NLRP6 Induces Lung Injury and Inflammation Early in Brucella and Influenza Coinfection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Bacterial and Virus Strains
2.3. Virus Titration
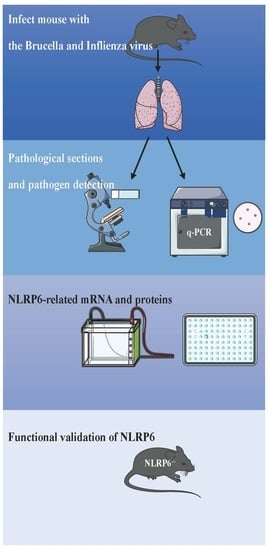
2.4. IAV and Brucella Coinfection In Vivo
2.5. Plate Colony Counting
2.6. RNA Extraction, cDNA Synthesis, and qPCR Analysis
2.7. Lung Injury Severity Scoring
2.8. Western-Blot
2.9. ELISA
2.10. Quantification and Statistical Analysis
3. Results
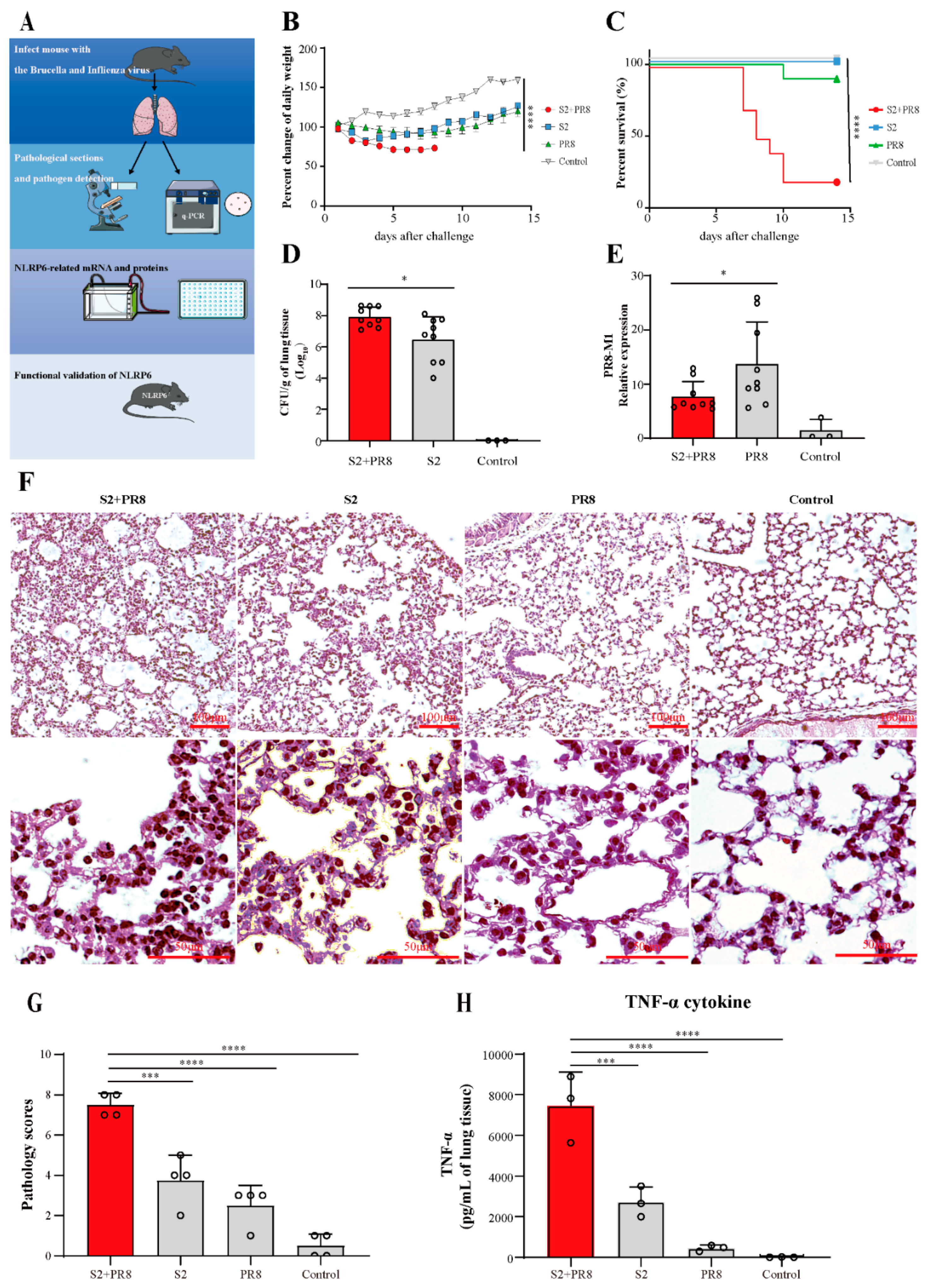
3.1. Coinfection Caused Acute Lung Injury, which Can Lead to Fatal Conditions
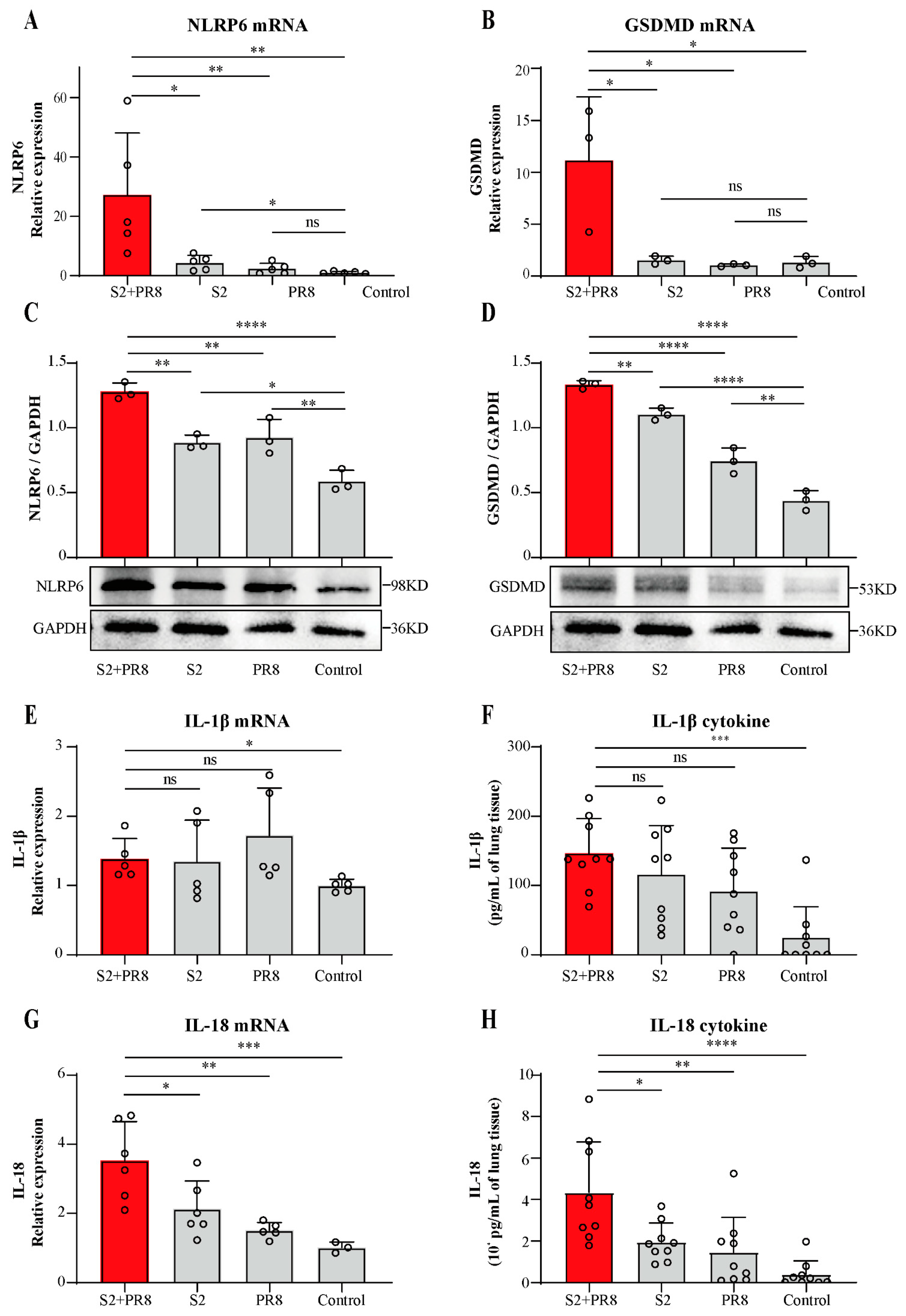
3.2. Coinfection Caused an Increase in NLRP6 Expression
3.3. Coinfection Caused an Increase in IL-18 Expression
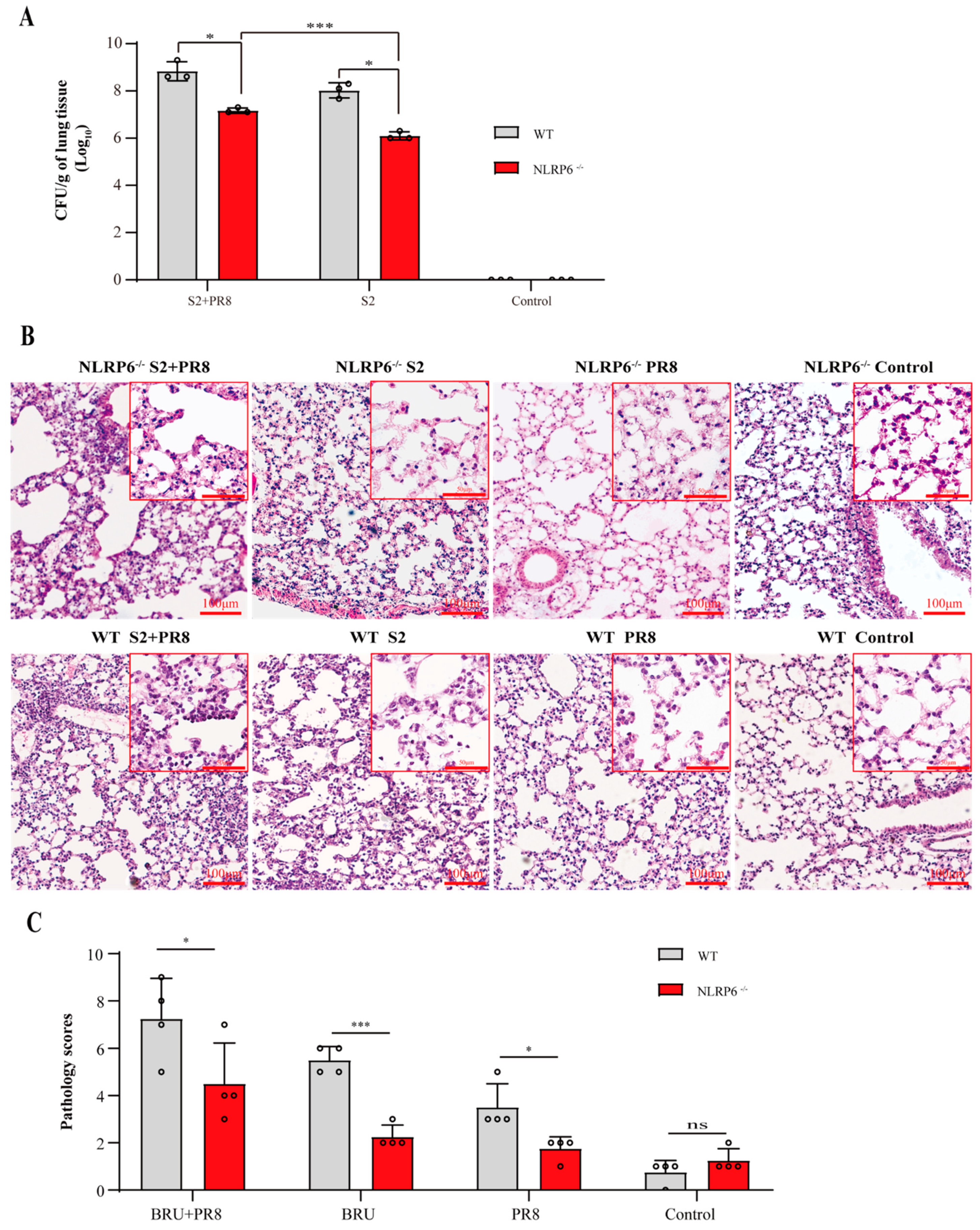
3.4. The Involvement of NLRP6 in Brucella and/or Influenza Virus Infections
3.5. NLRP6 Mediated the Upregulation of IL-18 and IL-1β
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balmer, M.L.; Slack, E.; de Gottardi, A.; Lawson, M.A.; Hapfelmeier, S.; Miele, L.; Grieco, A.; Van Vlierberghe, H.; Fahrner, R.; Patuto, N.; et al. The liver may act as a firewall mediating mutualism between the host and its gut commensal microbiota. Sci. Transl. Med. 2014, 6, 237ra66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacey, C.A.; Chambers, C.A.; Mitchell, W.J.; Skyberg, J.A. IFN-γ-dependent nitric oxide suppresses Brucella-induced arthritis by inhibition of inflammasome activation. J. Leukoc. Biol. 2019, 106, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Bossi, P.; Tegnell, A.; Baka, A.; Van Loock, F.; Hendriks, J.; Werner, A.; Maidhof, H.; Gouvras, G. Bichat guidelines for the clinical management of brucellosis and bioterrorism-related brucellosis. Eurosurveillance 2004, 9, E15–E16. [Google Scholar] [CrossRef] [PubMed]
- Pappas, G.; Papadimitriou, P.; Akritidis, N.; Christou, L.; Tsianos, E.V. The new global map of human brucellosis. Lancet. Infect. Dis. 2006, 6, 91–99. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Kumar, S.; Pal, V.; Bhardwaj, B.; Rai, G.P. Evaluation of the recombinant 10-kilodalton immunodominant region of the BP26 protein of Brucella abortus for specific diagnosis of bovine brucellosis. Clin. Vaccine Immunol. 2011, 18, 1760–1764. [Google Scholar] [CrossRef] [Green Version]
- Zheng, R.; Xie, S.; Niyazi, S.; Lu, X.; Sun, L.; Zhou, Y.; Zhang, Y.; Wang, K. Meta-Analysis of the Changes of Peripheral Blood T Cell Subsets in Patients with Brucellosis. J. Immunol. Res. 2018, 2018, 8439813. [Google Scholar] [CrossRef] [Green Version]
- Pathak, P.; Kumar, A.; Thavaselvam, D. Evaluation of recombinant porin (rOmp2a) protein as a potential antigen candidate for serodiagnosis of Human Brucellosis. BMC Infect. Dis. 2017, 17, 485. [Google Scholar] [CrossRef] [Green Version]
- Castaño, M.J.; Solera, J. Chronic brucellosis and persistence of Brucella melitensis DNA. J. Clin. Microbiol. 2009, 47, 2084–2089. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-González, E.A.; Moreno-Lafont, M.C.; Méndez-Tenorio, A.; Cancino-Díaz, M.E.; Estrada-García, I.; López-Santiago, R. Prediction of Structure and Molecular Interaction with DNA of BvrR, a Virulence-Associated Regulatory Protein of Brucella. Molecules 2019, 24, 3137. [Google Scholar] [CrossRef] [Green Version]
- Ashford, D.A.; di Pietra, J.; Lingappa, J.; Woods, C.; Noll, H.; Neville, B.; Weyant, R.; Bragg, S.L.; Spiegel, R.A.; Tappero, J.; et al. Adverse events in humans associated with accidental exposure to the livestock brucellosis vaccine RB51. Vaccine 2004, 22, 3435–3439. [Google Scholar] [CrossRef]
- Miller, C.D.; Songer, J.R.; Sullivan, J.F. A twenty-five year review of laboratory-acquired human infections at the National Animal Disease Center. Am. Ind. Hyg. Assoc. J. 1987, 48, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Traxler, R.M.; Lehman, M.W.; Bosserman, E.A.; Guerra, M.A.; Smith, T.L. A literature review of laboratory-acquired brucellosis. J. Clin. Microbiol. 2013, 51, 3055–3062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, W.W.; Shay, D.K.; Weintraub, E.; Brammer, L.; Cox, N.; Anderson, L.J.; Fukuda, K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003, 289, 179–186. [Google Scholar] [CrossRef]
- Metersky, M.L.; Masterton, R.G.; Lode, H.; File, T.M., Jr.; Babinchak, T. Epidemiology, microbiology, and treatment considerations for bacterial pneumonia complicating influenza. Int. J. Infect. Dis. 2012, 16, e321–e331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacIntyre, C.R.; Chughtai, A.A.; Barnes, M.; Ridda, I.; Seale, H.; Toms, R.; Heywood, A. The role of pneumonia and secondary bacterial infection in fatal and serious outcomes of pandemic influenza a(H1N1)pdm09. BMC Infect. Dis. 2018, 18, 637. [Google Scholar] [CrossRef] [Green Version]
- Rice, T.W.; Rubinson, L.; Uyeki, T.M.; Vaughn, F.L.; John, B.B.; Miller, R.R., 3rd; Higgs, E.; Randolph, A.G.; Smoot, B.E.; Thompson, B.T. Critical illness from 2009 pandemic influenza A virus and bacterial coinfection in the United States. Crit. Care Med. 2012, 40, 1487–1498. [Google Scholar] [CrossRef] [Green Version]
- Taubenberger, J.K.; Morens, D.M. The 1918 Influenza Pandemic and Its Legacy. Cold Spring Harb. Perspect. Med. 2020, 10, a038695. [Google Scholar] [CrossRef] [Green Version]
- Morens, D.M.; Taubenberger, J.K.; Fauci, A.S. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: Implications for pandemic influenza preparedness. J. Infect. Dis. 2008, 198, 962–970. [Google Scholar] [CrossRef]
- Masiá, M.; Padilla, S.; Antequera, P.; Ramos, J.M.; Ruiz, M.; Gutiérrez, F. Predictors of pneumococcal co-infection for patients with pandemic (H1N1) 2009. Emerg. Infect. Dis. 2011, 17, 1475–1478. [Google Scholar] [CrossRef]
- Blyth, C.C.; Webb, S.A.; Kok, J.; Dwyer, D.E.; van Hal, S.J.; Foo, H.; Ginn, A.N.; Kesson, A.M.; Seppelt, I.; Iredell, J.R. The impact of bacterial and viral co-infection in severe influenza. Influenza Other Respir. Viruses 2013, 7, 168–176. [Google Scholar] [CrossRef]
- Cillóniz, C.; Ewig, S.; Menéndez, R.; Ferrer, M.; Polverino, E.; Reyes, S.; Gabarrús, A.; Marcos, M.A.; Cordoba, J.; Mensa, J.; et al. Bacterial co-infection with H1N1 infection in patients admitted with community acquired pneumonia. J. Infect. 2012, 65, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Paddock, C.D.; Liu, L.; Denison, A.M.; Bartlett, J.H.; Holman, R.C.; Deleon-Carnes, M.; Emery, S.L.; Drew, C.P.; Shieh, W.J.; Uyeki, T.M.; et al. Myocardial injury and bacterial pneumonia contribute to the pathogenesis of fatal influenza B virus infection. J. Infect. Dis. 2012, 205, 895–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van-Tam, J.; Sellwood, C. Pandemic Influenza; CABI: Boston, MA, USA, 2012. [Google Scholar]
- Goel, N.; Ahmad, R.; Fatima, H.; Khare, S.K. New threatening of SARS-CoV-2 coinfection and strategies to fight the current pandemic. Med. Drug Discov. 2021, 10, 100089. [Google Scholar] [CrossRef]
- Soltani, S.; Faramarzi, S.; Zandi, M.; Shahbahrami, R.; Jafarpour, A.; Akhavan Rezayat, S.; Pakzad, I.; Abdi, F.; Malekifar, P.; Pakzad, R. Bacterial coinfection among coronavirus disease 2019 patient groups: An updated systematic review and meta-analysis. New Microbes New Infect. 2021, 43, 100910. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Paudel, S.; Jin, L.; Ghimire, L.; Taylor, C.M.; Wakamatsu, N.; Bhattarai, D.; Jeyaseelan, S. NLRP6 modulates neutrophil homeostasis in bacterial pneumonia-derived sepsis. Mucosal Immunol. 2021, 14, 574–584. [Google Scholar] [CrossRef]
- Ghimire, L.; Paudel, S.; Jin, L.; Baral, P.; Cai, S.; Jeyaseelan, S. NLRP6 negatively regulates pulmonary host defense in Gram-positive bacterial infection through modulating neutrophil recruitment and function. PLoS Pathog. 2018, 14, e1007308. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Wu, X.; Peng, L.; Chen, T.; Huang, Q.; Wang, Y.; Ye, C.; Peng, Y.; Hu, D.; Fang, R. The Critical Role of NLRP6 Inflammasome in Streptococcus pneumoniae Infection In Vitro and In Vivo. Int. J. Mol. Sci. 2021, 22, 3876. [Google Scholar] [CrossRef] [PubMed]
- Rungue, M.; Melo, V.; Martins, D.; Campos, P.C.; Leles, G.; Galvão, I.; Mendes, V.; Aganetti, M.; Pedersen, Á.; Assis, N.R.G.; et al. NLRP6-associated host microbiota composition impacts in the intestinal barrier to systemic dissemination of Brucella abortus. PLoS Negl. Trop. Dis. 2021, 15, e0009171. [Google Scholar] [CrossRef]
- Ghimire, L.; Paudel, S.; Jin, L.; Jeyaseelan, S. The NLRP6 inflammasome in health and disease. Mucosal Immunol. 2020, 13, 388–398. [Google Scholar] [CrossRef] [Green Version]
- Franco, M.P.; Mulder, M.; Gilman, R.H.; Smits, H.L. Human brucellosis. Lancet Infect. Dis. 2007, 7, 775–786. [Google Scholar] [CrossRef]
- Zheng, R.; Xie, S.; Lu, X.; Sun, L.; Zhou, Y.; Zhang, Y.; Wang, K. A Systematic Review and Meta-Analysis of Epidemiology and Clinical Manifestations of Human Brucellosis in China. BioMed Res. Int. 2018, 2018, 5712920. [Google Scholar] [CrossRef] [PubMed]
- Deqiu, S.; Donglou, X.; Jiming, Y. Epidemiology and control of brucellosis in China. Vet. Microbiol. 2002, 90, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Gonzalez-Juarbe, N.; Riegler, A.N.; Im, H.; Hale, Y.; Platt, M.P.; Croney, C.; Briles, D.E.; Orihuela, C.J. Streptococcus pneumoniae binds to host GAPDH on dying lung epithelial cells worsening secondary infection following influenza. Cell Rep. 2021, 35, 109267. [Google Scholar] [CrossRef]
- Sharma-Chawla, N.; Stegemann-Koniszewski, S.; Christen, H.; Boehme, J.D.; Kershaw, O.; Schreiber, J.; Guzmán, C.A.; Bruder, D.; Hernandez-Vargas, E.A. In vivo Neutralization of Pro-inflammatory Cytokines During Secondary Streptococcus pneumoniae Infection Post Influenza A Virus Infection. Front. Immunol. 2019, 10, 1864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; He, L.; Li, S.; He, W.; Zha, C.; Ou, W.; Hou, Q.; Wang, W.; Sun, X.; Liang, H. Combination of procalcitonin and C-reactive protein levels in the early diagnosis of bacterial co-infections in children with H1N1 influenza. Influenza Other Respir. Viruses 2019, 13, 184–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshihara, K.; Le, M.N.; Toizumi, M.; Nguyen, H.A.; Vo, H.M.; Odagiri, T.; Fujisaki, S.; Ariyoshi, K.; Moriuchi, H.; Hashizume, M.; et al. Influenza B associated paediatric acute respiratory infection hospitalization in central vietnam. Influenza Other Respir. Viruses 2019, 13, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Yang, W.; Luan, X.; Li, H.; Li, H.; Tian, D.; Fan, W.; Li, J.; Wang, B.; Liu, W.; et al. Induction of cyclophilin A by influenza A virus infection facilitates group A Streptococcus coinfection. Cell Rep. 2021, 35, 109159. [Google Scholar] [CrossRef]
- Zheng, D.; Kern, L.; Elinav, E. The NLRP6 inflammasome. Immunology 2021, 162, 281–289. [Google Scholar] [CrossRef]
- Ding, J.; Wang, K.; Liu, W.; She, Y.; Sun, Q.; Shi, J.; Sun, H.; Wang, D.C.; Shao, F. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 2016, 535, 111–116. [Google Scholar] [CrossRef]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Ydens, E.; Demon, D.; Lornet, G.; De Winter, V.; Timmerman, V.; Lamkanfi, M.; Janssens, S. Nlrp6 promotes recovery after peripheral nerve injury independently of inflammasomes. J. Neuroinflamm. 2015, 12, 143. [Google Scholar] [CrossRef] [Green Version]
- Jorgensen, I.; Zhang, Y.; Krantz, B.A.; Miao, E.A. Pyroptosis triggers pore-induced intracellular traps (PITs) that capture bacteria and lead to their clearance by efferocytosis. J. Exp. Med. 2016, 213, 2113–2128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voet, S.; Mc Guire, C.; Hagemeyer, N.; Martens, A.; Schroeder, A.; Wieghofer, P.; Daems, C.; Staszewski, O.; Vande Walle, L.; Jordao, M.J.C.; et al. A20 critically controls microglia activation and inhibits inflammasome-dependent neuroinflammation. Nat. Commun. 2018, 9, 2036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, W.T.; Wan, H.; Hu, L.; Chen, P.; Wang, X.; Huang, Z.; Yang, Z.H.; Zhong, C.Q.; Han, J. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015, 25, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Sansonetti, P.J.; Phalipon, A.; Arondel, J.; Thirumalai, K.; Banerjee, S.; Akira, S.; Takeda, K.; Zychlinsky, A. Caspase-1 activation of IL-1beta and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity 2000, 12, 581–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raupach, B.; Peuschel, S.K.; Monack, D.M.; Zychlinsky, A. Caspase-1-mediated activation of interleukin-1beta (IL-1beta) and IL-18 contributes to innate immune defenses against Salmonella enterica serovar Typhimurium infection. Infect. Immun. 2006, 74, 4922–4926. [Google Scholar] [CrossRef] [Green Version]
- Hara, H.; Seregin, S.S.; Yang, D.; Fukase, K.; Chamaillard, M.; Alnemri, E.S.; Inohara, N.; Chen, G.Y.; Núñez, G. The NLRP6 Inflammasome Recognizes Lipoteichoic Acid and Regulates Gram-Positive Pathogen Infection. Cell 2018, 175, 1651–1664.e14. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Kumar, R.; Tsakem Lenou, E.; Basrur, V.; Kontoyiannis, D.L.; Ioakeimidis, F.; Mosialos, G.; Theiss, A.L.; Flavell, R.A.; Venuprasad, K. Deubiquitination of NLRP6 inflammasome by Cyld critically regulates intestinal inflammation. Nat. Immunol. 2020, 21, 626–635. [Google Scholar] [CrossRef]
- Wang, P.; Zhu, S.; Yang, L.; Cui, S.; Pan, W.; Jackson, R.; Zheng, Y.; Rongvaux, A.; Sun, Q.; Yang, G.; et al. Nlrp6 regulates intestinal antiviral innate immunity. Science 2015, 350, 826–830. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, B.; Han, H.; Li, H.; Tan, L.; Li, X.; Wang, K.; Li, B.; He, W.; Tian, C.; Yan, F.; et al. NLRP6 Induces Lung Injury and Inflammation Early in Brucella and Influenza Coinfection. J. Pers. Med. 2022, 12, 2063. https://doi.org/10.3390/jpm12122063
Shi B, Han H, Li H, Tan L, Li X, Wang K, Li B, He W, Tian C, Yan F, et al. NLRP6 Induces Lung Injury and Inflammation Early in Brucella and Influenza Coinfection. Journal of Personalized Medicine. 2022; 12(12):2063. https://doi.org/10.3390/jpm12122063
Chicago/Turabian StyleShi, Bochang, Hui Han, Huabin Li, Lingyun Tan, Xinyu Li, Keyu Wang, Bo Li, Wei He, Chongyu Tian, Fang Yan, and et al. 2022. "NLRP6 Induces Lung Injury and Inflammation Early in Brucella and Influenza Coinfection" Journal of Personalized Medicine 12, no. 12: 2063. https://doi.org/10.3390/jpm12122063
APA StyleShi, B., Han, H., Li, H., Tan, L., Li, X., Wang, K., Li, B., He, W., Tian, C., Yan, F., Shi, Y., Zheng, Y., & Zhao, Z. (2022). NLRP6 Induces Lung Injury and Inflammation Early in Brucella and Influenza Coinfection. Journal of Personalized Medicine, 12(12), 2063. https://doi.org/10.3390/jpm12122063