Cabergoline Failure and a Spontaneous Pregnancy in a Microprolactinoma with High Prolactin Levels
Abstract
1. Introduction
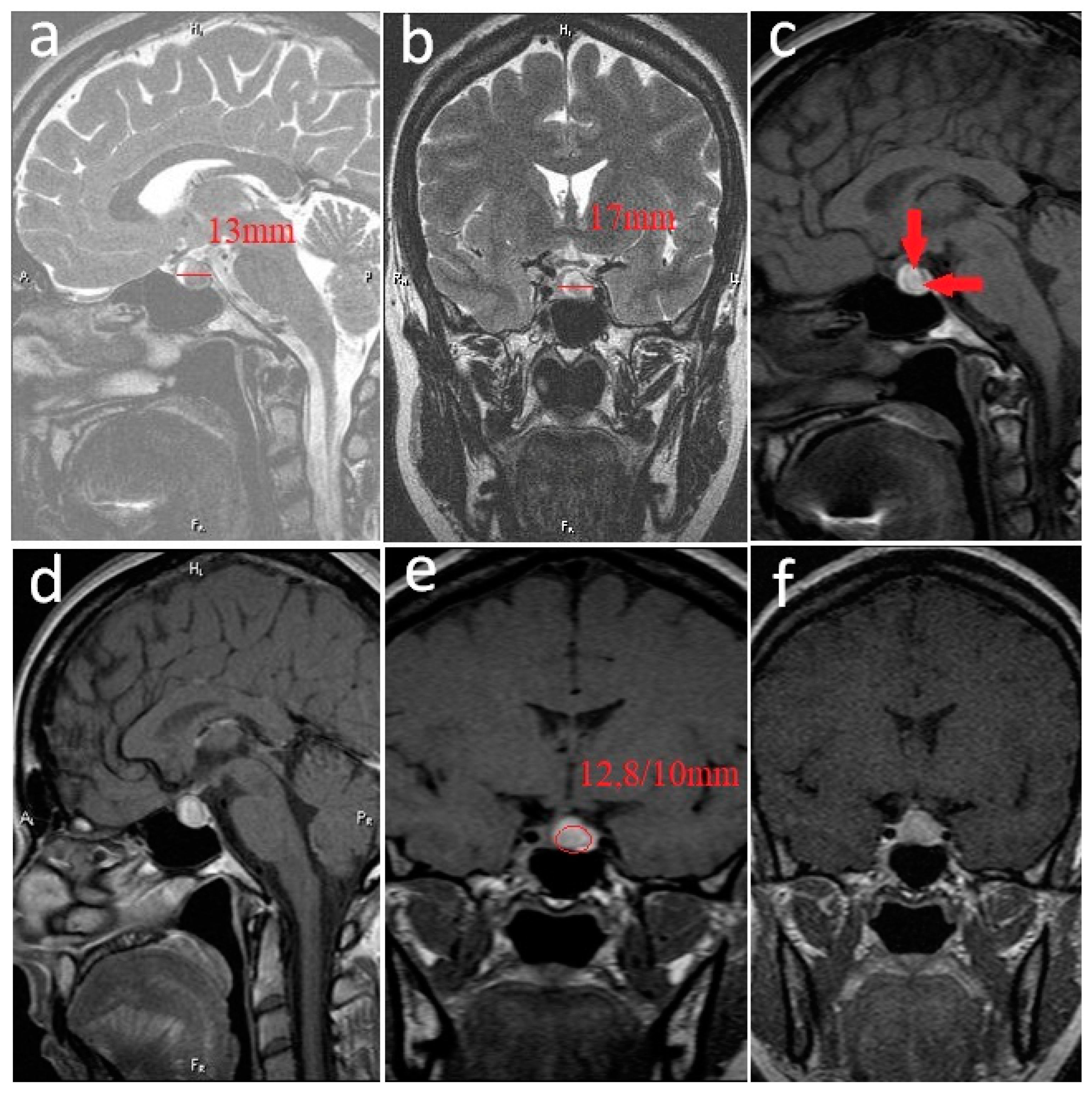
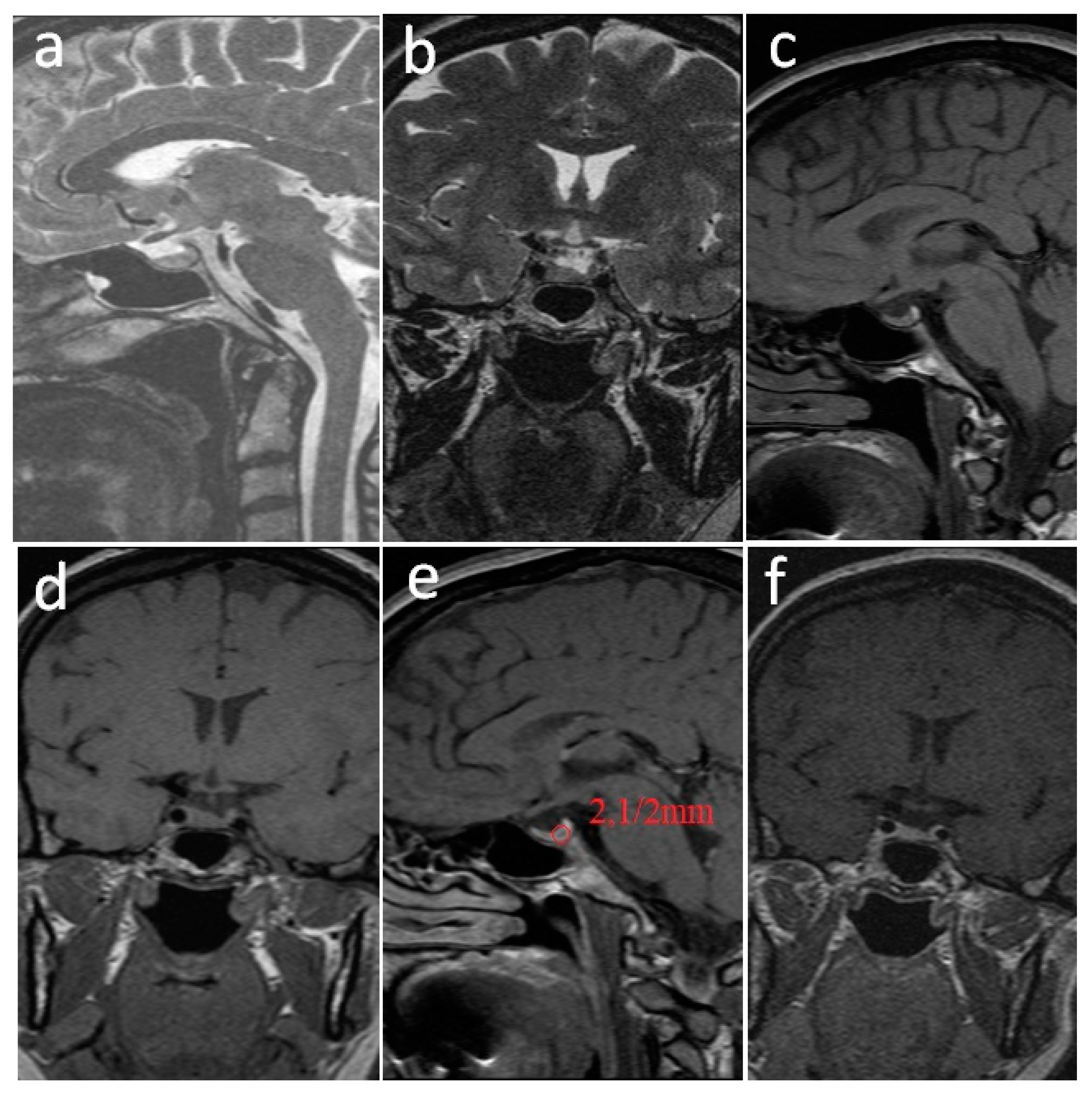
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Melmed, S. Chapter 15—Hypothalamic–Pituitary Regulation. In Conn’s Translational Neuroscience; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Bronstein, M.D. Prolactinomas and Pregnancy. Pituitary 2006, 8, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Yatavelli, R.K.R.; Bhusal, K. Prolactinoma. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459347/ (accessed on 20 May 2022).
- Colao, A. Pituitary tumours: The prolactinoma. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 575–596. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, V.K.; Davis, J.R. Hyperprolactinemia. Best Pract. Res. Clin. Obstet. Gynaecol. 2008, 22, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Martin, H.; Coates, P. Prolactin Biology and Laboratory Measurement: An Update on Physiology and Current Analytical Issues. Clin. Biochem. Rev. 2018, 39, 3–16. [Google Scholar]
- Maiter, D. Prolactinoma and pregnancy: From the wish of conception to lactation. Ann. Endocrinol. 2016, 77, 128–134. [Google Scholar] [CrossRef]
- Cunningham, F.G.; Gant, N.F.; Leveno, K.J.; Gilstrap, L.C., III; Hauth, J.C.; Wenstrom, K.D. (Eds.) Endocrine Disorders. In Williams Obstetrics; McGraw-Hill: New York, NY, USA, 2001; Volume 50, p. 1353. [Google Scholar]
- Samperi, I.; Lithgow, K.; Karavitaki, N. Hyperprolactinaemia. J. Clin. Med. 2019, 8, 2203. [Google Scholar] [CrossRef]
- Melmed, S.; Casanueva, F.F.; Hoffman, A.R.; Kleinberg, D.L.; Montori, V.M.; Schlechte, J.A.; Wass, J.A.H. Diagnosis and treatment of hyperprolactinemia: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 273–288. [Google Scholar] [CrossRef]
- Leca, B.M.; Mytilinaiou, M.; Tsoli, M.; Epure, A.; Aylwin, S.J.B.; Kaltsas, G.; Randeva, H.S.; Dimitriadis, G.K. Identification of an optimal prolactin threshold to determine prolactinoma size using receiver operating characteristic analysis. Sci. Rep. 2021, 11, 330–331. [Google Scholar] [CrossRef]
- Dawaher, W.; Patel, S.; Unjom, Z.; Moid, A.; Gilden, J.L.; Trendafilova, V. Discordance Between Prolactinoma Size and Prolactin Level: An Unusual Laboratory Finding. J. Endocrin. Soc. 2021, 5, A582. [Google Scholar] [CrossRef]
- Brown, R.S.; Herbison, A.E.; Grattan, D.R. Prolactin regulation of kisspeptin neurons in the mouse brain and its role in the lactation induced suppression of kisspeptin expression. J. Neuroendocrinol. 2014, 26, 898–908. [Google Scholar] [CrossRef]
- Sonigo, C.; Bouilly, J.; Carre, N.; Tolle, V.; Caraty, A.; Tello, J.; Simony-Conesa, F.J.; Millar, R.; Young, J.; Binart, N. Hyperprolactinemia-induced ovarian acyclicity is reversed by kisspeptin administration. J. Clin. Investig. 2012, 122, 3791–3795. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, A.; Mangal, N.S. Hyperprolactinemia. J. Hum. Reprod. Sci. 2013, 6, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Bernard, H.J. Evaluation of endocrine function. In Clinical Diagnosis and Management by Laboratory Methods, 20th ed.; ASM Press: Washington, DC, USA, 1998; pp. 303–304. [Google Scholar]
- Jayakrishnan, K. Chapter 11—Hyperprolactinemia and Infertility. In Insights Into Infertility Management; JP Medical Ltd.: New Delhi, India, 2011; p. 79. [Google Scholar]
- Fukuhara, N.; Nishiyama, M.; Iwasaki, Y. Update in Pathogenesis, Diagnosis, and Therapy of Prolactinoma. Cancers 2022, 14, 3604. [Google Scholar] [CrossRef]
- Al Dahmani, K.M.; Almalki, M.H.; Ekhzaimy, A.; Aziz, F.; Bashier, A.; Mahzari, M.M.; Beshyah, S.A. Proportion and predictors 344 of Hypogonadism Recovery in Men with Macroprolactinomas treated with dopamine agonists. Pituitary 2022, 25, 658–666. [Google Scholar] [CrossRef]
- Levin, G.; Rottenstreich, A. Prolactin, prolactin disorders, and dopamine agonists during pregnancy. Hormones 2019, 18, 137–139. [Google Scholar] [CrossRef]
- Caraty, A.; Martin, G.B.; Montgomery, G. A new method for studying pituitary responsiveness in vivo using pulses of LH-RH analogue in ewes passively immunized against native LH-RH. Reprod. Nutr. Dev. 1984, 24, 439–448. [Google Scholar] [CrossRef]
- Padmanabhan, V.; McFadden, K.; Mauger, D.T.; Karsch, F.J.; Midgley Jr, A.R. Neuroendocrine control of follicle-stimulating hormone (FSH) secretion. I. Direct evidence for separate episodic and basal components of FSH secretion. Endocrinology 1997, 138, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, U.B.; Jakubowiak, A.; Steinberger, A.; Chin, W.W. Differential effects of gonadotropin releasing hormone (GnRH) pulse frequency on gonadotropin subunit and GnRH receptor messenger ribonucleic acid levels in vitro. Endocrinology 1997, 138, 1224–1231. [Google Scholar] [CrossRef]
- Marquez, P.; Skorupskaite, K.; George, J.T.; Anderson, R.A. Physiology of GnRH and Gonadotropin Secretion. 2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279070/36 (accessed on 22 May 2022).
- Milenkovic, L.; D’Angelo, G.; Kelly, P.A.; Weiner, R.I. Inhibition of gonadotropin hormone releasing hormone release by prolactin from GT1 neuronal cell lines through prolactin receptors. Proc. Natl. Acad. Sci. USA 1994, 91, 1244–1247. [Google Scholar] [CrossRef]
- Donato, J., Jr.; Frazao, R. Interreactions between prolactin and kisspeptin to control reproduction. Arch. Endocrinol. Metabol. 2016, 60, 587–595. [Google Scholar] [CrossRef]
- Hoskova, K.; Bryant, N.K.; Chen, M.E.; Nachtigall, L.B.; Lippincott, M.J.; Balasubramanian, R.; Seminara, S.B. Kisspeptin Overcomes GnRH Neuronal Suppression Secondary to Hyperprolactinemia in Humans. J. Clin. Endocrinol. Metab. 2022, 107, e3515–e3525. [Google Scholar] [CrossRef]
- Li, Q.; Rao, A.; Pereira, A.; Clarke, I.J.; Smith, J.T. Kisspeptin Cells in the Ovine Arcuate Nucleus Express Prolactin Receptor but not Melatonin Receptor. J. Neuroendocrinol. 2011, 23, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Hrabovszky, E.; Ciofi, P.; Vida, B.; Horvath, M.C.; Keller, E.; Caraty, A.; Bloom, S.R.; Ghatei, M.A.; Dhillo, W.S.; Liposits, Z.; et al. The kisspeptin system of the human hypothalamus: Sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. Eur. J. Neurosci. 2010, 31, 1984–1998. [Google Scholar] [CrossRef] [PubMed]
- Lehman, M.N.; Coolen, L.M.; Goodman, R.L. Minireview: Kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: A central node in the control of gonadotropin releasing hormone secretion. Endocrinology 2010, 151, 3479–3489. [Google Scholar] [CrossRef] [PubMed]
- Skorupskaite, K.; George, J.T.; Anderson, R.A. The kisspeptin-GnRH pathway in human reproductive health and disease. Hum. Reprod. Update 2014, 20, 485–500. [Google Scholar] [CrossRef]
- Ikegami, K.; Minabe, S.; Ieda, N.; Goto, T.; Sugimoto, A.; Nakamura, S.; Inoue, N.; Oishi, S.; Maturana, A.D.; Sanbo, M.; et al. Evidence of involvement of neurone-glia/neurone-neurone communications via gap junctions in synchronised activity of KNDy neurones. J. Neuroendocrinol. 2017, 29, 1–14. [Google Scholar] [CrossRef]
- Herbison, A.E.; Skinner, D.C.; Robinson, J.E.; King, I.S. Androgen receptor-immunoreactive cells in ram hypothalamus: Distribution and co-localization patterns with gonadotropin releasing hormone, somatostatin and tyrosine hydroxylase. Neuroendocrinology 1996, 63, 120–131. [Google Scholar] [CrossRef]
- Kinsey-Jones, J.S.; Li, X.F.; Knox, A.M.; Wilkinson, E.S.; Zhu, X.L.; Chaudhary, A.A.; Milligan, S.R.; Lightman, S.L.; O’Byrne, K.T. Down-regulation of hypothalamic kisspeptin and its receptor, Kiss1r, mRNA expression is associated with stress-induced suppression of luteinizing hormone secretion in the female rat. J. Neuroendocrinol. 2009, 21, 20–29. [Google Scholar] [CrossRef]
- Wdowiak, A.; Raczkiewicz, D.; Janczyk, P.; Bojar, I.; Makara-Studzińska, M.; Wdowiak-Filip, A. Interactions of Cortisol and Prolactin with Other Selected Menstrual Cycle Hormones Affecting the Chances of Conception in Infertile Women. Int. J. Environ. Res. Public Health 2020, 17, 7537. [Google Scholar] [CrossRef]
- Backholer, K.; Smith, J.T.; Rao, A.; Pereira, A.; Iqbal, J.; Ogawa, S.; Li, Q.; Clarke, I.J. Kisspeptin cells in the ewe brain respond to leptin and communicate with neuropeptide Y and proopiomelanocortin cells. Endocrinology 2010, 151, 2233–2243. [Google Scholar] [CrossRef]
- Araujo, B.; Belo, S.; Carvalho, D. Pregnancy and Tumor Outcomes in Women with Prolactinoma. Exp. Clin. Endocrinol. Diabetes 2017, 125, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Colao, A.; Abs, R.; Bárcena, D.G.; Chanson, P.; Paulus, W.; Kleinberg, D.L. Pregnancy outcomes following cabergoline treatment: Extended results from a 12-year observational study. Clin. Endocrinol. 2008, 68, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tang, C.; Wen, G.; Zhong, C.; Yang, J.; Zhu, J.; Ma, C. The Mechanism and Pathways of Dopamine and Dopamine Agonists in Prolactinomas. Front. Endocrinol. 2019, 9, 768. [Google Scholar] [CrossRef] [PubMed]
- Crosignani, P.G. Management of hyperprolactinemic infertility. Middle East Fert. Soc. J. 2012, 17, 63–69. [Google Scholar] [CrossRef]
- Khaldi, S.; Saad, G.; Elfekih, H.; Abdelkrim, A.B.; Ach, T.; Kacem, M.; Chaieb, M.; Maaroufi, A.; Hasni, Y.; Ach, K. Pituitary apoplexy of a giant prolactinoma during pregnancy. Gynecol. Endocrinol. 2021, 37, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Souteiro, P.; Belo, S.; Carvalho, D. Dopamine agonists in prolactinomas: When to withdraw? Pituitary 2020, 23, 38–44. [Google Scholar] [CrossRef]
- Snyder, P.J. Patient Education: High Prolactin Levels and Prolactinomas (Beyond the Basics). Available online: https://www.uptodate.com/contents/high-prolactin-levels-and-prolactinomas-beyond-the-basics/contributors (accessed on 24 May 2022).
- Oride, A.; Kanasaki, H.; Okada, H.; Satoru Kyo, S. Reproductive prognosis of patients with hypogonadotropic hypogonadism: Retrospective review of 16 cases with amenorrhea. J. Obstet. Gynaecol. Res. 2021, 47, 3895–3902. [Google Scholar] [CrossRef]
- Tyson, J.E.; Hwang, P.; Guyda, H.; Friesen, H.G. Studies of prolactin secretion in human pregnancy. Am. J. Obstet. Gynecol. 1972, 113, 14–24. [Google Scholar] [CrossRef]
- Corlenblum, B. Pituitary Disease and Pregnancy. 2018. Available online: https://emedicine.medscape.com/article/127650-overview#a2 (accessed on 24 May 2022).
- Ho Yuen, B.; Cannon, W.; Lewis, J.; Sy, L.; Wooley, S. A possible role for prolactin in the control of human chorionic gonadotropin and estrogen secretion by fetoplacental unit. Am. J. Obstet. Gynecol. 1980, 136, 286–291. [Google Scholar] [CrossRef]
- Kletzky, O.A.; Marrs, R.P.; Howard, W.F.; McCormick, W.; Mishell, D.R., Jr. Prolactin synthesis and release during pregnancy and puerperium. Am. J. Obstet. Gynecol. 1980, 136, 545–550. [Google Scholar] [CrossRef]
- McCoshen, J.A.; Barc, J. Prolactin bioactivity following decidual synthesis and transport by amniochorion. Am. J. Obstet. Gynecol. 1985, 153, 217–223. [Google Scholar] [CrossRef]
- Narita, O.; Kimura, T.; Suganuma, N.; Osawa, M.; Mizutani, S.; Masahashi, T.; Asai, M.; Tomoda, T. Relationship between maternal prolactin levels during pregnancy and lactation in women with pituitary adenoma. Nihon Sanka Fujinka Gakkai Zasshi 1985, 37, 758–762. [Google Scholar] [PubMed]
- Speroff, L.; Glass, R.H.; Kase, N.G. The Endocrinology of Pregnancy. In Clinical Gynecologic Endocrinology and Infertility; Lippincott Williams& Wilkins: Philadelphia, PA, USA, 1999; Volume 8, p. 304. [Google Scholar]
- Flores-Espinosa, P. Selective immuno-modulatory effect of prolactin upon pro-inflammatory response in human fetal membranes. J. Reprod. Immunol. 2017, 123, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, B.H.; Betz, G. Prolactin-secreting pituitary tumors and pregnancy. In Contemporary Issues in Endocrinology and metabolism: Prolactinomas; Olefshy, J.M., Robinson, R.J., Eds.; Churchill Livingston: London, UK, 1986; Volume II, p. 195. [Google Scholar]
- Luger, A.; Broersen, L.H.A.; Biermasz, N.R.; Biller, B.M.K.; Buchfelder, M.; Chanson, P.; Jorgensen, J.O.L.; Kelestimur, F.; Llahana, S.; Maiter, D.; et al. ESE Clinical Practice Guideline on functioning and nonfunctioning pituitary adenomas in pregnancy. Eur. J. Endocrinol. 2021, 185, G1–G33. [Google Scholar] [CrossRef]
- Almalki, M.H.; Alzahrani, S.; Alshahrani, F.; Alsherbeni, S.; Almoharib, O.; Aljohani, N.; Almagamsi, A. Managing Prolactinomas during Pregnancy. Front. Endocrinol. 2015, 6, 85. [Google Scholar] [CrossRef]
- O’Sullivan, S.M.; Farrant, M.T.; Ogilvie, C.M.; Gunn, A.J.; Milsom, S.R. An observational study of pregnancy and post-partum outcomes in women with prolactinoma treated with dopamine agonists. Aust. N. Z. J. Obstet. Gynaecol. 2020, 60, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Glezer, A.; Bronstein, M.D. Prolactinomas: How to handle prior to and during pregnancy? Minerva Endocrinol. 2018, 43, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Duhamel, C.; Ilie, M.D.; Salle, H.; Nassouri, A.J.; Gaillard, S.; Deluche, W.; Assaker, R.; Mortier, L.; Cortet, C.; Raverot, G. Immunotherapy in Corticotroph and Lactotroph Aggressive Tumors and Carcinomas: Two Case Reports and a Review of the Literature. J. Pers. Med. 2020, 10, 88. [Google Scholar] [CrossRef]
- Kuhn, E.; Weinreich, A.A.; Biermasz, N.R.; Jorgensen, J.O.L.; Chanson, P. Apoplexy of microprolactinomas during pregnancy: Report of five cases and review of the literature. Eur. J. Endocrinol. 2021, 185, 99–108. [Google Scholar] [CrossRef]
- Crosignani, P.G.; Mattei, A.M.; Severini, V.; Cavioni, V.; Maggioni, P.; Testa, G. Long-term effects of time, medical treatment and pregnancy in 176 hyperproactinemic women. Eur. J. Obstet. Gynecol. Reprod. Biol. 1992, 44, 175–180. [Google Scholar] [CrossRef]
- Domingue, M.E.; Devuyst, F.; Alexopoulou, O.; Corvilain, B.; Maiter, D. Outcome of prolactinoma after pregnancy and lactation: A study on 73 patients. Clin. Endocrinol. 2014, 80, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Badawy, S.Z.; Marziale, J.C.; Rosenbaum, A.E.; Chang, J.K.; Joy, S.E. The long-term effects of pregnancy and bromocriptine treatment on prolactinomas--the value of radiologic studies. Early Pregnancy 1997, 3, 306–311. [Google Scholar] [PubMed]
- Swanson, J.A.; Chapler, F.K.; Sherman, B.M.; Crickard, K. Spontaneous pregnancy in women with a prolactin-producing pituitary adenoma. Fert. Sterilit. 1978, 29, 629–631. [Google Scholar] [CrossRef] [PubMed]
- Eren, M.A.; Cece, H.; Torun, A.N. Spontaneous and Uneventful Pregnancy Occurring in a Case of Macroprolactinoma Diagnosed With Subclinical Pituitary Apoplexy. Neurosurg. Q. 2012, 22, 43–45. [Google Scholar] [CrossRef]
- Ennaifer, H.; Jemel, M.; Kandar, H.; Grira, W.; Kammoun, I.; Ben Salem, L. Developed diplopia due to a pituitary macroadenoma during pregnancy. Pan Afr. Med. J. 2018, 29, 39. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tica, A.A.; Dumitrescu, D.; Tica, I.; Neamţu, C.; Tica, V.I.; Dumitrescu, C.I.; Tica, O.S. Cabergoline Failure and a Spontaneous Pregnancy in a Microprolactinoma with High Prolactin Levels. J. Pers. Med. 2022, 12, 2061. https://doi.org/10.3390/jpm12122061
Tica AA, Dumitrescu D, Tica I, Neamţu C, Tica VI, Dumitrescu CI, Tica OS. Cabergoline Failure and a Spontaneous Pregnancy in a Microprolactinoma with High Prolactin Levels. Journal of Personalized Medicine. 2022; 12(12):2061. https://doi.org/10.3390/jpm12122061
Chicago/Turabian StyleTica, Andrei Adrian, Daniela Dumitrescu, Irina Tica, Corina Neamţu, Vlad Iustin Tica, Cristiana Iulia Dumitrescu, and Oana Sorina Tica. 2022. "Cabergoline Failure and a Spontaneous Pregnancy in a Microprolactinoma with High Prolactin Levels" Journal of Personalized Medicine 12, no. 12: 2061. https://doi.org/10.3390/jpm12122061
APA StyleTica, A. A., Dumitrescu, D., Tica, I., Neamţu, C., Tica, V. I., Dumitrescu, C. I., & Tica, O. S. (2022). Cabergoline Failure and a Spontaneous Pregnancy in a Microprolactinoma with High Prolactin Levels. Journal of Personalized Medicine, 12(12), 2061. https://doi.org/10.3390/jpm12122061





