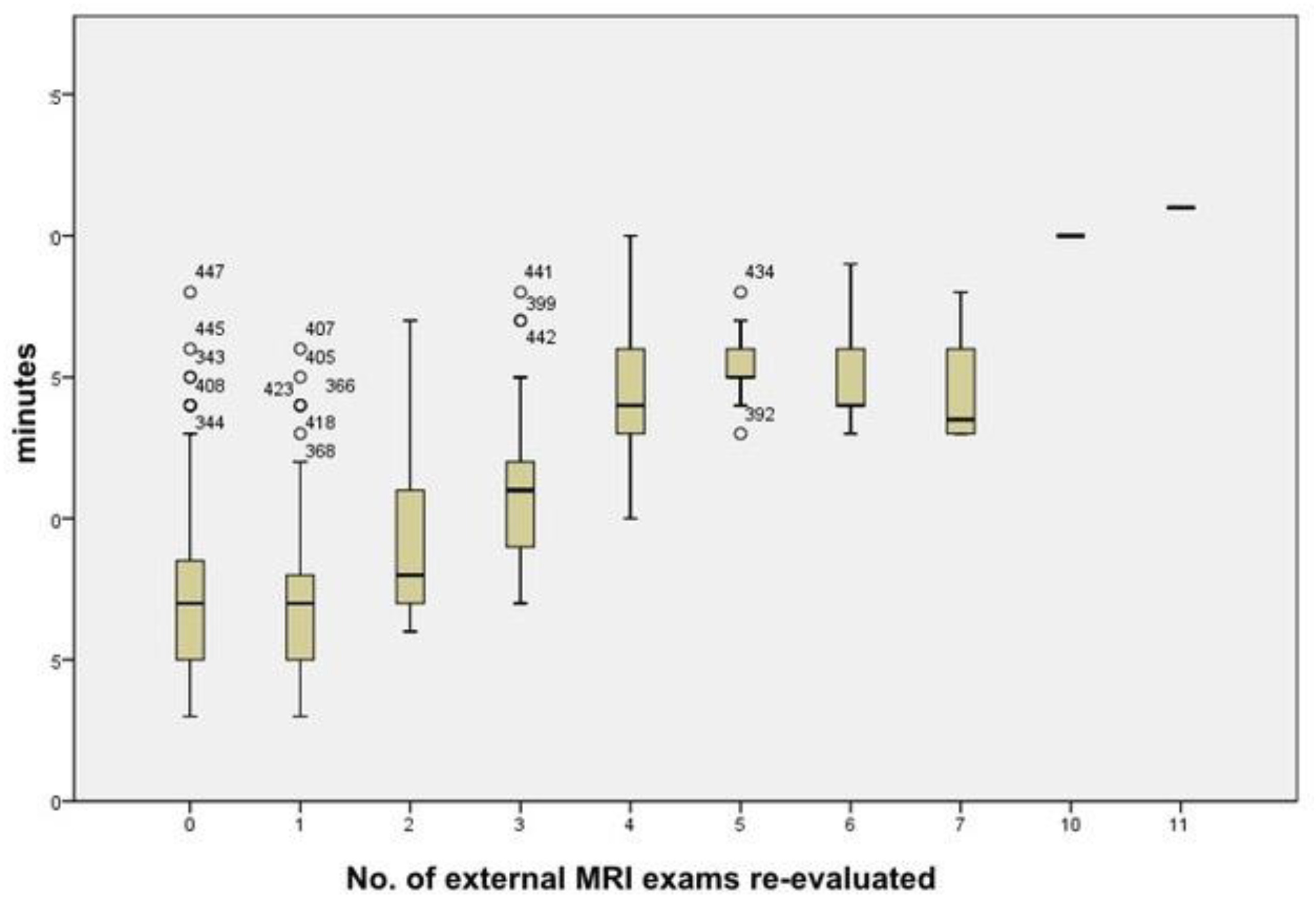
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fennell, M.L.; Das, I.P.; Clauser, S.; Petrelli, N.; Salner, A. The organization of multidisciplinary care teams: Modeling internal and external influences on cancer care quality. J. Natl. Cancer Inst. Monogr. 2010, 2010, 72–80. [Google Scholar] [CrossRef] [Green Version]
- Tripathy, D. Multidisciplinary care for breast cancer: Barriers and solutions. Breast J. 2003, 9, 60–63. [Google Scholar] [CrossRef]
- Gross, G.E. The role of the tumor board in a community hospital. CA Cancer J. Clin. 1987, 37, 88–92. [Google Scholar] [CrossRef] [PubMed]
- RCR The Royal College of Radiologists. Cancer Multidisciplinary Team Meetings-Standards for Clinical Radiologists 2014. Available online: https://www.rcr.ac.uk/publication/cancer-multidisciplinary-team-meetings-–-standards-clinical-radiologists (accessed on 15 January 2021).
- Chung, R.; Rosenkrantz, A.B.; Shanbhogue, K.P. Expert radiologist review at a hepatobiliary multidisciplinary tumor board: Impact on patient management. Abdom Radiol. 2020, 45, 3800–3808. [Google Scholar] [CrossRef] [PubMed]
- Keating, N.L.; Landrum, M.B.; Lamont, E.B.; Bozeman, S.R.; Shulman, L.N.; McNeil, B.J. Tumor boards and the quality of cancer care. J. Natl. Cancer Inst. 2013, 105, 113–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillay, B.; Wootten, A.C.; Crowe, H.; Corcoran, N.; Tran, B.; Bowden, P.; Crowe, J.; Costello, A.J. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: A systematic review of the literature. Cancer Treat. Rev. 2016, 42, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Forrest, L.M.; McMillan, D.C.; McArdle, C.S.; Dunlop, D.J. An evaluation of the impact of a multidisciplinary team, in a single centre, on treatment and survival in patients with inoperable non-small-cell lung cancer. Br. J. Cancer 2005, 93, 977–978. [Google Scholar] [CrossRef] [Green Version]
- El Saghir, N.S.; Charara, R.N.; Kreidieh, F.Y.; Eaton, V.; Litvin, K.; Farhat, R.A.; Khoury, K.E.; Breidy, J.; Tamim, H.; Eid, T.A. Global Practice and Efficiency of Multidisciplinary Tumor Boards: Results of an American Society of Clinical Oncology International Survey. J. Glob. Oncol. 2015, 1, 57–64. [Google Scholar] [CrossRef]
- Tagliaferri, L.; Garganese, G.; D’Aviero, A.; Lancellotta, V.; Fragomeni, S.M.; Fionda, B.; Casà, C.; Gui, B.; Perotti, G.; Gentileschi, S.; et al. Multidisciplinary personalized approach in the management of vulvar cancer-the Vul.Can Team experience. Int. J. Gynecol. Cancer 2020, 30, 932–938. [Google Scholar] [CrossRef]
- Ioannidis, A.; Konstantinidis, M.; Apostolakis, S.; Koutserimpas, C.; Machairas, N.; Konstantinidis, K.M. Impact of multidisciplinary tumor boards on patients with rectal cancer. Mol. Clin. Oncol. 2018, 9, 135–137. [Google Scholar] [CrossRef] [Green Version]
- Lesslie, M.; Parikh, J.R. Implementing a Multidisciplinary Tumor Board in the Community Practice Setting. Diagnostics 2017, 7, 55. [Google Scholar] [CrossRef]
- Ruhstaller, T.; Roe, H.; Thürlimann, B.; Nicoll, J.J. The multidisciplinary meeting: An indispensable aid to communication between different specialities. Eur. J. Cancer 2006, 42, 2459–2462. [Google Scholar] [CrossRef]
- Lamb, B.W.; Green, J.S.; Benn, J.; Brown, K.F.; Vincent, C.A.; Sevdalis, N. Improving decision making in multidisciplinary tumor boards: Prospective longitudinal evaluation of a multicomponent intervention for 1421 patients. J. Am. Coll Surg. 2013, 217, 412–420. [Google Scholar] [CrossRef]
- Quero, G.; Salvatore, L.; Fiorillo, C.; Bagalà, C.; Menghi, R.; Maria, B.; Cina, C.; Laterza, V.; Di Stefano, B.; Maratta, M.G.; et al. The impact of the multidisciplinary tumor board (MDTB) on the management of pancreatic diseases in a tertiary referral center. ESMO Open 2021, 6, 100010. [Google Scholar] [CrossRef] [PubMed]
- Charara, R.N.; Kreidieh, F.Y.; Farhat, R.A.; Al-Feghali, K.A.; Khoury, K.E.; Haydar, A.; Nassar, L.; Berjawi, G.; Shamseddine, A.; El Saghir, N.S. Practice and Impact of Multidisciplinary Tumor Boards on Patient Management: A Prospective Study. J. Glob. Oncol. 2016, 3, 242–249. [Google Scholar] [CrossRef]
- Liu, J.C.; Kaplon, A.; Blackman, E.; Miyamoto, C.; Savior, D.; Ragin, C. The impact of the multidisciplinary tumor board on head and neck cancer outcomes. Laryngoscope 2020, 130, 946–950. [Google Scholar] [CrossRef]
- Shäfer, N.; Bumes, B.; Eberle, F.; Fox, V.; Gessler, F.; Giordano, F.A.; Konczalla, J.; Onken, J.; Ottenhausen, M.; Scherer, M.; et al. Implementation, relevance, and virtual adaptation of neuro-oncological tumor boards during the COVID-19 pandemic: A nationwide provider survey. J. Neurooncol. 2021, 153, 479–485. [Google Scholar] [CrossRef]
- Snyder, J.; Schultz, L.; Walbert, T. The role of tumor board conferences in neuro-oncology: A nationwide providersurvey. J. Neurooncol. 2017, 133, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lutterbach, J.; Pagenstecher, A.; Spreer, J.; Hetzel, A.; van Velthoven, V.; Nikkhah, G.; Frommhold, H.; Volk, B.; Schumacher, M.; Lücking, C.; et al. The brain tumor board: Lessons to be learned from an interdisciplinary conference. Oncol. Res. Treat. 2005, 28, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Specchia, M.L.; Frisicale, E.M.; Carini, E.; Di Pilla, A.; Cappa, D.; Barbara, A.; Ricciardi, W.; Damiani, G. The impact of tumor board on cancer care: Evidence from an umbrella review. BMC Health Serv. Res. 2020, 20, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalafallah, A.M.; Jimenez, A.E.; Romo, C.G.; Kamson, D.O.; Kleinberg, L.; Weingart, J.; Brem, H.; Grossman, S.A.; Mukherjee, D. Quantifying the utility of a multidisciplinary neuro-oncology tumor board. J. Neurosurg. 2020, 1, 1–6. [Google Scholar] [CrossRef]
- Kehl, K.L.; Landrum, M.B.; Kahn, K.L.; Gray, S.W.; Chen, A.B.; Keating, N.L. Tumor board participation among physicians caring for patients with lung or colorectal cancer. J. Oncol. Pract. 2015, 11, e267–e278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeiner, P.S.; Kinzig, M.; Divé, I.; Maurer, G.D.; Filipski, K.; Harter, P.N.; Senft, C.; Bähr, O.; Hattingen, E.; Steinbach, J.P.; et al. Regorafenib CSF Penetration, Efficacy, and MRI Patterns in Recurrent Malignant Glioma Patients. J. Clin. Med. 2019, 8, 2031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thust, S.C.; Heiland, S.; Falini, A.; Jäger, H.R.; Waldman, A.D.; Sundgren, P.C.; Godi, C.; Katsaros, V.K.; Ramos, A.; Bargallo, N.; et al. Glioma imaging in Europe: A survey of 220 centres and recommendations for best clinical practice. Eur. Radiol. 2018, 28, 3306–3317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Physician | Requesting | Recipient |
|---|---|---|
| Radiation oncologist | 263 | 38 |
| Neurosurgeon | 181 | 50 |
| Pathologists | 2 | 29 |
| Neuroradiologist | 1 | 330 |
| Consensus Recommendation | Total (n = 447) | Percent |
|---|---|---|
| Imaging follow-up | 176 | 39.4 |
| Surgery | 65 | 14.5 |
| Radiation treatment | 84 | 18.8 |
| Chemotherapy | 52 | 11.6 |
| Multimodal treatment | 19 | 4.3 |
| Palliative care | 29 | 6.5 |
| Molecular studies | 16 | 3.6 |
| Nuclear medicine | 6 | 1.3 |
| Number of MRI Exams/Patient (n = 1514) | Total No. of Patients (n = 447) | Percent |
|---|---|---|
| 1 | 58 | 13 |
| 2 | 121 | 27.1 |
| 3 | 103 | 23 |
| 4 | 60 | 13.4 |
| 5 | 41 | 9.2 |
| 6 | 29 | 6.5 |
| 7 | 13 | 2.9 |
| 8 | 13 | 2.9 |
| 9 | 4 | 0.9 |
| 10 | 1 | 0.2 |
| 11 | 3 | 0.7 |
| 12 | 1 | 0.2 |
| Tumor Types | Minutes Average | SD | Min | Max |
|---|---|---|---|---|
| GB | 9.5 | 3.6 | 3 | 17 |
| AA | 9.9 | 4.4 | 4 | 20 |
| ODG | 12.7 | 5.0 | 4 | 21 |
| Meningioma | 7.8 | 3.2 | 3 | 14 |
| Metastasis | 9.0 | 3.7 | 3 | 18 |
| Low grade Astro. | 9.1 | 3.8 | 4 | 17 |
| Other high grade T | 7.4 | 2.8 | 4 | 16 |
| Other low grade T | 10.7 | 2.6 | 7 | 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaudino, S.; Giordano, C.; Magnani, F.; Cottonaro, S.; Infante, A.; Sabatino, G.; La Rocca, G.; Della Pepa, G.M.; D’Alessandris, Q.G.; Pallini, R.; et al. Neuro-Oncology Multidisciplinary Tumor Board: The Point of View of the Neuroradiologist. J. Pers. Med. 2022, 12, 135. https://doi.org/10.3390/jpm12020135
Gaudino S, Giordano C, Magnani F, Cottonaro S, Infante A, Sabatino G, La Rocca G, Della Pepa GM, D’Alessandris QG, Pallini R, et al. Neuro-Oncology Multidisciplinary Tumor Board: The Point of View of the Neuroradiologist. Journal of Personalized Medicine. 2022; 12(2):135. https://doi.org/10.3390/jpm12020135
Chicago/Turabian StyleGaudino, Simona, Carolina Giordano, Francesca Magnani, Simone Cottonaro, Amato Infante, Giovanni Sabatino, Giuseppe La Rocca, Giuseppe Maria Della Pepa, Quintino Giorgio D’Alessandris, Roberto Pallini, and et al. 2022. "Neuro-Oncology Multidisciplinary Tumor Board: The Point of View of the Neuroradiologist" Journal of Personalized Medicine 12, no. 2: 135. https://doi.org/10.3390/jpm12020135
APA StyleGaudino, S., Giordano, C., Magnani, F., Cottonaro, S., Infante, A., Sabatino, G., La Rocca, G., Della Pepa, G. M., D’Alessandris, Q. G., Pallini, R., Olivi, A., Balducci, M., Chiesa, S., Gessi, M., Guadalupi, P., Russo, R., Schiarelli, C., Ausili Cefaro, L., Di Lella, G. M., & Colosimo, C. (2022). Neuro-Oncology Multidisciplinary Tumor Board: The Point of View of the Neuroradiologist. Journal of Personalized Medicine, 12(2), 135. https://doi.org/10.3390/jpm12020135









