Comparison of Maternal–Fetal Outcomes among Unvaccinated and Vaccinated Pregnant Women with COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Outcomes
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.; Yuan, S.; Kok, K.H.; To, K.K.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.; Poon, R.W. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johns Hopkins University. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) 2020. Available online: https://coronavirus.jhu.edu/map.html (accessed on 15 October 2022).
- Rasmussen, S.A.; Smulian, J.C.; Lednicky, J.A.; Wen, T.S.; Jamieson, D.J. Coronavirus Disease 2019 (COVID-19) and pregnancy: What obstetricians need to know. Am. J. Obstet. Gynecol. 2020, 222, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Yan, J.; Guo, J.; Fan, C.; Juan, J.; Yu, X.; Li, J.; Feng, L.; Li, C.; Chen, H.; Qiao, Y. Coronavirus disease 2019 in pregnant women: A report based on 116 cases. Am. J. Obstet. Gynecol. 2020, 223, 111.e1–111.e14. [Google Scholar] [CrossRef]
- Shimabukuro, T.T.; Kim, S.Y.; Myers, T.R.; Moro, P.L.; Oduyebo, T.; Panagiotakopoulos, L.; Marquez, P.L.; Olson, C.K.; Liu, R.; Chang, K.T. Preliminary Findings of mRNA COVID-19 Vaccine Safety in Pregnant Persons. N. Engl. J. Med. 2021, 384, 2273–2282. [Google Scholar] [CrossRef]
- Jamieson, D.J.; Rasmussen, S.A. An update on COVID-19 and pregnancy. Am. J. Obstet. Gynecol. 2022, 226, 177–186. [Google Scholar] [CrossRef]
- Rasmussen, S.A.; Jamieson, D.J. COVID-19 Vaccination during Pregnancy—Two for the Price of One. N. Engl. J. Med. 2022, 387, 178–179. [Google Scholar] [CrossRef]
- Safadi, M.A.P.; Spinardi, J.; Swerdlow, D.; Srivastava, A. COVID-19 disease and vaccination in pregnant and lactating women. Am. J. Reprod. Immunol. 2022, 88, e13550. [Google Scholar] [CrossRef]
- Villar, J.; Ariff, S.; Gunier, R.B.; Thiruvengadam, R.; Rauch, S.; Kholin, A.; Roggero, P.; Prefumo, F.; Vale, M.S.d.; Cardona-Perez, J.A. Maternal and Neonatal Morbidity and Mortality Among Pregnant Women With and Without COVID-19 Infection: The INTERCOVID Multinational Cohort Study. JAMA Pediatr. 2021, 175, 817–826. [Google Scholar] [CrossRef]
- Zambrano, L.D.; Ellington, S.; Strid, P.; Galang, R.R.; Oduyebo, T.; Tong, V.T.; Woodworth, K.R.; Nahabedian, J.F., III; Azziz-Baumgartner, E.; Gilboa, S.M. Update: Characteristics of Symptomatic Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status—United States, January 22–October 3, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Badr, D.A.; Mattern, J.; Carlin, A.; Cordier, A.; Maillart, E.; el Hachem, L.; el Kenz, H.; Andronikof, M.; de Bels, D.; Damoisel, C. Are clinical outcomes worse for pregnant women at ≥20 weeks’ gestation infected with coronavirus disease 2019? A multicenter case-control study with propensity score matching. Am. J. Obstet. Gynecol. 2020, 223, 764–768. [Google Scholar] [CrossRef] [PubMed]
- Allotey, J.; Stallings, E.; Bonet, M.; Yap, M.; Chatterjee, S.; Kew, T.; Zhou, D.; Coomar, D.; Sheikh, J.; Lawson, H. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ 2020, 370, m3320. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.Q.; Bilodeau-Bertrand, M.; Liu, S.; Auger, N. The impact of COVID-19 on pregnancy outcomes: A systematic review and meta-analysis. CMAJ 2021, 193, E540–E548. [Google Scholar] [CrossRef]
- Di Mascio, D.; Khalil, A.; Saccone, G.; Rizzo, G.; Buca, D.; Liberati, M.; Vecchiet, J.; Nappi, L.; Scambia, G.; Berghella, V. Outcome of Coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2020, 2, 100107. [Google Scholar] [CrossRef]
- Male, V. SARS-CoV-2 infection and COVID-19 vaccination in pregnancy. Nat. Rev. Immunol. 2022, 22, 277–282. [Google Scholar] [CrossRef]
- Khalil, A.; Kalafat, E.; Benlioglu, C.; O’Brien, P.; Morris, E.; Draycott, T.; Thangaratinam, S.; Le Doare, K.; Heath, P.; Ladhani, S. SARS-CoV-2 infection in pregnancy: A systematic review and meta-analysis of clinical features and pregnancy outcomes. EClinicalMedicine 2020, 25, 100446. [Google Scholar] [CrossRef]
- Papageorghiou, A.T.; Deruelle, P.; Gunier, R.B.; Rauch, S.; García-May, P.K.; Mhatre, M.; Usman, M.A.; Abd-Elsalam, S.; Etuk, S.; Simmons, L.E. Preeclampsia and COVID-19: Results from the INTERCOVID prospective longitudinal study. Am. J. Obstet. Gynecol. 2021, 225, 289.e1–289.e17. [Google Scholar] [CrossRef]
- Trostle, M.E.; Limaye, M.A.; Avtushka, V.; Lighter, J.L.; Penfield, C.A.; Roman, A.S. COVID-19 vaccination in pregnancy: Early experience from a single institution. Am. J. Obstet. Gynecol. MFM 2021, 3, 100464. [Google Scholar] [CrossRef]
- Badell, M.L.; Dude, C.M.; Rasmussen, S.A.; Jamieson, D.J. COVID-19 vaccination in pregnancy. BMJ 2022, 378, e069741. [Google Scholar] [CrossRef]
- Beharier, O.; Mayo, R.P.; Raz, T.; Sacks, K.N.; Schreiber, L.; Suissa-Cohen, Y.; Chen, R.; Gomez-Tolub, R.; Hadar, E.; Gabbay-Benziv, R.; et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J. Clin. Investig. 2021, 131, e150319. [Google Scholar] [CrossRef] [PubMed]
- Blakeway, H.; Prasad, S.; Kalafat, E.; Heath, P.T.; Ladhani, S.N.; Le Doare, K.; Magee, L.A.; O’Brien, P.; Rezvani, A.; von Dadelszen, P.; et al. COVID-19 vaccination during pregnancy: Coverage and safety. Am. J. Obstet. Gynecol. 2022, 226, 236.e1–236.e14. [Google Scholar] [CrossRef] [PubMed]
- DeSilva, M.; Haapala, J.; Vazquez-Benitez, G.; Vesco, K.K.; Daley, M.F.; Getahun, D.; Zerbo, O.; Naleway, A.; Nelson, J.C.; Williams, J.T.; et al. Evaluation of Acute Adverse Events after COVID-19 Vaccination during Pregnancy. N. Engl. J. Med. 2022, 387, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Bookstein Peretz, S.; Regev, N.; Novick, L.; Nachshol, M.; Goffer, E.; Ben-David, A.; Asraf, K.; Doolman, R.; Gal Levin, E.; Regev Yochay, G.; et al. Short-term outcome of pregnant women vaccinated with BNT162b2 mRNA COVID-19 vaccine. Ultrasound Obstet. Gynecol. 2021, 58, 450–456. [Google Scholar] [CrossRef]
- Burd, I.; Kino, T.; Segars, J. The Israeli study of Pfizer BNT162b2 vaccine in pregnancy: Considering maternal and neonatal benefits. J. Clin. Investig. 2021, 131, e150790. [Google Scholar] [CrossRef]
- Zauche, L.H.; Wallace, B.; Smoots, A.N.; Olson, C.K.; Oduyebo, T.; Kim, S.Y.; Petersen, E.E.; Ju, J.; Beauregard, J.; Wilcox, A.J.; et al. Receipt of mRNA COVID-19 Vaccines and Risk of Spontaneous Abortion. N. Engl. J. Med. 2021, 385, 1533–1535. [Google Scholar] [CrossRef]
- Kachikis, A.; Englund, J.A.; Singleton, M.; Covelli, I.; Drake, A.L.; Eckert, L.O. Short-term Reactions Among Pregnant and Lactating Individuals in the First Wave of the COVID-19 Vaccine Rollout. JAMA Netw. Open 2021, 4, e2121310. [Google Scholar] [CrossRef]
- Magnus, M.C.; Gjessing, H.K.; Eide, H.N.; Wilcox, A.J.; Fell, D.B.; Håberg, S.E. COVID-19 Vaccination during Pregnancy and First-Trimester Miscarriage. N. Engl. J. Med. 2021, 385, 2008–2010. [Google Scholar] [CrossRef]
- Singh, V.; Choudhary, A.; Datta, M.R.; Ray, A. Maternal and Neonatal Outcomes of COVID-19 in Pregnancy: A Single-Centre Observational Study. Cureus 2021, 13, e13184. [Google Scholar] [CrossRef]
- Kiserud, T.; Piaggio, G.; Carroli, G.; Widmer, M.; Carvalho, J.; Jensen, L.N.; Giordano, D.; Cecatti, J.G.; Aleem, H.A.; Talegawkar, S.A.; et al. The World Health Organization Fetal Growth Charts: A Multinational Longitudinal Study of Ultrasound Biometric Measurements and Estimated Fetal Weight. PLoS Med. 2017, 14, e1002220. [Google Scholar]
- Figueras, F.; Gratacós, E. Update on the Diagnosis and Classification of Fetal Growth Restriction and Proposal of a Stage-Based Management Protocol. Fetal Diagn. Ther. 2014, 36, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Figueras, F.; Gratacos, E. An integrated approach to fetal growth restriction. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 38, 48–58. [Google Scholar] [CrossRef]
- Seely, E.W.; Ecker, J. Chronic hypertension in pregnancy. Circulation 2014, 129, 1254–1261. [Google Scholar] [CrossRef] [Green Version]
- Chaiworapongsa, T.; Chaemsaithong, P.; Yeo, L.; Romero, R. Pre-eclampsia part 1: Current understanding of its pathophysiology. Nat. Rev. Nephrol. 2014, 10, 466–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dröge, L.; Herraìz, I.; Zeisler, H.; Schlembach, D.; Stepan, H.; Küssel, L.; Henrich, W.; Galindo, A.; Verlohren, S. Maternal serum sFlt-1/PlGF ratio in twin pregnancies with and without pre-eclampsia in comparison with singleton pregnancies. Ultrasound Obstet. Gynecol. 2015, 45, 286–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeisler, H.; Llurba, E.; Chantraine, F.; Vatish, M.; Staff, A.C.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H.; Allegranza, D.; et al. Predictive Value of the sFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. N. Engl. J. Med. 2016, 374, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Webster, K.; Fishburn, S.; Maresh, M.; Findlay, S.C.; Chappell, L.C. Diagnosis and management of hypertension in pregnancy: Summary of updated NICE guidance. BMJ 2019, 366, 15119. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Gestational Diabetes Mellitus. Diabetes Care 2003, 26 (Suppl. S1), s103–s105. [Google Scholar] [CrossRef] [Green Version]
- Akgöl, E.; Abuşoğlu, S.; Gün, F.D.; Ünlü, A. Prevalence of gestational diabetes mellitus according to the different criterias. J. Turk. Soc. Obstet. Gynecol. 2017, 14, 18–22. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Cobo, T.; Aldecoa, V.; Bartha, J.L.; Bugatto, F.; Carrillo-Badillo, M.P.; Comas, C.; Diago-Almeda, V.; Ferrero, S.; Goya, M.; Herraiz, I.; et al. Assessment of an intervention to optimise antenatal management of women admitted with preterm labour and intact membranes using amniocentesis-based predictive risk models: Study protocol for a randomised controlled trial (OPTIM-PTL Study). BMJ Open 2021, 11, e054711. [Google Scholar] [CrossRef] [PubMed]
- Baños, N.; Julià, C.; Lorente, N.; Ferrero, S.; Cobo, T.; Gratacos, E.; Palacio, M. Mid-Trimester Cervical Consistency Index and Cervical Length to Predict Spontaneous Preterm Birth in a High-Risk Population. Am. J. Perinatol. Rep. 2018, 8, e43–e50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SARS-CoV-2 Variants in Spain. [Internet]. 2022. Available online: https://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/variantes.htm (accessed on 23 October 2022).
- Poon, L.C.; Yang, H.; Kapur, A.; Melamed, N.; Dao, B.; Divakar, H.; McIntyre, H.D.; Kihara, A.B.; Ayres-De-Campos, D.; Ferrazzi, E.M.; et al. Global interim guidance on coronavirus disease 2019 (COVID-19) during pregnancy and puerperium from FIGO and allied partners: Information for healthcare professionals. Int. J. Gynecol. Obstet. 2020, 149, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Poon, L.C.; Yang, H.; Dumont, S.; Lee, J.C.S.; Copel, J.A.; Danneels, L.; Wright, A.; Costa, F.D.S.; Leung, T.Y.; Zhang, Y.; et al. ISUOG Interim Guidance on coronavirus disease 2019 (COVID-19) during pregnancy and puerperium: Information for healthcare professionals–an update. Ultrasound Obstet. Gynecol. 2020, 55, 848–862. [Google Scholar] [CrossRef]
- COVID WWGo. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection. Ultrasound Obstet. Gynecol. 2021, 57, 232–241. [Google Scholar] [CrossRef]
- Metz, T.D.; Clifton, R.G.; Hughes, B.L.; Sandoval, G.; Saade, G.R.; Grobman, W.A.; Manuck, T.A.; Miodovnik, M.; Sowles, A.; Clark, K.; et al. Disease Severity and Perinatal Outcomes of Pregnant Patients With Coronavirus Disease 2019 (COVID-19). Obstet. Gynecol. 2021, 137, 571–580. [Google Scholar] [CrossRef]
- Flannery, D.D.; Gouma, S.; Dhudasia, M.B.; Mukhopadhyay, S.; Pfeifer, M.R.; Woodford, E.C.; Triebwasser, J.E.; Gerber, J.S.; Morris, J.S.; Weirick, M.E.; et al. Assessment of Maternal and Neonatal Cord Blood SARS-CoV-2 Antibodies and Placental Transfer Ratios. JAMA Pediatr. 2021, 175, 594–600. [Google Scholar] [CrossRef]
- Song, D.; Prahl, M.; Gaw, S.L.; Narasimhan, S.R.; Rai, D.S.; Huang, A.; Flores, C.V.; Lin, C.Y.; Jigmeddagva, U.; Alan Wu, A.; et al. Passive and active immunity in infants born to mothers with SARS-CoV-2 infection during pregnancy: Prospective cohort study. BMJ Open 2021, 11, e053036. [Google Scholar] [CrossRef]
- Collier, A.-R.Y.; McMahan, K.; Yu, J.; Tostanoski, L.H.; Aguayo, R.; Ansel, J.; Chandrashekar, A.; Patel, S.; Bondzie, E.A.; Sellers, D.; et al. Immunogenicity of COVID-19 mRNA Vaccines in Pregnant and Lactating Women. JAMA 2021, 325, 2370. [Google Scholar] [CrossRef]
- Shook, L.L.; Atyeo, C.G.; Yonker, L.M.; Fasano, A.; Gray, K.J.; Alter, G.; Edlow, A.G. Durability of Anti-Spike Antibodies in Infants After Maternal COVID-19 Vaccination or Natural Infection. JAMA 2022, 327, 1087–1089. [Google Scholar] [CrossRef]
- Shamshirsaz, A.A.; Hessami, K.; Morain, S.; Afshar, Y.; Nassr, A.A.; Arian, S.E.; Asl, N.M.; Aagaard, K. Intention to Receive COVID-19 Vaccine during Pregnancy: A Systematic Review and Meta-analysis. Am. J. Perinatol. 2021, 39, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, J. COVID-19 mRNA Vaccine Booster During Pregnancy Increases Maternal and Fetal Antibodies. JAMA 2022, 328, 120. [Google Scholar] [CrossRef] [PubMed]
- Stafford, I.A.; Parchem, J.G.; Sibai, B.M. The coronavirus disease 2019 vaccine in pregnancy: Risks, benefits, and recommendations. Am. J. Obstet. Gynecol. 2021, 224, 484–495. [Google Scholar] [CrossRef]
- Kalafat, E.; Heath, P.; Prasad, S.; O Brien, P.; Khalil, A. COVID-19 vaccination in pregnancy. Am. J. Obstet. Gynecol. 2022, 227, 136–147. [Google Scholar] [CrossRef]
- Eid, J.; Abdelwahab, M.; Williams, H.; Caplan, M.; Hajmurad, S.; Venkatesh, K.K.; Costantine, M.M.; Rood, K.M. Decreased severity of COVID-19 in vaccinated pregnant individuals during predominance of different SARS-CoV-2 variants. Am. J. Reprod. Immunol. 2022, 88, e13596. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, E.H.; SoRelle, J.A.; McIntire, D.D.; Spong, C.Y. Increasing severity of COVID-19 in pregnancy with Delta (B.1.617.2) variant surge. Am. J. Obstet. Gynecol. 2021, 226, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Seasely, A.R.; Blanchard, C.T.; Arora, N.; Battarbee, A.N.; Casey, B.M.; Dionne-Odom, J.; Sixto, M., Jr.; Moates, D.B.; Sinkey, R.G.; Szychowski, J.M.; et al. Maternal and Perinatal Outcomes Associated with the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Delta (B.1.617.2) Variant. Obstet. Gynecol. 2021, 138, 842–844. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.M.; Berry, M.; Moutos, C.P.; Omere, C.; Clark, S.M.; Harirah, H.M.; Jain, S.; Olson, G.L.; Pacheco, L.D.; Saade, G.R.; et al. Association of the Delta (B.1.617.2) Variant of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) with Pregnancy Outcomes. Obstet. Gynecol. 2021, 138, 838–841. [Google Scholar] [CrossRef]
- Morgan, J.A.; Biggio, J.R., Jr.; Martin, J.K.; Mussarat, N.; Chawla, H.K.; Puri, P.; Williams, F.B. Maternal Outcomes After Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection in Vaccinated Compared with Unvaccinated Pregnant Patients. Obstet. Gynecol. 2022, 139, 107–109. [Google Scholar] [CrossRef]
- Adhikari, E.H.; MacDonald, L.; SoRelle, J.A.; Morse, J.; Pruszynski, J.; Spong, C.Y. COVID-19 Cases and Disease Severity in Pregnancy and Neonatal Positivity Associated with Delta (B.1.617.2) and Omicron (B.1.1.529) Variant Predominance. JAMA 2022, 327, 1500. [Google Scholar] [CrossRef]
- Ilter, P.B.; Prasad, S.; Berkkan, M.; Mutlu, M.A.; Tekin, A.B.; Celik, E.; Ata, B.; Turgal, M.; Yildiz, S.; Turkgeldi, E.; et al. Clinical severity of SARS-CoV-2 infection among vaccinated and unvaccinated pregnancies during the Omicron wave. Ultrasound Obstet. Gynecol. 2022, 59, 560–562. [Google Scholar] [CrossRef] [PubMed]


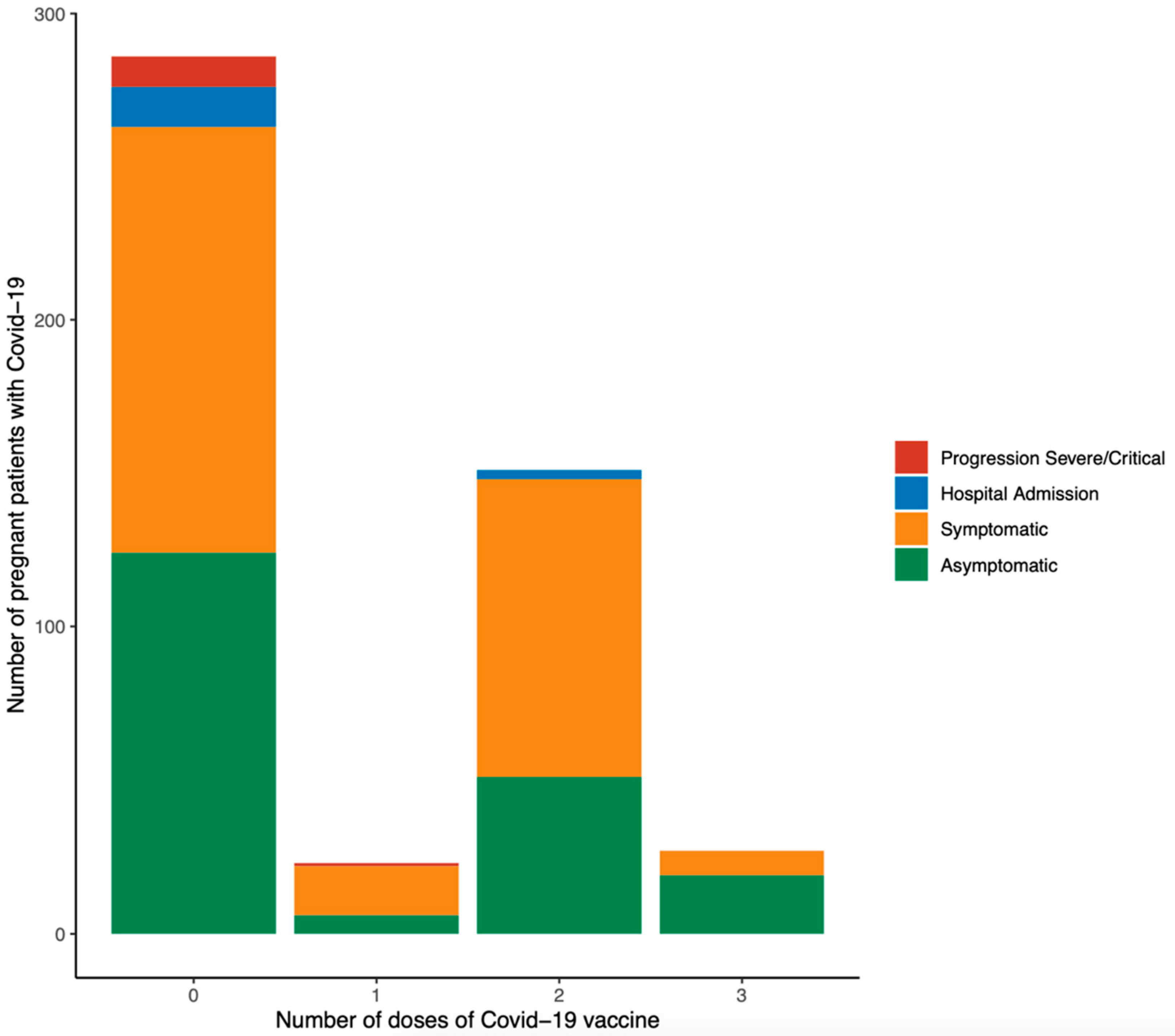
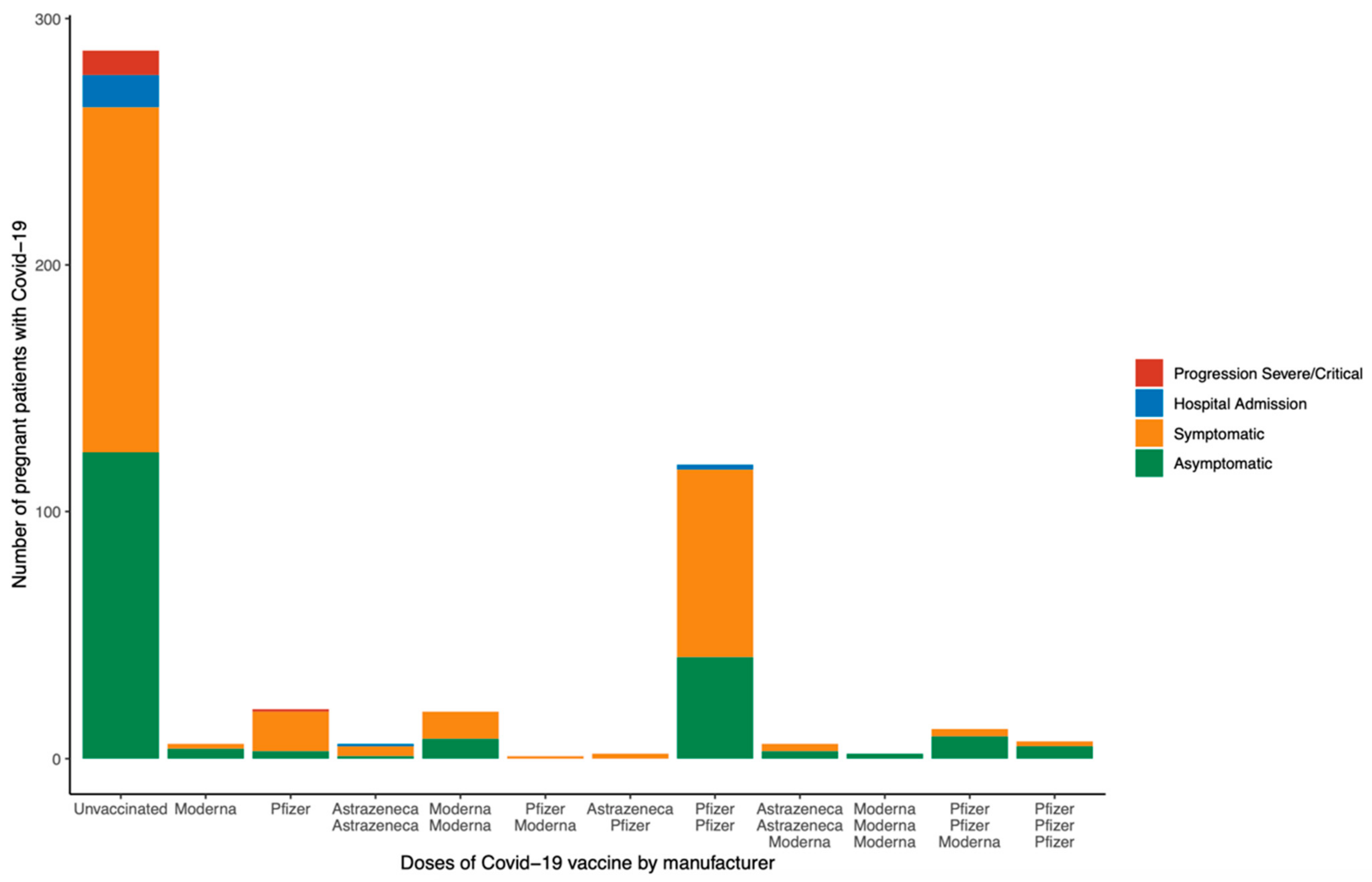

| Total Sample (n = 487) | Symptomatic (n = 287) | Asymptomatic (n = 200) | p | |
|---|---|---|---|---|
| Maternal age (years) | 32.0 ± 5.5 | 32.0 ± 5.7 | 31.9 ± 5.3 | 0.96 |
| Pre-existing chronic disease * | 93/486 (19.1 (15.7–22.9)) | 58/286 (20.3 (15.8–25.4)) | 35/200 (17.5 (12.5–23.5)) | 0.483 |
| Smoking habit | 32/485 (6.6 (4.6–9.2)) | 21/286 (7.3 (4.6–11.0)) | 11/199 (5.5 (2.8–9.7)) | 0.463 |
| Weight (kg) | 66.4 ± 14.1 | 65.7 ± 14.2 | 67.4 ± 14.0 | 0.213 |
| Obesity ** | 47/338 (13.9 (10.4–18.1)) | 23/200 (11.5 (7.4–16.8)) | 24/138 (17.4 (11.5–24.8)) | 0.15 |
| Nulliparity | 240/483 (49.7 (45.1–54.2)) | 147/283 (51.9 (46.0–57.9)) | 93/200 (46.5 (39.4–53.7)) | 0.268 |
| Twin pregnancy | 16/487 (3.3 (1.9–5.3)) | 9/287 (3.1 (1.4–5.9)) | 7/200 (3.5 (1.4–7.1)) | 0.803 |
| Assisted reproductive techniques | 36/487 (7.4 (5.2–10.1)) | 28/287 (9.8 (6.6–13.8)) | 8/200 (4.0 (1.7–7.7)) | 0.021 |
| Artificial insemination | 2/487 (0.4 (0.0–1.5)) | 0/287 (0.0 (0.0–1.3)) | 2/200 (1.0 (0.1–3.6)) | 0.168 |
| In vitro fertilization | 34/487 (7.0 (4.9–9.6)) | 28/287 (9.8 (6.6–13.8)) | 6/200 (3.0 (1.1–6.4)) | 0.004 |
| Egg donation | 4/487 (0.8 (0.2–2.1)) | 4/287 (1.4 (0.4–3.5)) | 0/200 (0.0 (0.0–1.8)) | 0.148 |
| SARS-CoV-2 infection during 1st trimester | 59/487 (12.1 (9.4–15.3)) | 34/287 (11.8 (8.3–16.2)) | 25/200 (12.5 (8.3–17.9)) | 0.888 |
| SARS-CoV-2 infection during 2nd trimester | 149/487 (30.6 (26.5–34.9)) | 109/287 (38.0 (32.3–43.9)) | 40/200 (20.0 (14.7–26.2)) | <0.001 |
| SARS-CoV-2 infection during 3rd trimester | 279/487 (57.3 (52.8–61.7)) | 144/287 (50.2 (44.2–56.1)) | 135/200 (67.5 (60.5–73.9)) | <0.001 |
| Total Sample (n = 487) | Symptomatic (n = 287) | Asymptomatic (n = 200) | p | |
|---|---|---|---|---|
| Maternal outcome | ||||
| Pneumonia | 28/487 (5.7 (3.9–8.2)) | 28/287 (9.8 (6.6–13.8)) | 0/200 (0.0 (0.0–1.8)) | <0.001 |
| Hospital admission due to COVID-19 | 27/487 (5.5 (3.7–8.0)) | 27/287 (9.4 (6.3–13.4)) | 0/200 (0.0 (0.0–1.8)) | <0.001 |
| Admission into the intensive care unit | 11/27 (40.7 (22.4–61.2)) | 11/27 (40.7 (22.4–61.2)) | 0/0 (0.0 (0.0–0.0)) | 1.0 |
| Composite adverse maternal outcome * | 11/487 (2.3 (1.1–4.0)) | 11/287 (3.8 (1.9–6.8)) | 0/200 (0.0 (0.0–1.8)) | 0.004 |
| Antibiotic treatment | 13/487 (2.7 (1.4–4.5)) | 13/287 (4.5 (2.4–7.6)) | 0/200 (0.0 (0.0–1.8)) | 0.001 |
| Corticosteroid drug | 14/487 (2.9 (1.6–4.8)) | 14/287 (4.9 (2.7–8.0)) | 0/200 (0.0 (0.0–1.8)) | <0.001 |
| Antiviral drug | 10/487 (2.1 (1.0–3.7)) | 10/287 (3.5 (1.7–6.3)) | 0/200 (0.0 (0.0–1.8)) | 0.007 |
| Oxygen therapy | 16/487 (3.3 (1.9–5.3)) | 16/287 (5.6 (3.2–8.9)) | 0/200 (0.0 (0.0–1.8)) | <0.001 |
| Intubation | 7/487 (1.4 (0.6–2.9)) | 7/287 (2.4 (1.0–5.0)) | 0/200 (0.0 (0.0–1.8)) | 0.045 |
| Extracorporeal membrane oxygenation | 1/487 (0.2 (0.0–1.1)) | 1/287 (0.3 (0.0–1.9)) | 0/200 (0.0 (0.0–1.8)) | 1.0 |
| Perinatal outcome | ||||
| Ectopic pregnancy | 1/487 (0.2 (0.0–1.1)) | 0/287 (0.0 (0.0–1.3)) | 1/200 (0.5 (0.0–2.8)) | 0.411 |
| Miscarriage | 9/487 (1.8 (0.8–3.5)) | 4/287 (1.4 (0.4–3.5)) | 5/200 (2.5 (0.8–5.7)) | 0.498 |
| Stillbirth | 2/487 (0.4 (0.0–1.5)) | 1/287 (0.3 (0.0–1.9)) | 1/200 (0.5 (0.0–2.8)) | 1.0 |
| Completed pregnancies (excluding ectopic pregnancy and miscarriage) | ||||
| Threatened preterm labor | 5/477 (1.0 (0.3–2.4)) | 3/283 (1.1 (0.2–3.1)) | 2/194 (1.0 (0.1–3.7)) | 1.0 |
| Small for gestational age | 4/477 (0.8 (0.2–2.1)) | 1/283 (0.4 (0.0–2.0)) | 3/194 (1.5 (0.3–4.5)) | 0.309 |
| Fetal growth restriction | 16/477 (3.4 (1.9–5.4)) | 10/283 (3.5 (1.7–6.4)) | 6/194 (3.1 (1.1–6.6)) | 1.0 |
| Gestational diabetes mellitus | 30/477 (6.3 (4.3–8.9)) | 21/283 (7.4 (4.7–11.1)) | 9/194 (4.6 (2.1–8.6)) | 0.253 |
| Gestational hypertension | 6/477 (1.3 (0.5–2.7)) | 1/283 (0.4 (0.0–2.0)) | 5/194 (2.6 (0.8–5.9)) | 0.043 |
| Preeclampsia | 4/477 (0.8 (0.2–2.1)) | 2/283 (0.7 (0.1–2.5)) | 2/194 (1.0 (0.1–3.7)) | 1.0 |
| Preterm premature rupture of membranes | 3/477 (0.6 (0.1–1.8)) | 1/283 (0.4 (0.0–2.0)) | 2/194 (1.0 (0.1–3.7)) | 0.569 |
| Gestational age at delivery (weeks) | 39.2 ± 1.7 | 39.2 ± 1.8 | 39.1 ± 1.6 | 0.168 |
| Cesarean section | 121/477 (25.4 (21.5–29.5)) | 74/283 (26.1 (21.1–31.7)) | 47/194 (24.2 (18.4–30.9)) | 0.669 |
| Preterm birth | 38/475 (8.0 (5.7–10.8)) | 20/282 (7.1 (4.4–10.7)) | 18/193 (9.3 (5.6–14.3)) | 0.393 |
| Singleton pregnancy | ||||
| Gestational age at delivery (weeks) | 39.3 ± 1.7 | 39.3 ± 1.8 | 39.2 ± 1.5 | 0.187 |
| Preterm birth | 29/459 (6.3 (4.3–8.9)) | 16/273 (5.9 (3.4–9.3)) | 13/186 (7.0 (3.8–11.7)) | 0.697 |
| Birth weight (g) | 3254.2 ± 480.6 | 3263.3 ± 500.4 | 3240.9 ± 451.0 | 0.483 |
| 1 min Apgar | 9.2 ± 1.1 | 9.2 ± 1.0 | 9.2 ± 1.2 | 0.717 |
| 5 min Apgar | 9.9 ± 0.5 | 9.9 ± 0.5 | 9.9 ± 0.4 | 0.602 |
| 5 min Apgar < 7 | 2/458 (0.4 (0.1–1.6)) | 2/273 (0.7 (0.1–2.6)) | 0/185 (0.0 (0.0–2.0)) | 0.517 |
| Umbilical artery pH | 7.2 ± 0.1 | 7.2 ± 0.1 | 7.3 ± 0.1 | 0.202 |
| Umbilical artery pH < 7.1 | 6/447 (1.3 (0.5–2.9)) | 3/269 (1.1 (0.2–3.2)) | 3/178 (1.7 (0.3–4.8)) | 0.686 |
| Umbilical venous pH | 7.3 ± 0.1 | 7.3 ± 0.1 | 7.3 ± 0.1 | 0.254 |
| Neonatal morbidity ** | 43/459 (9.4 (6.9–12.4)) | 28/273 (10.3 (6.9–14.5)) | 15/186 (8.1 (4.6–13.0)) | 0.515 |
| Neonatal death | 1/459 (0.2 (0.0–1.2)) | 1/273 (0.4 (0.0–2.0)) | 0/186 (0.0 (0.0–2.0)) | 1.0 |
| Positive RT-PCR SARS-CoV-2 | 2/122 (1.6 (0.2–5.8)) | 0/48 (0.0 (0.0–7.4)) | 2/74 (2.7 (0.3–9.4)) | 0.519 |
| Positive cord-blood SARS-CoV-2 immunoglobulin G antibodies | 52/84 (61.9 (50.7–72.3)) | 30/41 (73.2 (57.1–85.8)) | 22/43 (51.2 (35.5–66.7)) | 0.045 |
| Twin pregnancy | ||||
| Gestational age at delivery (weeks) | 36.4 ± 0.9 | 36.7 ± 0.9 | 36.0 ± 0.9 | 0.135 |
| Preterm birth | 9/16 (56.3 (29.9–80.2)) | 4/9 (44.4 (13.7–78.8)) | 5/7 (71.4 (29.0–96.3)) | 0.358 |
| Birth weight (g) of the first twin | 2408.6 ± 363.0 | 2441.7 ± 364.2 | 2366.0 ± 385.8 | 0.874 |
| Birth weight (g) of the second twin | 2402.5 ± 332.8 | 2389.4 ± 374.6 | 2419.3 ± 298.8 | 0.634 |
| 1 min Apgar of the first twin | 9.4 ± 0.9 | 9.6 ± 0.7 | 9.1 ± 1.1 | 0.373 |
| 1 min Apgar of the second twin | 8.9 ± 1.2 | 8.9 ± 1.3 | 8.9 ± 1.2 | 0.912 |
| 5 min Apgar of the first twin | 9.7 ± 0.8 | 9.8 ± 0.4 | 9.6 ± 1.1 | 0.816 |
| 5 min Apgar of the second twin | 9.8 ± 0.4 | 9.9 ± 0.3 | 9.7 ± 0.5 | 0.39 |
| 5 min Apgar < 7 of the first twin | 0/16 (0.0 (0.0–20.6)) | 0/9 (0.0 (0.0–33.6)) | 0/7 (0.0 (0.0–41.0)) | 1.0 |
| 5 min Apgar < 7 of the second twin | 0/16 (0.0 (0.0–20.6)) | 0/9 (0.0 (0.0–33.6)) | 0/7 (0.0 (0.0–41.0)) | 1.0 |
| Umbilical artery pH of the first twin | 7.3 ± 0.1 | 7.3 ± 0.0 | 7.2 ± 0.0 | 0.002 |
| Umbilical artery pH of the second twin | 7.3 ± 0.1 | 7.3 ± 0.0 | 7.2 ± 0.1 | 0.345 |
| Umbilical artery pH < 7.1 of the first twin | 0/15 (0.0 (0.0–21.8)) | 0/9 (0.0 (0.0–33.6)) | 0/6 (0.0 (0.0–45.9)) | 1.0 |
| Umbilical artery pH < 7.1 of the second twin | 0/15 (0.0 (0.0–21.8)) | 0/9 (0.0 (0.0–33.6)) | 0/6 (0.0 (0.0–45.9)) | 1.0 |
| Umbilical venous pH of the first twin | 7.3 ± 0.1 | 7.3 ± 0.0 | 7.3 ± 0.1 | 0.022 |
| Umbilical venous pH of the second twin | 7.3 ± 0.1 | 7.3 ± 0.0 | 7.3 ± 0.1 | 0.123 |
| Neonatal morbidity ** of the first twin | 2/16 (12.5 (1.6–38.3)) | 0/9 (0.0 (0.0–33.6)) | 2/7 (28.6 (3.7–71.0)) | 0.175 |
| Neonatal morbidity ** of the second twin | 2/16 (12.5 (1.6–38.3)) | 1/9 (11.1 (0.3–48.2)) | 1/7 (14.3 (0.4–57.9)) | 1.0 |
| Neonatal death | 0/16 (0.0 (0.0–20.6)) | 0/9 (0.0 (0.0–33.6)) | 0/7 (0.0 (0.0–41.0)) | 1.0 |
| Positive RT-PCR SARS-CoV-2, first twin | 1/6 (16.7 (0.4–64.1)) | 0/2 (0.0 (0.0–84.2)) | 1/4 (25.0 (0.6–80.6)) | 1.0 |
| Positive RT-PCR SARS-CoV-2, second twin | 1/3 (33.3 (0.8–90.6)) | 0/0 (0.0 (0.0–0.0)) | 1/3 (33.3 (0.8–90.6)) | 1.0 |
| Positive cord-blood SARS-CoV-2 immunoglobulin G antibodies, first twin | 1/5 (20.0 (0.5–71.6)) | 0/2 (0.0 (0.0–84.2)) | 1/3 (33.3 (0.8–90.6)) | 1.0 |
| Positive cord-blood SARS-CoV-2 immunoglobulin G antibodies, second twin | 2/3 (66.7 (9.4–99.2)) | 1/1 (100.0 (2.5–100.0)) | 1/2 (50.0 (1.3–98.7)) | 1.0 |
| Variable | OR (95% CI) | aOR (95% CI) |
|---|---|---|
| Preterm birth | 0.742 (0.382–1.443) | 0.745 (0.313–1.776) |
| Fetal growth restriction | 1.148 (0.410–3.212) | 0.904 (0.245–3.340) |
| Preeclampsia | 0.683 (0.095–4.892) | 1.204 (0.103–14.074) |
| Stillbirth | 0.696 (0.043–11.190) | 0.925 (0.054–15.962) |
| Cesarean section | 1.107 (0.726–1.689) | 1.060 (0.630–1.782) |
| Umbilical artery pH < 7.1 | 1.519 (0.303–7.611) | 1.217 (0.158–9.357) |
| Neonatal morbidity * | 1.141 (0.606–2.148) | 0.914 (0.447–1.868) |
| Total Sample (n = 487) | Vaccinated (n = 201) | Not Vaccinated (n = 286) | p | |
|---|---|---|---|---|
| Maternal age (years) | 32.0 ± 5.5 | 33.0 ± 4.9 | 31.2 ± 5.8 | <0.001 |
| Pre-existing chronic disease * | 93/486 (19.1 (15.7–22.9)) | 44/201 (21.9 (16.4–28.3)) | 49/285 (17.2 (13.0–22.1)) | 0.2 |
| Smoking habit | 32/485 (6.6 (4.6–9.2)) | 12/199 (6.0 (3.2–10.3)) | 20/286 (7.0 (4.3–10.6)) | 0.714 |
| Weight (kg) | 66.4 ± 14.1 | 65.3 ± 13.6 | 67.4 ± 14.5 | 0.118 |
| Obesity ** | 47/338 (13.9 (10.4–18.1)) | 19/159 (11.9 (7.4–18.0)) | 28/179 (15.6 (10.7–21.8)) | 0.349 |
| Nulliparity | 240/483 (49.7 (45.1–54.2)) | 105/198 (53.0 (45.8–60.1)) | 135/285 (47.4 (41.5–53.3)) | 0.23 |
| Twin pregnancy | 16/487 (3.3 (1.9–5.3)) | 5/201 (2.5 (0.8–5.7)) | 11/286 (3.8 (1.9–6.8)) | 0.452 |
| Assisted reproductive techniques | 36/487 (7.4 (5.2–10.1)) | 24/201 (11.9 (7.8–17.2)) | 12/286 (4.2 (2.2–7.2)) | 0.002 |
| Artificial insemination | 2/487 (0.4 (0.0–1.5)) | 2/201 (1.0 (0.1–3.5)) | 0/286 (0.0 (0.0–1.3)) | 0.17 |
| In vitro fertilization | 34/487 (7.0 (4.9–9.6)) | 22/201 (10.9 (7.0–16.1)) | 12/286 (4.2 (2.2–7.2)) | 0.006 |
| Egg donation | 4/487 (0.8 (0.2–2.1)) | 3/201 (1.5 (0.3–4.3)) | 1/286 (0.3 (0.0–1.9)) | 0.311 |
| SARS-CoV-2 infection during 1st trimester | 59/487 (12.1 (9.4–15.3)) | 11/201 (5.5 (2.8–9.6)) | 48/286 (16.8 (12.6–21.6)) | <0.001 |
| SARS-CoV-2 infection during 2nd trimester | 149/487 (30.6 (26.5–34.9)) | 74/201 (36.8 (30.1–43.9)) | 75/286 (26.2 (21.2–31.7)) | 0.016 |
| SARS-CoV-2 infection during 3rd trimester | 279/487 (57.3 (52.8–61.7)) | 116/201 (57.7 (50.6–64.6)) | 163/286 (57.0 (51.0–62.8)) | 0.926 |
| Symptomatic SARS-CoV-2 infection | 287/487 (58.9 (54.4–63.3)) | 125/201 (62.2 (55.1–68.9)) | 162/286 (56.6 (50.7–62.5)) | 0.226 |
| Total Sample (n = 487) | Vaccinated (n = 201) | Not Vaccinated (n = 286) | p | |
|---|---|---|---|---|
| Maternal outcome | ||||
| Pneumonia | 28/487 (5.7 (3.9–8.2)) | 4/201 (2.0 (0.5–5.0)) | 24/286 (8.4 (5.5–12.2)) | 0.003 |
| Hospital admission due to SARS-CoV-2 infection | 27/487 (5.5 (3.7–8.0)) | 4/201 (2.0 (0.5–5.0)) | 23/286 (8.0 (5.2–11.8)) | 0.004 |
| Admission into the intensive care unit | 11/27 (40.7 (22.4–61.2)) | 1/4 (25.0 (0.6–80.6)) | 10/23 (43.5 (23.2–65.5)) | 0.624 |
| Composite adverse maternal outcome * | 11/487 (2.3 (1.1–4.0)) | 1/201 (0.5 (0.0–2.7)) | 10/286 (3.5 (1.7–6.3)) | 0.031 |
| Antibiotic treatment | 13/487 (2.7 (1.4–4.5)) | 1/201 (0.5 (0.0–2.7)) | 12/286 (4.2 (2.2–7.2)) | 0.019 |
| Corticosteroid drug | 14/487 (2.9 (1.6–4.8)) | 1/201 (0.5 (0.0–2.7)) | 13/286 (4.5 (2.4–7.6)) | 0.01 |
| Antiviral drug | 10/487 (2.1 (1.0–3.7)) | 1/201 (0.5 (0.0–2.7)) | 9/286 (3.1 (1.4–5.9)) | 0.052 |
| Oxygen therapy | 16/487 (3.3 (1.9–5.3)) | 1/201 (0.5 (0.0–2.7)) | 15/286 (5.2 (3.0–8.5)) | 0.003 |
| Intubation | 7/487 (1.4 (0.6–2.9)) | 1/201 (0.5 (0.0–2.7)) | 6/286 (2.1 (0.8–4.5)) | 0.248 |
| Extracorporeal membrane oxygenation | 1/487 (0.2 (0.0–1.1)) | 0/201 (0.0 (0.0–1.8)) | 1/286 (0.3 (0.0–1.9)) | 1.0 |
| Perinatal outcome | ||||
| Ectopic pregnancy | 1/487 (0.2 (0.0–1.1)) | 0/201 (0.0 (0.0–1.8)) | 1/286 (0.3 (0.0–1.9)) | 1.0 |
| Miscarriage | 9/487 (1.8 (0.8–3.5)) | 3/201 (1.5 (0.3–4.3)) | 6/286 (2.1 (0.8–4.5)) | 0.742 |
| Stillbirth | 2/487 (0.4 (0.0–1.5)) | 2/201 (1.0 (0.1–3.5)) | 0/286 (0.0 (0.0–1.3)) | 0.17 |
| Completed pregnancies (excluding ectopic pregnancy and miscarriage) | ||||
| Threatened preterm labor | 5/477 (1.0 (0.3–2.4)) | 2/198 (1.0 (0.1–3.6)) | 3/279 (1.1 (0.2–3.1)) | 1.0 |
| Small for gestational age | 4/477 (0.8 (0.2–2.1)) | 4/198 (2.0 (0.6–5.1)) | 0/279 (0.0 (0.0–1.3)) | 0.029 |
| Fetal growth restriction | 16/477 (3.4 (1.9–5.4)) | 6/198 (3.0 (1.1–6.5)) | 10/279 (3.6 (1.7–6.5)) | 0.802 |
| Gestational diabetes mellitus | 30/477 (6.3 (4.3–8.9)) | 11/198 (5.6 (2.8–9.7)) | 19/279 (6.8 (4.1–10.4)) | 0.703 |
| Gestational hypertension | 6/477 (1.3 (0.5–2.7)) | 1/198 (0.5 (0.0–2.8)) | 5/279 (1.8 (0.6–4.1)) | 0.408 |
| Preeclampsia | 4/477 (0.8 (0.2–2.1)) | 1/198 (0.5 (0.0–2.8)) | 3/279 (1.1 (0.2–3.1)) | 0.645 |
| Preterm premature rupture of membranes | 3/477 (0.6 (0.1–1.8)) | 2/198 (1.0 (0.1–3.6)) | 1/279 (0.4 (0.0–2.0)) | 0.573 |
| Gestational age at delivery (weeks) | 39.2 ± 1.7 | 39.2 ± 1.6 | 39.2 ± 1.8 | 0.507 |
| Cesarean section | 121/477 (25.4 (21.5–29.5)) | 59/198 (29.8 (23.5–36.7)) | 62/279 (22.2 (17.5–27.6)) | 0.07 |
| Preterm birth | 38/475 (8.0 (5.7–10.8)) | 13/196 (6.6 (3.6–11.1)) | 25/279 (9.0 (5.9–12.9)) | 0.395 |
| Singleton pregnancy | ||||
| Gestational age at delivery (weeks) | 39.3 ± 1.7 | 39.3 ± 1.6 | 39.3 ± 1.8 | 0.343 |
| Preterm birth | 29/459 (6.3 (4.3–8.9)) | 11/191 (5.8 (2.9–10.1)) | 18/268 (6.7 (4.0–10.4)) | 0.846 |
| Birth weight (g) | 3254.2 ± 480.6 | 3236.3 ± 424.2 | 3267.0 ± 517.5 | 0.308 |
| 1 min Apgar | 9.2 ± 1.1 | 9.2 ± 0.9 | 9.2 ± 1.2 | 0.349 |
| 5 min Apgar | 9.9 ± 0.5 | 9.9 ± 0.5 | 9.9 ± 0.5 | 0.04 |
| 5 min Apgar < 7 | 2/458 (0.4 (0.1–1.6)) | 1/190 (0.5 (0.0–2.9)) | 1/268 (0.4 (0.0–2.1)) | 1.0 |
| Umbilical artery pH | 7.2 ± 0.1 | 7.2 ± 0.1 | 7.2 ± 0.1 | 0.976 |
| Umbilical artery pH < 7.1 | 6/447 (1.3 (0.5–2.9)) | 3/186 (1.6 (0.3–4.6)) | 3/261 (1.1 (0.2–3.3)) | 0.697 |
| Umbilical venous pH | 7.3 ± 0.1 | 7.3 ± 0.1 | 7.3 ± 0.1 | 0.23 |
| Neonatal morbidity ** | 43/459 (9.4 (6.9–12.4)) | 25/191 (13.1 (8.7–18.7)) | 18/268 (6.7 (4.0–10.4)) | 0.023 |
| Neonatal death | 1/459 (0.2 (0.0–1.2)) | 0/191 (0.0 (0.0–1.9)) | 1/268 (0.4 (0.0–2.1)) | 1.0 |
| Positive RT-PCR SARS-CoV-2 | 2/122 (1.6 (0.2–5.8)) | 0/28 (0.0 (0.0–12.3)) | 2/94 (2.1 (0.3–7.5)) | 1.0 |
| Positive cord-blood SARS-CoV-2 immunoglobulin G antibodies | 52/84 (61.9 (50.7–72.3)) | 1/1 (100.0 (2.5–100.0)) | 51/83 (61.4 (50.1–71.9)) | 1.0 |
| Twin pregnancy | ||||
| Gestational age at delivery (weeks) | 36.4 ± 0.9 | 36.6 ± 0.6 | 36.3 ± 1.0 | 0.278 |
| Preterm birth | 9/16 (56.3 (29.9–80.2)) | 2/5 (40.0 (5.3–85.3)) | 7/11 (63.6 (30.8–89.1)) | 0.596 |
| Birth weight (g) of the first twin | 2408.6 ± 363.0 | 2398.0 ± 352.8 | 2413.4 ± 384.4 | 0.777 |
| Birth weight (g) of the second twin | 2402.5 ± 332.8 | 2339.0 ± 309.3 | 2431.4 ± 353.5 | 0.61 |
| 1 min Apgar of the first twin | 9.4 ± 0.9 | 9.4 ± 0.9 | 9.4 ± 0.9 | 0.949 |
| 1 min Apgar of the second twin | 8.9 ± 1.2 | 7.8 ± 1.3 | 9.4 ± 0.8 | 0.021 |
| 5 min Apgar of the first twin | 9.7 ± 0.8 | 9.6 ± 0.5 | 9.7 ± 0.9 | 0.212 |
| 5 min Apgar of the second twin | 9.8 ± 0.4 | 9.6 ± 0.5 | 9.9 ± 0.3 | 0.155 |
| 5 min Apgar < 7 of the first twin | 0/16 (0.0 (0.0–20.6)) | 0/5 (0.0 (0.0–52.2)) | 0/11 (0.0 (0.0–28.5)) | 1.0 |
| 5 min Apgar < 7 of the second twin | 0/16 (0.0 (0.0–20.6)) | 0/5 (0.0 (0.0–52.2)) | 0/11 (0.0 (0.0–28.5)) | 1.0 |
| Umbilical artery pH of the first twin | 7.3 ± 0.1 | 7.3 ± 0.0 | 7.3 ± 0.1 | 0.387 |
| Umbilical artery pH of the second twin | 7.3 ± 0.1 | 7.3 ± 0.1 | 7.3 ± 0.0 | 0.713 |
| Umbilical artery pH < 7.1 of the first twin | 0/15 (0.0 (0.0–21.8)) | 0/5 (0.0 (0.0–52.2)) | 0/10 (0.0 (0.0–30.8)) | 1.0 |
| Umbilical artery pH < 7.1 of the second twin | 0/15 (0.0 (0.0–21.8)) | 0/5 (0.0 (0.0–52.2)) | 0/10 (0.0 (0.0–30.8)) | 1.0 |
| Umbilical venous pH of the first twin | 7.3 ± 0.1 | 7.3 ± 0.0 | 7.3 ± 0.1 | 0.776 |
| Umbilical venous pH of the second twin | 7.3 ± 0.1 | 7.3 ± 0.1 | 7.3 ± 0.1 | 0.776 |
| Neonatal morbidity ** of the first twin | 2/16 (12.5 (1.6–38.3)) | 1/5 (20.0 (0.5–71.6)) | 1/11 (9.1 (0.2–41.3)) | 1.0 |
| Neonatal morbidity ** of the second twin | 2/16 (12.5 (1.6–38.3)) | 0/5 (0.0 (0.0–52.2)) | 2/11 (18.2 (2.3–51.8)) | 1.0 |
| Neonatal death | 0/16 (0.0 (0.0–20.6)) | 0/5 (0.0 (0.0–52.2)) | 0/11 (0.0 (0.0–28.5)) | 1.0 |
| Positive RT-PCR SARS-CoV-2, first twin | 1/6 (16.7 (0.4–64.1)) | 0/0 (0.0 (0.0–0.0)) | 1/6 (16.7 (0.4–64.1)) | 1.0 |
| Positive RT-PCR SARS-CoV-2, second twin | 1/3 (33.3 (0.8–90.6)) | 0/0 (0.0 (0.0–0.0)) | 1/3 (33.3 (0.8–90.6)) | 1.0 |
| Positive cord-blood SARS-CoV-2 immunoglobulin G antibodies, first twin | 1/5 (20.0 (0.5–71.6)) | 0/0 (0.0 (0.0–0.0)) | 1/5 (20.0 (0.5–71.6)) | 1.0 |
| Positive cord-blood SARS-CoV-2 immunoglobulin G antibodies, second twin | 2/3 (66.7 (9.4–99.2)) | 0/0 (0.0 (0.0–0.0)) | 2/3 (66.7 (9.4–99.2)) | 1.0 |
| Variable | OR (95% CI) | aOR (95% CI) |
|---|---|---|
| Pneumonia | 0.222 (0.076–0.649) | 0.209 (0.044–0.985) |
| Hospital admission due to COVID-19 | 0.232 (0.079–0.682) | 0.209 (0.044–0.985) |
| Preterm birth | 0.722 (0.360–1.449) | 1.024 (0.433–2.424) |
| Fetal growth restriction | 0.841 (0.300–2.352) | 1.047 (0.289–3.787) |
| Preeclampsia | 0.467 (0.048–4.523) | 0.517 (0.045–5.940) |
| Cesarean section | 1.486 (0.981–2.250) | 1.200 (0.723–1.993) |
| Umbilical artery pH < 7.1 | 0.701 (0.140–3.513) | 0.893 (0.120–6.626) |
| Neonatal morbidity * | 2.093 (1.123–3.900) | 1.982 (0.957–4.103) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Varea, A.; Satorres, E.; Florez, S.; Domenech, J.; Desco-Blay, J.; Monfort-Pitarch, S.; Hueso, M.; Perales-Marín, A.; Diago-Almela, V. Comparison of Maternal–Fetal Outcomes among Unvaccinated and Vaccinated Pregnant Women with COVID-19. J. Pers. Med. 2022, 12, 2008. https://doi.org/10.3390/jpm12122008
Martínez-Varea A, Satorres E, Florez S, Domenech J, Desco-Blay J, Monfort-Pitarch S, Hueso M, Perales-Marín A, Diago-Almela V. Comparison of Maternal–Fetal Outcomes among Unvaccinated and Vaccinated Pregnant Women with COVID-19. Journal of Personalized Medicine. 2022; 12(12):2008. https://doi.org/10.3390/jpm12122008
Chicago/Turabian StyleMartínez-Varea, Alicia, Elena Satorres, Sandra Florez, Josep Domenech, Julia Desco-Blay, Sagrario Monfort-Pitarch, María Hueso, Alfredo Perales-Marín, and Vicente Diago-Almela. 2022. "Comparison of Maternal–Fetal Outcomes among Unvaccinated and Vaccinated Pregnant Women with COVID-19" Journal of Personalized Medicine 12, no. 12: 2008. https://doi.org/10.3390/jpm12122008
APA StyleMartínez-Varea, A., Satorres, E., Florez, S., Domenech, J., Desco-Blay, J., Monfort-Pitarch, S., Hueso, M., Perales-Marín, A., & Diago-Almela, V. (2022). Comparison of Maternal–Fetal Outcomes among Unvaccinated and Vaccinated Pregnant Women with COVID-19. Journal of Personalized Medicine, 12(12), 2008. https://doi.org/10.3390/jpm12122008







