Exercise Modulates Brain Glucose Utilization Response to Acute Cocaine
Abstract
:1. Introduction
2. Methods
2.1. Animals
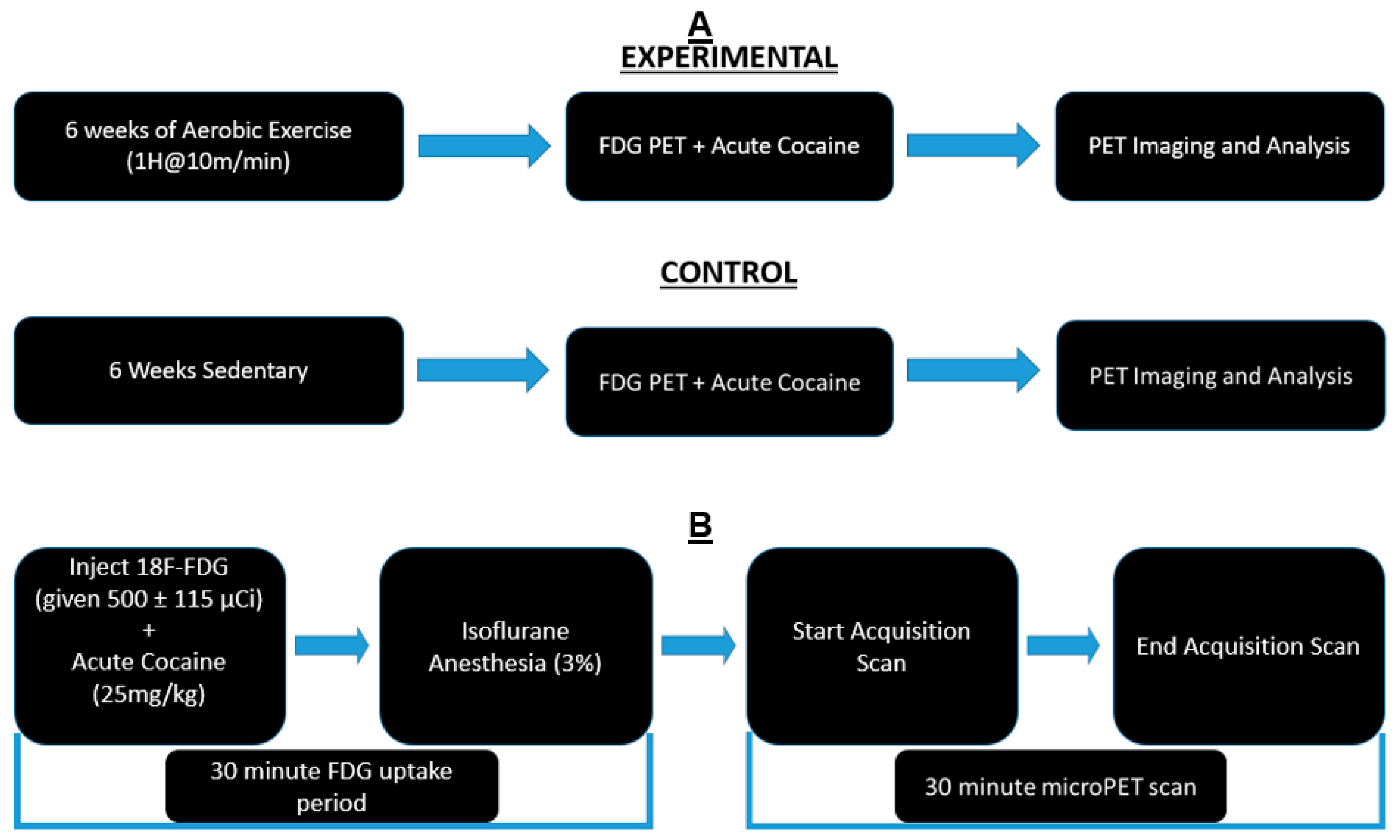
2.2. Exercise Regimen
2.3. PET Imaging and Cocaine Injection
2.4. Imaging and Statistical Analysis
3. Results
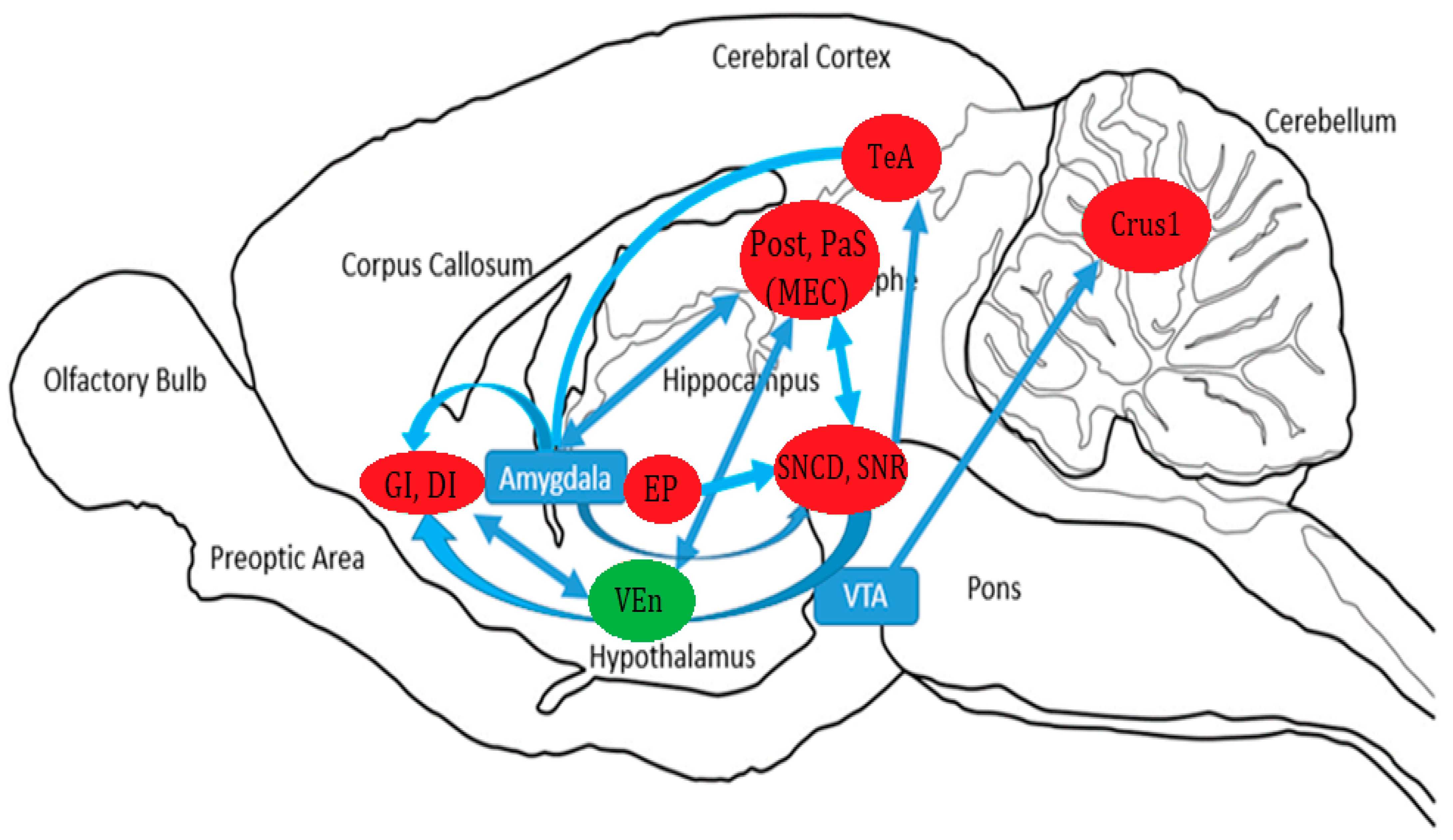
4. Discussion
4.1. Parahippocampal Activation
4.2. Insular Cortex Activation
4.3. Substantia Nigra Activation
4.4. Activation of the Temporal Association Area
4.5. Entopeduncular Nucleus
4.6. Crus 1 of the Ansiform Lobule
4.7. Inhibition of the Ventral Endopiriform Nucleus
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kampman, K.M. The treatment of cocaine use disorder. Sci. Adv. 2019, 5, eaax1532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, A.J.; Rehm, J.; Fischer, B. Health outcomes associated with crack-cocaine use: Systematic review and meta-analyses. Drug Alcohol Depend. 2017, 180, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Warner, E.A. Cocaine abuse. Ann. Intern. Med. 1993, 119, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Sordo, L.; Indave, B.I.; Barrio, G.; Degenhardt, L.; de la Fuente, L.; Bravo, M.J. Cocaine use and risk of stroke: A systematic review. Drug Alcohol Depend. 2014, 142, 1–13. [Google Scholar] [CrossRef]
- Cury, P.R.; Oliveira, M.G.; de Andrade, K.M.; de Freitas, M.D.; Dos Santos, J.N. Dental health status in crack/cocaine-addicted men: A cross-sectional study. Environ. Sci. Pollut. Res. Int. 2017, 24, 7585–7590. [Google Scholar] [CrossRef]
- Kim, S.T.; Park, T. Acute and chronic effects of cocaine on cardiovascular health. Int. J. Mol. Sci. 2019, 20, 584. [Google Scholar] [CrossRef] [Green Version]
- Thanos, P.K.; Stamos, J.; Robison, L.S.; Heyman, G.; Tucci, A.; Wang, G.J.; Robinson, J.K.; Anderson, B.J.; Volkow, N.D. Daily treadmill exercise attenuates cocaine cue-induced reinstatement and cocaine induced locomotor response but increases cocaine-primed reinstatement. Behav. Brain Res. 2013, 239, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Thanos, P.K.; Tucci, A.; Stamos, J.; Robison, L.; Wang, G.J.; Anderson, B.J.; Volkow, N.D. Chronic forced exercise during adolescence decreases cocaine conditioned place preference in lewis rats. Behav. Brain Res. 2010, 215, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Blum, K.; Chen, T.J.; Morse, S.; Giordano, J.; Chen, A.L.; Thompson, J.; Allen, C.; Smolen, A.; Lubar, J.; Stice, E.; et al. Overcoming qeeg abnormalities and reward gene deficits during protracted abstinence in male psychostimulant and polydrug abusers utilizing putative dopamine d2 agonist therapy: Part 2. Postgrad. Med. 2010, 122, 214–226. [Google Scholar] [CrossRef] [Green Version]
- Robison, L.S.; Alessi, L.; Thanos, P.K. Chronic forced exercise inhibits stress-induced reinstatement of cocaine conditioned place preference. Behav. Brain Res. 2018, 353, 176–184. [Google Scholar] [CrossRef]
- Robison, L.S.; Swenson, S.; Hamilton, J.; Thanos, P.K. Exercise reduces dopamine d1r and increases d2r in rats: Implications for addiction. Med. Sci. Sport. Exerc. 2018, 50, 1596–1602. [Google Scholar] [CrossRef] [PubMed]
- Hanna, C.; Hamilton, J.; Arnavut, E.; Blum, K.; Thanos, P.K. Brain mapping the effects of chronic aerobic exercise in the rat brain using fdg pet. J. Pers. Med. 2022, 12, 860. [Google Scholar] [CrossRef] [PubMed]
- Paolucci, E.M.; Loukov, D.; Bowdish, D.M.E.; Heisz, J.J. Exercise reduces depression and inflammation but intensity matters. Biol. Psychol. 2018, 133, 79–84. [Google Scholar] [CrossRef]
- Boecker, H.; Drzezga, A. A perspective on the future role of brain pet imaging in exercise science. NeuroImage 2016, 131, 73–80. [Google Scholar] [CrossRef]
- Wallace, E.A.; Wisniewski, G.; Zubal, G.; vanDyck, C.H.; Pfau, S.E.; Smith, E.O.; Rosen, M.I.; Sullivan, M.C.; Woods, S.W.; Kosten, T.R. Acute cocaine effects on absolute cerebral blood flow. Psychopharmacology 1996, 128, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Porrino, L.J.; Hampson, R.E.; Opris, I.; Deadwyler, S.A. Acute cocaine induced deficits in cognitive performance in rhesus macaque monkeys treated with baclofen. Psychopharmacology 2013, 225, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Nicolucci, C.; Pais, M.L.; Santos, A.C.; Ribeiro, F.M.; Encarnação, P.; Silva, A.L.M.; Castro, I.F.; Correia, P.M.M.; Veloso, J.; Reis, J.; et al. Single low dose of cocaine-structural brain injury without metabolic and behavioral changes. Front. Neurosci. 2020, 14, 589897. [Google Scholar] [CrossRef]
- Volkow, N.D.; Fowler, J.S.; Wang, G.J.; Hitzemann, R.; Logan, J.; Schlyer, D.J.; Dewey, S.L.; Wolf, A.P. Decreased dopamine d2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synap. (New York N.Y.) 1993, 14, 169–177. [Google Scholar] [CrossRef]
- Volkow, N.D.; Hitzemann, R.; Wang, G.J.; Fowler, J.S.; Wolf, A.P.; Dewey, S.L.; Handlesman, L. Long-term frontal brain metabolic changes in cocaine abusers. Synap. (New York N.Y.) 1992, 11, 184–190. [Google Scholar] [CrossRef]
- Cannella, N.; Cosa-Linan, A.; Roscher, M.; Takahashi, T.T.; Vogler, N.; Wängler, B.; Spanagel, R. [18f]-fluorodeoxyglucose-positron emission tomography in rats with prolonged cocaine self-administration suggests potential brain biomarkers for addictive behavior. Front. Psychiatry 2017, 8, 218. [Google Scholar] [CrossRef]
- Noble, E.P.; Blum, K.; Khalsa, M.E.; Ritchie, T.; Montgomery, A.; Wood, R.C.; Fitch, R.J.; Ozkaragoz, T.; Sheridan, P.J.; Anglin, M.D.; et al. Allelic association of the d2 dopamine receptor gene with cocaine dependence. Drug Alcohol Depend. 1993, 33, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Thanos, P.K.; Michaelides, M.; Benveniste, H.; Wang, G.J.; Volkow, N.D. The effects of cocaine on regional brain glucose metabolism is attenuated in dopamine transporter knockout mice. Synap. (New York N.Y.) 2008, 62, 319–324. [Google Scholar] [CrossRef] [Green Version]
- Noble, E.P.; Gottschalk, L.A.; Fallon, J.H.; Ritchie, T.L.; Wu, J.C. D2 dopamine receptor polymorphism and brain regional glucose metabolism. Am. J. Med. Genet. 1997, 74, 162–166. [Google Scholar] [CrossRef]
- McGregor, M.; Richer, K.; Ananth, M.; Thanos, P.K. The functional networks of a novel environment: Neural activity mapping in awake unrestrained rats using positron emission tomography. Brain Behav. 2020, 10, e01646. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Blum, K.; Gondré-Lewis, M.C.; Baron, D.; Thanos, P.K.; Braverman, E.R.; Neary, J.; Elman, I.; Badgaiyan, R.D. Introducing precision addiction management of reward deficiency syndrome, the construct that underpins all addictive behaviors. Front. Psychiatry 2018, 9, 548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sammons, R.P.; Parthier, D.; Stumpf, A.; Schmitz, D. Electrophysiological and molecular characterization of the parasubiculum. J. Neurosci. Off. J. Soc. Neurosci. 2019, 39, 8860–8876. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Burgalossi, A.; Ebbesen, C.L.; Sanguinetti-Scheck, J.I.; Schmidt, H.; Tukker, J.J.; Naumann, R.; Ray, S.; Preston-Ferrer, P.; Schmitz, D.; et al. Functional architecture of the rat parasubiculum. J. Neurosci. Off. J. Soc. Neurosci. 2016, 36, 2289–2301. [Google Scholar] [CrossRef] [Green Version]
- Taube, J.S.; Muller, R.U.; Ranck, J.B., Jr. Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J. Neurosci. Off. J. Soc. Neurosci. 1990, 10, 420–435. [Google Scholar] [CrossRef] [Green Version]
- Boccara, C.N.; Sargolini, F.; Thoresen, V.H.; Solstad, T.; Witter, M.P.; Moser, E.I.; Moser, M.B. Grid cells in pre- and parasubiculum. Nat. Neurosci. 2010, 13, 987–994. [Google Scholar] [CrossRef]
- Hafting, T.; Fyhn, M.; Molden, S.; Moser, M.B.; Moser, E.I. Microstructure of a spatial map in the entorhinal cortex. Nature 2005, 436, 801–806. [Google Scholar] [CrossRef]
- Peckford, G.; Dwyer, J.A.; Snow, A.C.; Thorpe, C.M.; Martin, G.M.; Skinner, D.M. The effects of lesions to the postsubiculum or the anterior dorsal nucleus of the thalamus on spatial learning in rats. Behav. Neurosci. 2014, 128, 654–665. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Jarrard, L.E.; Bilkey, D.K. Excitotoxic lesions of the pre- and parasubiculum disrupt object recognition and spatial memory processes. Behav. Neurosci. 2001, 115, 112–124. [Google Scholar] [CrossRef]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leiter, O.; Zhuo, Z.; Rust, R.; Wasielewska, J.M.; Grönnert, L.; Kowal, S.; Overall, R.W.; Adusumilli, V.S.; Blackmore, D.G.; Southon, A.; et al. Selenium mediates exercise-induced adult neurogenesis and reverses learning deficits induced by hippocampal injury and aging. Cell Metab 2022, 34, 408–423.e8. [Google Scholar] [CrossRef]
- Hutten, N.R.; Kuypers, K.P.; van Wel, J.H.; Theunissen, E.L.; Toennes, S.W.; Verkes, R.J.; Ramaekers, J.G. A single dose of cocaine enhances prospective memory performance. J. Psychopharmacol. 2018, 32, 883–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kutlu, M.G.; Gould, T.J. Effects of drugs of abuse on hippocampal plasticity and hippocampus-dependent learning and memory: Contributions to development and maintenance of addiction. Learn. Mem. 2016, 23, 515–533. [Google Scholar] [CrossRef] [Green Version]
- Maćkowiak, M.; Markowicz-Kula, K.; Fijał, K.; Wedzony, K. Acute and repeated administration of cocaine differentially regulates expression of psa-ncam-positive neurons in the rat hippocampus. Brain Res. 2005, 1055, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuys, R. The insular cortex: A review. Prog. Brain Res. 2012, 195, 123–163. [Google Scholar]
- Kobayashi, M. Macroscopic connection of rat insular cortex: Anatomical bases underlying its physiological functions. Int. Rev. Neurobiol. 2011, 97, 285–303. [Google Scholar]
- El Rawas, R.; Klement, S.; Kummer, K.K.; Fritz, M.; Dechant, G.; Saria, A.; Zernig, G. Brain regions associated with the acquisition of conditioned place preference for cocaine vs. Social interaction. Front. Behav. Neurosci. 2012, 6, 63. [Google Scholar] [CrossRef] [Green Version]
- Forget, B.; Pushparaj, A.; Le Foll, B. Granular insular cortex inactivation as a novel therapeutic strategy for nicotine addiction. Biol. Psychiatry 2010, 68, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Pushparaj, A.; Le Foll, B. Involvement of the caudal granular insular cortex in alcohol self-administration in rats. Behav. Brain Res. 2015, 293, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, Y.; Tachibana, Y.; Sato, F.; Furuta, T.; Ohara, H.; Tomita, A.; Fujita, M.; Moritani, M.; Yoshida, A. Cortical and subcortical projections from granular insular cortex receiving orofacial proprioception. Neuroscience 2018, 388, 317–329. [Google Scholar] [CrossRef]
- Melo, C.A.A.; Guimarães, H.R.G.; Medeiros, R.C.F.; Souza, G.C.A.; Santos, P.; Tôrres, A. Oral changes in cocaine abusers: An integrative review. Braz. J. Otorhinolaryngol. 2021. [Google Scholar] [CrossRef]
- Höistad, M.; Barbas, H. Sequence of information processing for emotions through pathways linking temporal and insular cortices with the amygdala. NeuroImage 2008, 40, 1016–1033. [Google Scholar] [CrossRef] [Green Version]
- Grady, F.; Peltekian, L.; Iverson, G.; Geerling, J.C. Direct parabrachial-cortical connectivity. Cerebral Cortex 2020, 30, 4811–4833. [Google Scholar] [CrossRef] [PubMed]
- Lamb, K.; Gallagher, K.; McColl, R.; Mathews, D.; Querry, R.; Williamson, J.W. Exercise-induced decrease in insular cortex rcbf during postexercise hypotension. Med. Sci. Sport. Exerc. 2007, 39, 672–679. [Google Scholar] [CrossRef]
- Williamson, J.W.; McColl, R.; Mathews, D.; Ginsburg, M.; Mitchell, J.H. Activation of the insular cortex is affected by the intensity of exercise. J. Appl. Physiol. 1999, 87, 1213–1219. [Google Scholar] [CrossRef] [Green Version]
- Birtwistle, J.; Baldwin, D. Role of dopamine in schizophrenia and parkinson’s disease. Br. J. Nurs. 1998, 7, 832–834, 836, 838–841. [Google Scholar] [CrossRef]
- Tolosa, E.; Wenning, G.; Poewe, W. The diagnosis of parkinson’s disease. Lancet. Neurol. 2006, 5, 75–86. [Google Scholar] [CrossRef]
- Chinta, S.J.; Andersen, J.K. Dopaminergic neurons. Int. J. Biochem. Cell Biol. 2005, 37, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin, G.M.J., 3rd; Gomez, J.A.; Perkins, J.; Bland, J.L.; Petko, A.K.; Paladini, C.A. Cocaine selectively reorganizes excitatory inputs to substantia nigra pars compacta dopamine neurons. J. Neurosci. Off. J. Soc. Neurosci. 2018, 38, 1151–1159. [Google Scholar] [CrossRef] [Green Version]
- Quinlan, M.G.; Sharf, R.; Lee, D.Y.; Wise, R.A.; Ranaldi, R. Blockade of substantia nigra dopamine d1 receptors reduces intravenous cocaine reward in rats. Psychopharmacology 2004, 175, 53–59. [Google Scholar] [CrossRef]
- Zhang, Y.; Larcher, K.M.; Misic, B.; Dagher, A. Anatomical and functional organization of the human substantia nigra and its connections. eLife 2017, 6, e26653. [Google Scholar] [CrossRef] [PubMed]
- Baik, J.H. Dopamine signaling in reward-related behaviors. Front. Neural Circuits 2013, 7, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Self, D.W.; Nestler, E.J. Molecular mechanisms of drug reinforcement and addiction. Annu. Rev. Neurosci. 1995, 18, 463–495. [Google Scholar] [CrossRef]
- Minozzi, S.; Amato, L.; Pani, P.P.; Solimini, R.; Vecchi, S.; De Crescenzo, F.; Zuccaro, P.; Davoli, M. Dopamine agonists for the treatment of cocaine dependence. Cochrane Database Syst. Rev. 2015, 2015, Cd003352. [Google Scholar] [CrossRef]
- San Luciano, M.; Saunders-Pullman, R. Substance abuse and movement disorders. Curr. Drug Abus. Rev. 2009, 2, 273–278. [Google Scholar] [CrossRef]
- Tasaka, G.I.; Feigin, L.; Maor, I.; Groysman, M.; DeNardo, L.A.; Schiavo, J.K.; Froemke, R.C.; Luo, L.; Mizrahi, A. The temporal association cortex plays a key role in auditory-driven maternal plasticity. Neuron 2020, 107, 566–579.e567. [Google Scholar] [CrossRef]
- Purves, D.; Augustine, G.; Fitzpatrick, D. Neuroscience, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2001. [Google Scholar]
- McDaniel, W.; Thomas, R. Temporal and parietal association cortex lesions and spatial and black-white reversal learning in the rat. Physiol. Psychol. 1978, 6, 300–305. [Google Scholar] [CrossRef] [Green Version]
- Cassilhas, R.C.; Lee, K.S.; Venâncio, D.P.; Oliveira, M.G.; Tufik, S.; de Mello, M.T. Resistance exercise improves hippocampus-dependent memory. Braz. J. Med. Biol. Res. Rev. Bras. De Pesqui. Med. E Biol. 2012, 45, 1215–1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsang, A.R.; Rajakumar, N.; Jog, M.S. Botulinum toxin a injection into the entopeduncular nucleus improves dynamic locomotory parameters in hemiparkinsonian rats. PloS ONE 2019, 14, e0223450. [Google Scholar] [CrossRef] [PubMed]
- Dybdal, D.; Gale, K. Postural and anticonvulsant effects of inhibition of the rat subthalamic nucleus. J. Neurosci. Off. J. Soc. Neurosci. 2000, 20, 6728–6733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavian, H.; Loewenstern, Y.; Madar, R.; Almog, M.; Bar-Gad, I.; Okun, E.; Korngreen, A. Dopamine receptors in the rat entopeduncular nucleus. Brain Struct. Funct. 2018, 223, 2673–2684. [Google Scholar] [CrossRef]
- Li, H.; Eid, M.; Pullmann, D.; Chao, Y.S.; Thomas, A.A.; Jhou, T.C. Entopeduncular nucleus projections to the lateral habenula contribute to cocaine avoidance. J. Neurosci. Off. J. Soc. Neurosci. 2021, 41, 298–306. [Google Scholar] [CrossRef]
- Mullié, Y.; Arto, I.; Yahiaoui, N.; Drew, T. Contribution of the entopeduncular nucleus and the globus pallidus to the control of locomotion and visually guided gait modifications in the cat. Cerebral Cortex. 2020, 30, 5121–5146. [Google Scholar] [CrossRef]
- Jackson, A.; Crossman, A.R. Nucleus tegmenti pedunculopontinus: Efferent connections with special reference to the basal ganglia, studied in the rat by anterograde and retrograde transport of horseradish peroxidase. Neuroscience 1983, 10, 725–765. [Google Scholar] [CrossRef]
- Beh, S.C.; Frohman, T.C.; Frohman, E.M. Cerebellar control of eye movements. J. Neuro-Ophthalmol. Off. J. North Am. Neuro-Ophthalmol. Soc. 2017, 37, 87–98. [Google Scholar] [CrossRef]
- Voogd, J.; Schraa-Tam, C.K.; van der Geest, J.N.; De Zeeuw, C.I. Visuomotor cerebellum in human and nonhuman primates. Cerebellum 2012, 11, 392–410. [Google Scholar] [CrossRef] [Green Version]
- Diano, M.; D’Agata, F.; Cauda, F.; Costa, T.; Geda, E.; Sacco, K.; Duca, S.; Torta, D.M.; Geminiani, G.C. Cerebellar clustering and functional connectivity during pain processing. Cerebellum 2016, 15, 343–356. [Google Scholar] [CrossRef] [Green Version]
- Hayakawa, Y.; Nakajima, T.; Takagi, M.; Fukuhara, N.; Abe, H. Human cerebellar activation in relation to saccadic eye movements: A functional magnetic resonance imaging study. Ophthalmologica. J. Int. D’ophtalmologie. Int. J. Ophthalmol. Z. Fur Augenheilkd. 2002, 216, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Fulbright, R.K.; Jenner, A.R.; Mencl, W.E.; Pugh, K.R.; Shaywitz, B.A.; Shaywitz, S.E.; Frost, S.J.; Skudlarski, P.; Constable, R.T.; Lacadie, C.M.; et al. The cerebellum’s role in reading: A functional mr imaging study. AJNR. Am. J. Neuroradiol. 1999, 20, 1925–1930. [Google Scholar] [PubMed]
- Dieterich, M.; Bucher, S.F.; Seelos, K.C.; Brandt, T. Cerebellar activation during optokinetic stimulation and saccades. Neurology 2000, 54, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Ikai, Y.; Takada, M.; Shinonaga, Y.; Mizuno, N. Dopaminergic and non-dopaminergic neurons in the ventral tegmental area of the rat project, respectively, to the cerebellar cortex and deep cerebellar nuclei. Neuroscience 1992, 51, 719–728. [Google Scholar] [CrossRef]
- Douma, E.H.; de Kloet, E.R. Stress-induced plasticity and functioning of ventral tegmental dopamine neurons. Neurosci. Biobehav. Rev. 2020, 108, 48–77. [Google Scholar] [CrossRef]
- Majak, K.; Moryś, J. Endopiriform nucleus connectivities: The implications for epileptogenesis and epilepsy. Folia Morphol. 2007, 66, 267–271. [Google Scholar]
- Behan, M.; Haberly, L.B. Intrinsic and efferent connections of the endopiriform nucleus in rat. J. Comp. Neurol. 1999, 408, 532–548. [Google Scholar] [CrossRef]
- Yoshimura, H.; Hasumoto-Honjo, M.; Sugai, T.; Segami, N.; Kato, N. Enhancement of oscillatory activity in the endopiriform nucleus of rats raised under abnormal oral conditions. Neurosci. Lett. 2014, 561, 162–165. [Google Scholar] [CrossRef]
- Beneyto, M.; Prieto, J.J. Connections of the auditory cortex with the claustrum and the endopiriform nucleus in the cat. Brain Res. Bull. 2001, 54, 485–498. [Google Scholar] [CrossRef]
- Swenson, S.; Blum, K.; McLaughlin, T.; Gold, M.S.; Thanos, P.K. The therapeutic potential of exercise for neuropsychiatric diseases: A review. J. Neurol. Sci. 2020, 412, 116763. [Google Scholar] [CrossRef]
- Rahman, N.; Mihalkovic, A.; Geary, O.; Haffey, R.; Hamilton, J.; Thanos, P.K. Chronic aerobic exercise: Autoradiographic assessment of gaba(a) and mu-opioid receptor binding in adult rats. Pharmacol. Biochem. Behav. 2020, 196, 172980. [Google Scholar] [CrossRef] [PubMed]
- Swenson, S.; Hamilton, J.; Robison, L.; Thanos, P.K. Chronic aerobic exercise: Lack of effect on brain cb1 receptor levels in adult rats. Life Sci. 2019, 230, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Thanos, P.K.; Hamilton, J.; O’Rourke, J.R.; Napoli, A.; Febo, M.; Volkow, N.D.; Blum, K.; Gold, M. Dopamine d2 gene expression interacts with environmental enrichment to impact lifespan and behavior. Oncotarget 2016, 7, 19111–19123. [Google Scholar] [CrossRef] [PubMed]
- Blum, K.; Thanos, P.K.; Gold, M.S. Dopamine and glucose, obesity, and reward deficiency syndrome. Front. Psychol. 2014, 5, 919. [Google Scholar] [CrossRef] [Green Version]
- Blum, K.; Cadet, J.L.; Gold, M.S. Psychostimulant use disorder emphasizing methamphetamine and the opioid -dopamine connection: Digging out of a hypodopaminergic ditch. J. Neurol. Sci. 2021, 420, 117252. [Google Scholar] [CrossRef]
- Blum, K.; Badgaiyan, R.D.; Braverman, E.R.; Dushaj, K.; Li, M.; Thanos, P.K.; Demetrovics, Z.; Febo, M. Hypothesizing that, a pro-dopamine regulator (kb220z) should optimize, but not hyper-activate the activity of trace amine-associated receptor 1 (taar-1) and induce anti-craving of psychostimulants in the long-term. J. Reward Defic. Syndr. Addict. Sci. 2016, 2, 14–21. [Google Scholar] [CrossRef]
- Blum, K.; Baron, D.; Badgaiyan, R.D.; Gold, M.S. Can chronic consumption of caffeine by increasing d2/d3 receptors offer benefit to carriers of the drd2 a1 allele in cocaine abuse? EC Psychol. Psychiatry 2019, 8, 318–321. [Google Scholar]
- Blum, K.; Chen, T.J.; Meshkin, B.; Downs, B.W.; Gordon, C.A.; Blum, S.; Mangucci, J.F.; Braverman, E.R.; Arcuri, V.; Deutsch, R.; et al. Genotrim, a DNA-customized nutrigenomic product, targets genetic factors of obesity: Hypothesizing a dopamine-glucose correlation demonstrating reward deficiency syndrome (rds). Med. Hypotheses 2007, 68, 844–852. [Google Scholar] [CrossRef]
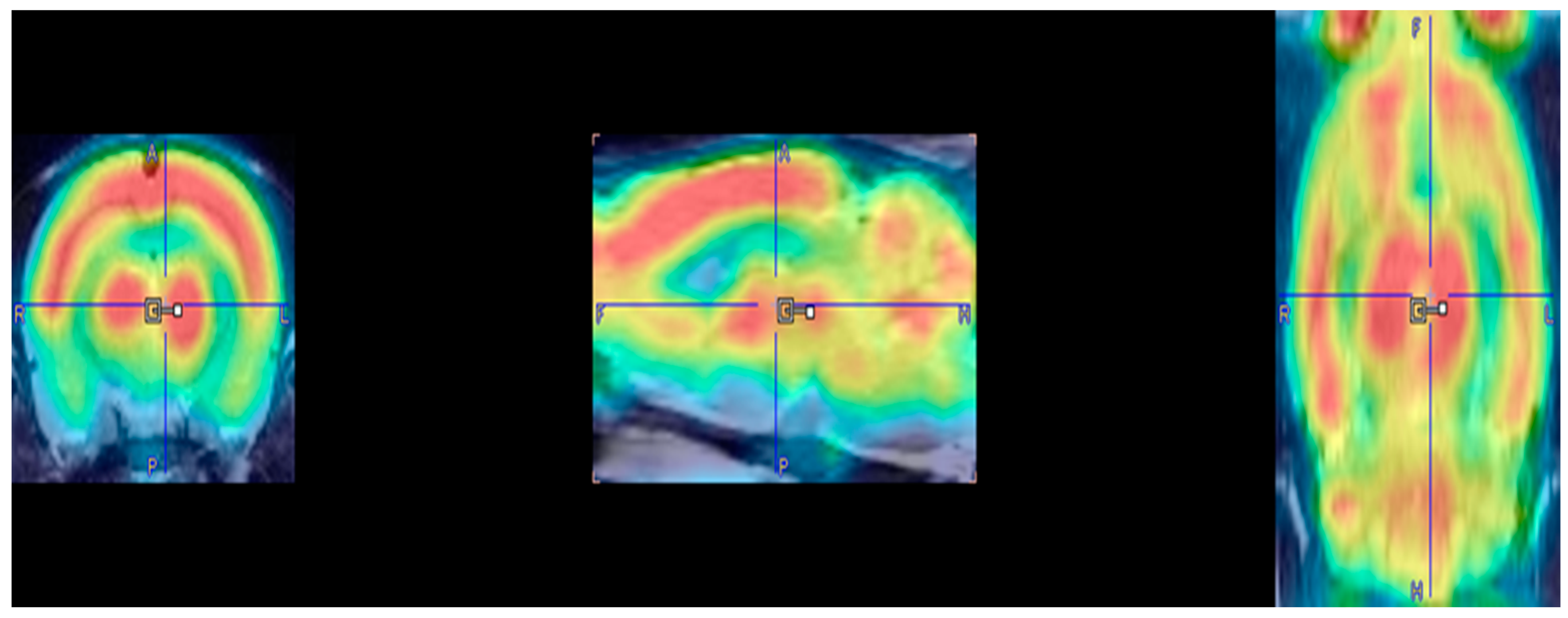

| Brain Region(s) | Significant Effect | Medial-Lateral (mm) | Anterior-Posterior (mm) | Dorsal-Ventral (mm) | t-Value | z-Score | KE |
|---|---|---|---|---|---|---|---|
| Post PaS | + | 3.6 | −8.0 | 3.4 | 7.92 | 4.49 | 120 |
| GI DI | + | −6.0 | −0.4 | 6.6 | 7.92 | 4.49 | 311 |
| SNR SNCD | + | −1.2 | −5.6 | 8.2 | 5.85 | 3.86 | 64 |
| TeA | + | −4.8 | −7.6 | 3.4 | 4.14 | 3.15 | 132 |
| EP | + | −1.8 | −2.0 | 7.6 | 5.50 | 3.74 | 244 |
| Crus 1 | + | 2.2 | −11.6 | 4.2 | 4.98 | 3.53 | 64 |
| VEn | - | 5.2 | −1.8 | 9.0 | 5.42 | 3.71 | 79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanna, C.; Hamilton, J.; Blum, K.; Badgaiyan, R.D.; Thanos, P.K. Exercise Modulates Brain Glucose Utilization Response to Acute Cocaine. J. Pers. Med. 2022, 12, 1976. https://doi.org/10.3390/jpm12121976
Hanna C, Hamilton J, Blum K, Badgaiyan RD, Thanos PK. Exercise Modulates Brain Glucose Utilization Response to Acute Cocaine. Journal of Personalized Medicine. 2022; 12(12):1976. https://doi.org/10.3390/jpm12121976
Chicago/Turabian StyleHanna, Colin, John Hamilton, Kenneth Blum, Rajendra D. Badgaiyan, and Panayotis K. Thanos. 2022. "Exercise Modulates Brain Glucose Utilization Response to Acute Cocaine" Journal of Personalized Medicine 12, no. 12: 1976. https://doi.org/10.3390/jpm12121976
APA StyleHanna, C., Hamilton, J., Blum, K., Badgaiyan, R. D., & Thanos, P. K. (2022). Exercise Modulates Brain Glucose Utilization Response to Acute Cocaine. Journal of Personalized Medicine, 12(12), 1976. https://doi.org/10.3390/jpm12121976









