The Soluble Folate Receptor in Autism Spectrum Disorder: Relation to Autism Severity and Leucovorin Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. sFBP Assay
2.2. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Effectiveness of Leucovorin Treatment
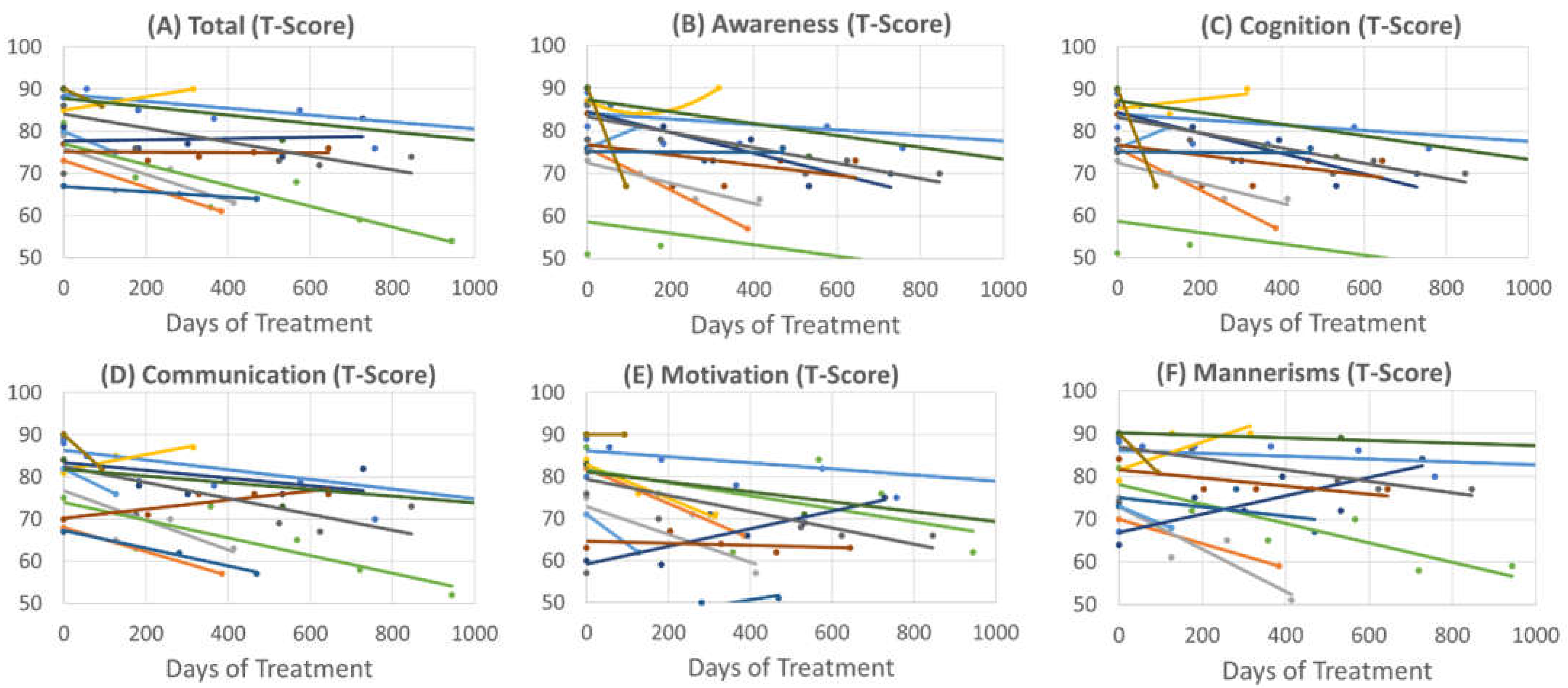
3.2.1. Social Responsiveness Scale
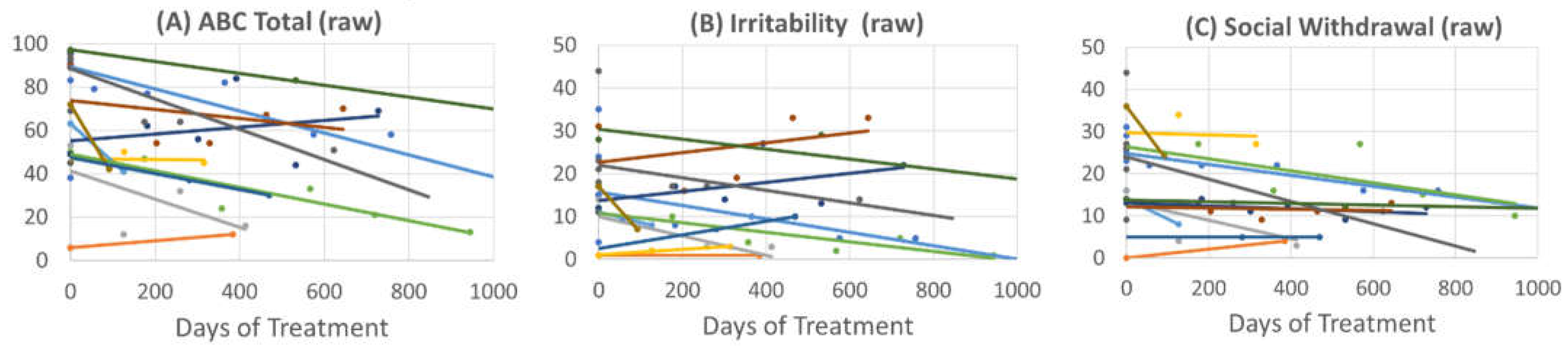
3.2.2. Aberrant Behavior Checklist
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maenner, M.J.; Shaw, K.A.; Baio, J.; Washington, A.; Patrick, M.; DiRienzo, M.; Christensen, D.L.; Wiggins, L.D.; Pettygrove, S.; Jennifer, G.; et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR Surveill Summ. 2020, 69, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, D.A.; Frye, R.E. A review of research trends in physiological abnormalities in autism spectrum disorders: Immune dysregulation, inflammation, oxidative stress, mitochondrial dysfunction and environmental toxicant exposures. Mol. Psychiatry 2012, 17, 389–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frye, R.E.; Rossignol, D.A. Treatments for biomedical abnormalities associated with autism spectrum disorder. Front. Pediatr. 2014, 2, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frustaci, A.; Neri, M.; Cesario, A.; Adams, J.B.; Domenici, E.; Dalla Bernardina, B.; Bonassi, S. Oxidative stress-related biomarkers in autism: Systematic review and meta-analyses. Free Radic. Biol. Med. 2012, 52, 2128–2141. [Google Scholar] [CrossRef] [PubMed]
- Mahmuda, N.A.; Yokoyama, S.; Huang, J.J.; Liu, L.; Munesue, T.; Nakatani, H.; Hayashi, K.; Yagi, K.; Yamagishi, M.; Higashida, H. A Study of Single Nucleotide Polymorphisms of the SLC19A1/RFC1 Gene in Subjects with Autism Spectrum Disorder. Int. J. Mol. Sci. 2016, 17, 772. [Google Scholar] [CrossRef] [Green Version]
- Haghiri, R.; Mashayekhi, F.; Bidabadi, E.; Salehi, Z. Analysis of methionine synthase (rs1805087) gene polymorphism in autism patients in Northern Iran. Acta Neurobiol. Exp. 2016, 76, 318–323. [Google Scholar] [CrossRef] [Green Version]
- James, S.J.; Melnyk, S.; Jernigan, S.; Cleves, M.A.; Halsted, C.H.; Wong, D.H.; Cutler, P.; Bock, K.; Boris, M.; Bradstreet, J.J.; et al. Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. Off. Publ. Int. Soc. Psychiatr. Genet. 2006, 141b, 947–956. [Google Scholar] [CrossRef] [Green Version]
- James, S.J.; Melnyk, S.; Jernigan, S.; Pavliv, O.; Trusty, T.; Lehman, S.; Seidel, L.; Gaylor, D.W.; Cleves, M.A. A functional polymorphism in the reduced folate carrier gene and DNA hypomethylation in mothers of children with autism. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. Off. Publ. Int. Soc. Psychiatr. Genet. 2010, 153b, 1209–1220. [Google Scholar] [CrossRef] [Green Version]
- Guo, B.Q.; Ding, S.B.; Li, H.B. Blood biomarker levels of methylation capacity in autism spectrum disorder: A systematic review and meta-analysis. Acta Psychiatr. Scand. 2020, 141, 492–509. [Google Scholar] [CrossRef]
- Guo, B.Q.; Li, H.B.; Ding, S.B. Blood homocysteine levels in children with autism spectrum disorder: An updated systematic review and meta-analysis. Psychiatry Res. 2020, 291, 113283. [Google Scholar] [CrossRef]
- Howsmon, D.P.; Kruger, U.; Melnyk, S.; James, S.J.; Hahn, J. Classification and adaptive behavior prediction of children with autism spectrum disorder based upon multivariate data analysis of markers of oxidative stress and DNA methylation. PLoS Comput. Biol. 2017, 13, e1005385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howsmon, D.P.; Vargason, T.; Rubin, R.A.; Delhey, L.; Tippett, M.; Rose, S.; Bennuri, S.C.; Slattery, J.C.; Melnyk, S.; James, S.J.; et al. Multivariate techniques enable a biochemical classification of children with autism spectrum disorder versus typically-developing peers: A comparison and validation study. Bioeng. Transl. Med. 2018, 3, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Vogel Ciernia, A.; LaSalle, J. The landscape of DNA methylation amid a perfect storm of autism aetiologies. Nat. Rev. Neurosci. 2016, 17, 411–423. [Google Scholar] [CrossRef] [Green Version]
- Bakulski, K.M.; Dou, J.F.; Feinberg, J.I.; Aung, M.T.; Ladd-Acosta, C.; Volk, H.E.; Newschaffer, C.J.; Croen, L.A.; Hertz-Picciotto, I.; Levy, S.E.; et al. Autism-Associated DNA Methylation at Birth From Multiple Tissues Is Enriched for Autism Genes in the Early Autism Risk Longitudinal Investigation. Front. Mol. Neurosci. 2021, 14, 775390. [Google Scholar] [CrossRef] [PubMed]
- Garrido, N.; Cruz, F.; Egea, R.R.; Simon, C.; Sadler-Riggleman, I.; Beck, D.; Nilsson, E.; Ben Maamar, M.; Skinner, M.K. Sperm DNA methylation epimutation biomarker for paternal offspring autism susceptibility. Clin. Epigenetics 2021, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, D.A.; Frye, R.E. Cerebral Folate Deficiency, Folate Receptor Alpha Autoantibodies and Leucovorin (Folinic Acid) Treatment in Autism Spectrum Disorders: A Systematic Review and Meta-Analysis. J. Pers. Med. 2021, 11, 1141. [Google Scholar] [CrossRef] [PubMed]
- Holm, J.; Hansen, S.I. Characterization of soluble folate receptors (folate binding proteins) in humans. Biological roles and clinical potentials in infection and malignancy. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140466. [Google Scholar] [CrossRef]
- Duvekot, J.; van der Ende, J.; Verhulst, F.C.; Greaves-Lord, K. The Screening Accuracy of the Parent and Teacher-Reported Social Responsiveness Scale (SRS): Comparison with the 3Di and ADOS. J. Autism Dev. Disord. 2015, 45, 1658–1672. [Google Scholar] [CrossRef]
- Murray, M.J.; Mayes, S.D.; Smith, L.A. Brief report: Excellent agreement between two brief autism scales (Checklist for Autism Spectrum Disorder and Social Responsiveness Scale) completed independently by parents and the Autism Diagnostic Interview-Revised. J. Autism Dev. Disord. 2011, 41, 1586–1590. [Google Scholar] [CrossRef]
- Frye, R.E.; Slattery, J.; Delhey, L.; Furgerson, B.; Strickland, T.; Tippett, M.; Sailey, A.; Wynne, R.; Rose, S.; Melnyk, S.; et al. Folinic acid improves verbal communication in children with autism and language impairment: A randomized double-blind placebo-controlled trial. Mol. Psychiatry 2018, 23, 247–256. [Google Scholar] [CrossRef]
- Connery, K.; Tippett, M.; Delhey, L.M.; Rose, S.; Slattery, J.C.; Kahler, S.G.; Hahn, J.; Kruger, U.; Cunningham, M.W.; Shimasaki, C.; et al. Intravenous immunoglobulin for the treatment of autoimmune encephalopathy in children with autism. Transl. Psychiatry 2018, 8, 148. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; Sequeira, J.M.; Quadros, E.V.; James, S.J.; Rossignol, D.A. Cerebral folate receptor autoantibodies in autism spectrum disorder. Mol. Psychiatry 2013, 18, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, J.M.; Ramaekers, V.T.; Quadros, E.V. The diagnostic utility of folate receptor autoantibodies in blood. Clin. Chem. Lab. Med. 2013, 51, 545–554. [Google Scholar] [CrossRef]
- Jensen, A.R.; Lane, A.L.; Werner, B.A.; McLees, S.E.; Fletcher, T.S.; Frye, R.E. Modern Biomarkers for Autism Spectrum Disorder: Future Directions. Mol. Diagn Ther. 2022, 26, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Almekkawi, A.K.; AlJardali, M.W.; Daadaa, H.M.; Lane, A.L.; Worner, A.R.; Karim, M.A.; Scheck, A.C.; Frye, R.E. Folate Pathway Gene Single Nucleotide Polymorphisms and Neural Tube Defects: A Systematic Review and Meta-Analysis. J. Pers. Med. 2022, 12, 1609. [Google Scholar] [CrossRef]
- Franco, C.N.; Seabrook, L.J.; Nguyen, S.T.; Leonard, J.T.; Albrecht, L.V. Simplifying the B Complex: How Vitamins B6 and B9 Modulate One Carbon Metabolism in Cancer and Beyond. Metabolites 2022, 12, 961. [Google Scholar] [CrossRef]
- Kovalev, I.S.; Zyryanov, G.V.; Santra, S.; Majee, A.; Varaksin, M.V.; Charushin, V.N. Folic Acid Antimetabolites (Antifolates): A Brief Review on Synthetic Strategies and Application Opportunities. Molecules 2022, 27, 6229. [Google Scholar] [CrossRef]
- Fallah, M.S.; Shaikh, M.R.; Neupane, B.; Rusiecki, D.; Bennett, T.A.; Beyene, J. Atypical Antipsychotics for Irritability in Pediatric Autism: A Systematic Review and Network Meta-Analysis. J. Child. Adolesc. Psychopharmacol. 2019, 29, 168–180. [Google Scholar] [CrossRef]

| Biomarker | Description |
|---|---|
| Blocking Antibody | This is an antibody (any type, IgG, IgM, etc) that blocks the ability of folate to bind to the folate receptor alpha |
| Binding Antibody | This is an antibody (any type, IgG, IgM, etc) that binds anywhere on the folate receptor alpha |
| Soluble Folate Binding Proteins | These are proteins that binds folate. Their presence is inferred by an interference with the blocking assay. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frye, R.E.; Lane, A.; Worner, A.; Werner, B.A.; McCarty, P.J.; Scheck, A.C.; Collins, H.L.; Adelman, S.J.; Quadros, E.V.; Rossignol, D.A. The Soluble Folate Receptor in Autism Spectrum Disorder: Relation to Autism Severity and Leucovorin Treatment. J. Pers. Med. 2022, 12, 2033. https://doi.org/10.3390/jpm12122033
Frye RE, Lane A, Worner A, Werner BA, McCarty PJ, Scheck AC, Collins HL, Adelman SJ, Quadros EV, Rossignol DA. The Soluble Folate Receptor in Autism Spectrum Disorder: Relation to Autism Severity and Leucovorin Treatment. Journal of Personalized Medicine. 2022; 12(12):2033. https://doi.org/10.3390/jpm12122033
Chicago/Turabian StyleFrye, Richard E., Alison Lane, Ashley Worner, Brianna A. Werner, Patrick J. McCarty, Adrienne C. Scheck, Heidi L. Collins, Steven J. Adelman, Edward V. Quadros, and Daniel A. Rossignol. 2022. "The Soluble Folate Receptor in Autism Spectrum Disorder: Relation to Autism Severity and Leucovorin Treatment" Journal of Personalized Medicine 12, no. 12: 2033. https://doi.org/10.3390/jpm12122033
APA StyleFrye, R. E., Lane, A., Worner, A., Werner, B. A., McCarty, P. J., Scheck, A. C., Collins, H. L., Adelman, S. J., Quadros, E. V., & Rossignol, D. A. (2022). The Soluble Folate Receptor in Autism Spectrum Disorder: Relation to Autism Severity and Leucovorin Treatment. Journal of Personalized Medicine, 12(12), 2033. https://doi.org/10.3390/jpm12122033









