Abstract
Despite the treatment of lung adenocarcinoma (LUAD) having partially improved in recent years, LUAD patients still have poor prognosis rates. Therefore, it is especially important to explore effective biomarkers and exploit novel therapeutic developments. High-throughput technologies are widely used as systematic approaches to explore differences in expressions of thousands of genes for both biological and genomic systems. Recently, using big data analyses in biomedicine research by integrating several high-throughput databases and tools, including The Cancer Genome Atlas (TCGA), cBioportal, Oncomine, and Kaplan–Meier plotter, is an important strategy to identify novel biomarkers for cancer therapy. Here, we used two different comprehensive bioinformatics analysis and revealed protein tyrosine phosphatase non-receptor type (PTPN) family genes, especially PTPN1 and PTPN22, were downregulated in lung cancer tissue in comparison with normal samples. The survival curves indicated that LUAD patients with high transcription levels of PTPN5 were significantly associated with a good prognosis. Meanwhile, Gene Ontology (GO) and MetaCore analyses indicated that co-expression of the PTPN1, PTPN5, and PTPN21 genes was significantly enriched in cancer development-related pathways, including GTPase activity, regulation of small GTPase-mediated signal transduction, response to mechanical stimuli, vasculogenesis, organ morphogenesis, regulation of stress fiber assembly, mitogen-activated protein kinase (MAPK) cascade, cell migration, and angiogenesis. Collectively, this study revealed that PTPN family members are both significant prognostic biomarkers for lung cancer progression and promising clinical therapeutic targets, which provide new targets for treating LUAD patients.
1. Introduction
Lung cancer is the leading cause of cancer deaths worldwide, accounting for 18.0% of total cancer deaths [1]. Lung cancer is composed of small-cell lung carcinoma (SCLC) and non-SCLC (NSCLC). NSCLC comprises around 85% of lung cancer cases and it is further classified into three subtypes, squamous-cell carcinoma, adenocarcinoma, and large-cell carcinoma [2]. Lung adenocarcinoma (LUAD) is the main subtype of NSCLC, accounting for 40% of all cases of lung cancer. Despite the treatment of LUAD having partially improved in recent years, LUAD patients still have a relatively poor prognosis, and the 5-year relative survival rate is less than 21% [3,4]. Therefore, it is especially important to explore effective biomarkers and exploit novel therapeutic developments [5,6,7,8,9].
The tyrosine phosphorylation of proteins plays a key role in controlling several signaling pathways involved in cell proliferation, apoptosis, migration, and invasion [10]. The process of signal transduction is coordinately mediated by protein tyrosine phosphatases (PTPs) and protein tyrosine kinases (PTKs), and it is orchestrated by a cascade of molecular events involving protein phosphorylation by PTKs and de-phosphorylation by PTPs [11]. Notably, published research suggests that PTPs may have essential roles in tumor invasion and metastasis [12]. The PTP family comprises 107 members that can be divided into four separate classes according to differences in amino acid sequence of the catalytic domain. The protein tyrosine phosphatase non-receptor type (PTPN) family is part of class I cysteine-based PTPs [13]. There are 17 different members involved in the PTPN family in humans, and accumulating evidence suggests that PTPN family members have critical roles in the progression of a variety of human cancers. For instance, it was demonstrated that PTPN3 acts as a suppressor in tumorigenesis, and it could modulate the transforming growth factor (TGF)-β signaling in liver cancer [14]. Published research has shown that mutations and genetic variants of certain genes in the PTPN2 pathway are related to the risk and survival of lung cancer [15]. The loss of PTPN4 by activation of signal transduction and activator of transcription 3 (STAT3) might cause tumorigenesis of colorectal cancer [16]. In addition, the current study revealed that PTPN11 has an oncogenic function in breast cancer by regulating the phosphatidylinositol 3-kinase (PI3K)/AKT/glycogen synthase kinase 3β (GSK3β) signaling pathway. [17] However, relationships between PTPN family members and LUAD have not been extensively studied.
In the present study, we utilized large-scale bioinformatics databases to identify the expression status of different PTPN members in LUAD patients [18,19,20,21,22,23]. The main purpose of this study was to determine expression patterns and molecular mechanisms of PTPN for LUAD, define predictive biomarkers, and develop new therapeutic targets.
2. Materials and Methods
2.1. UALCAN Analysis
The Cancer Genome Atlas (TCGA) level 3 RNA-sequencing (RNA-Seq) together with clinical data acquired from around 30 different types of cancer are combined in UALCAN [24,25,26,27,28]. Expression values of more than 25,000 genes within this database were calculated using the RNA-Seq by Expectation Maximization (RSEM) tool. Transcripts per million (TPM) measurements were applied to determine whether there were statistically significant differences in gene expression levels among groups. This platform was also employed to extract TCGA data, which included 515 primary LUAD samples and 59 normal samples. In our study, we examined messenger (m)RNA levels of five PTPN family genes in LUAD and their relationships to clinicopathological characteristics and tumor progression presented at different stages.
2.2. Cancer Cell Line Encyclopedia (CCLE) Analysis
The CCLE project was employed to further examine expression level patterns of all PTPN members on a broader scale. This web-based application gave users access to more than 1000 human cancer cell lines and 130 different datasets that are both genetically and pharmacologically characterized [29,30,31,32]. Additionally, independent LUAD cancer cell lines were subjected to the integrated RNA-Seq Aligned Reads method to plot the expression of each PTPN gene.
2.3. Functional Enrichment Analysis of PTPN Target Genes
The InteractiVenn tool was selected to generate a one-way Venn diagram that presents the overlap and number of genes linked with the expression of PTPN target genes across the two given datasets obtained from TCGA database (available at the cBioPortal platform), and together with the MetaCore platform was employed to map the intersection between these two sets of data in terms of related pathways and involved networks. A p value of <0.05 was as previously described [33,34,35,36,37].
2.4. Kaplan–Meier (KM) Overall Survival (OS) Analysis
The KM database is an integrated online database available at https://kmplot.com/ (accessed on 14 April 2021) which prominently features in assessing target genes in survivors of more than 20 different types of cancer. This tool was subsequently leveraged to expand on LUAD prognosis-related issues, including OS, first progression (FP), and post-progression survival (PPS) [31]. The independent prognostic values of PTPN target genes on patients diagnosed with LUAD as mentioned above were represented as two comparative KM survival curves, one for LUAD patients and one for the healthy population, by concurrently integrating messenger (m)RNA expression levels and clinical data obtained from the target genes. Comparisons of the two patient cohorts were performed with 95% confidence intervals of hazard ratios and a fixed log-rank p value [38,39,40].
3. Results
3.1. Expression Patterns of PTPN Family Members in LUAD Patients
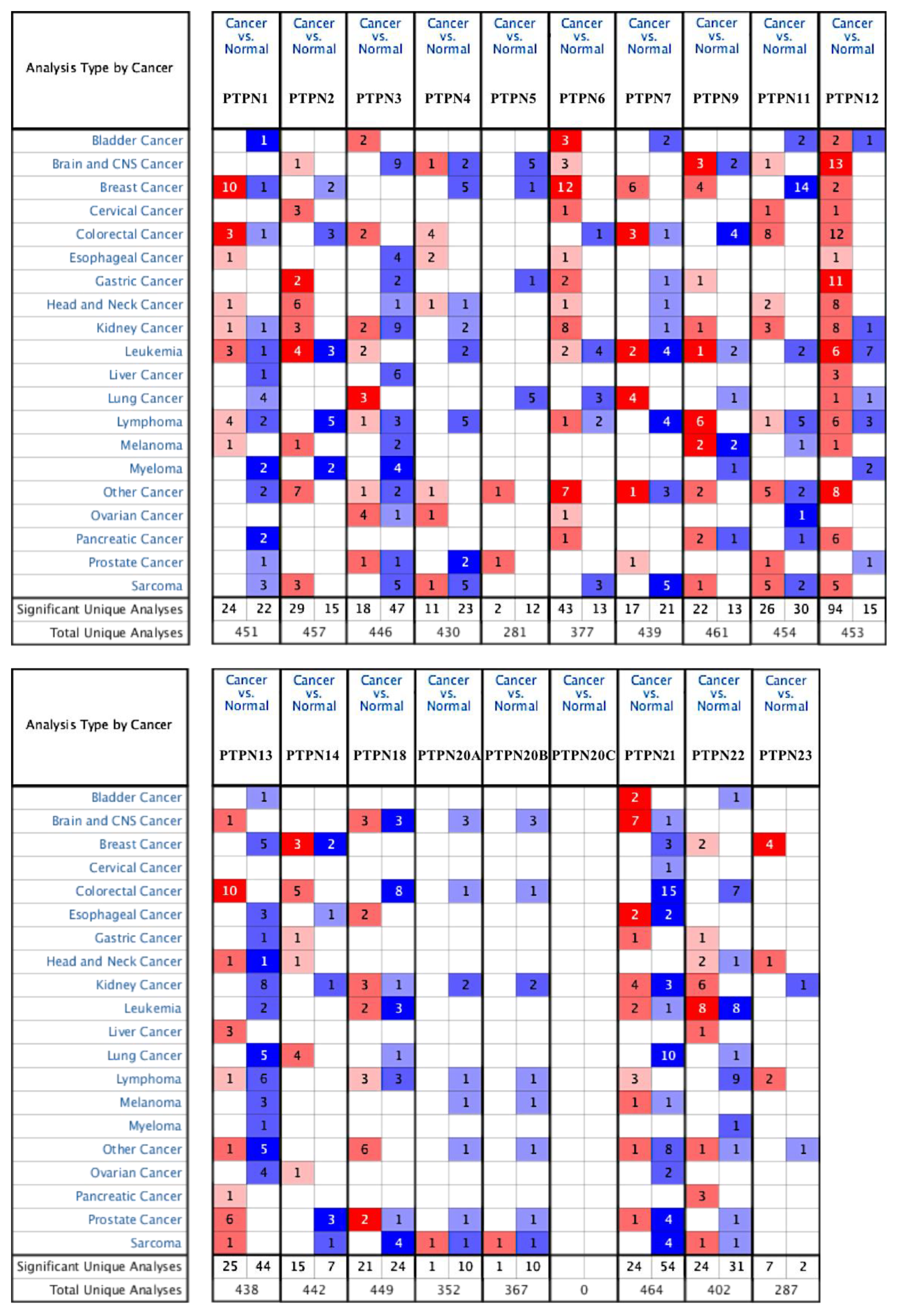
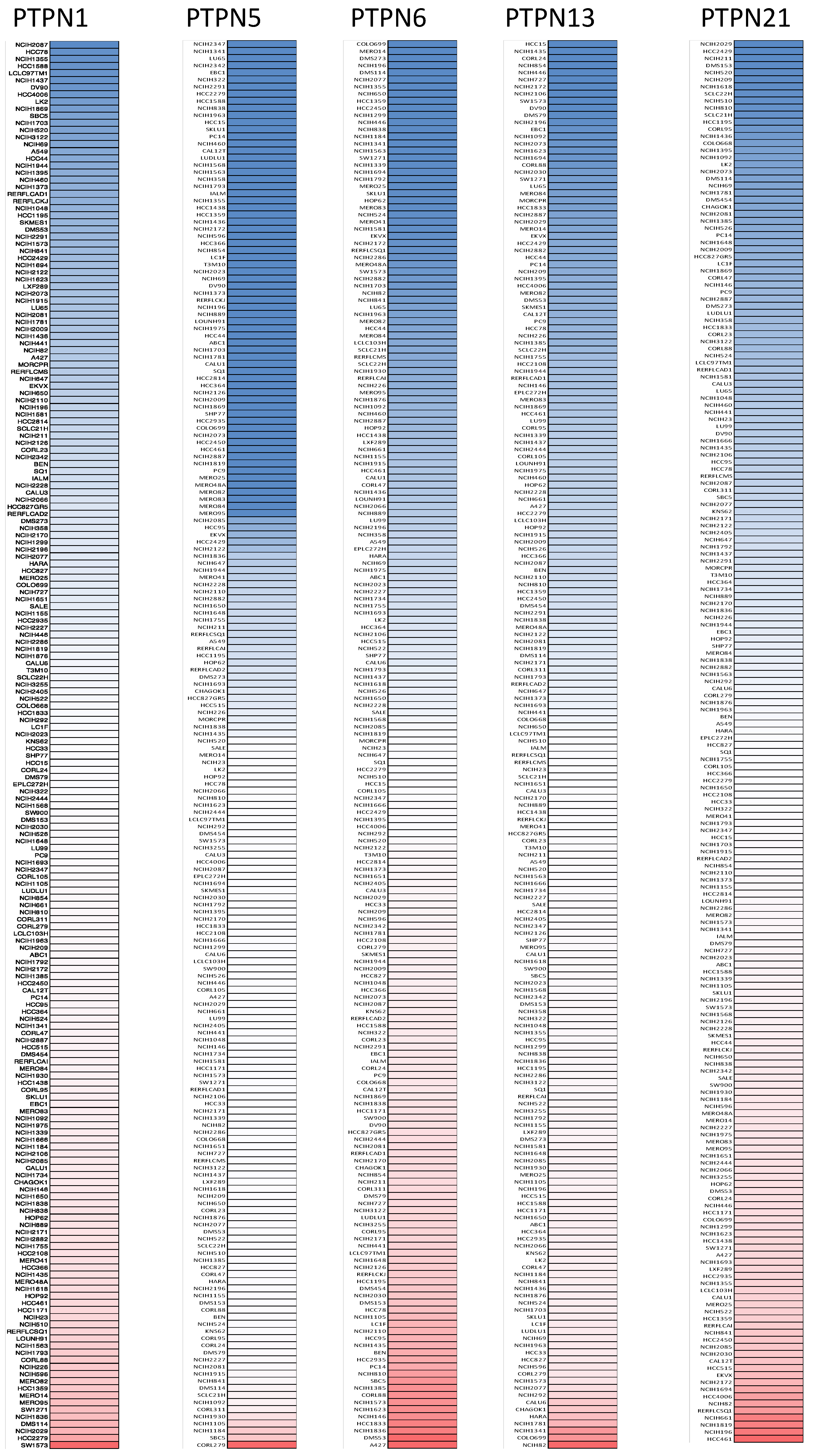
First, we used the ONCOMINE database to compare levels of different PTPN members in various cancers and normal tissue samples (Figure 1). Results showed that PTPN3, PTPN7, PTPN12, and PTPN14 were expressed at higher levels, while expressions of PTPN1, PTPN5, PTPN6, PTPN9, PTPN12, PTPN13, PTPN18, PTPN21, and PTPN22 were downregulated in lung cancer. The CCLE dataset was used to investigate mRNA expression levels in cancer cell lines in order to confirm the involvement of these PTPN family members. Values of the expression profiles were log-transformed and then displayed using a heatmap (Figure 2). Additionally, we further checked the analyses that met the threshold for PTPN genes according to the histopathological type of lung cancer (Supplementary Table S1). These datasets showed that PTPN1/5/6/9/13/21 expressions were significantly lower in LUAD tissues with the following thresholds: p value = 0.01; fold change = 1.5; and gene rank = 10%. These results suggested that these PTPN genes may play roles as tumor-suppressor genes in LUAD patients.
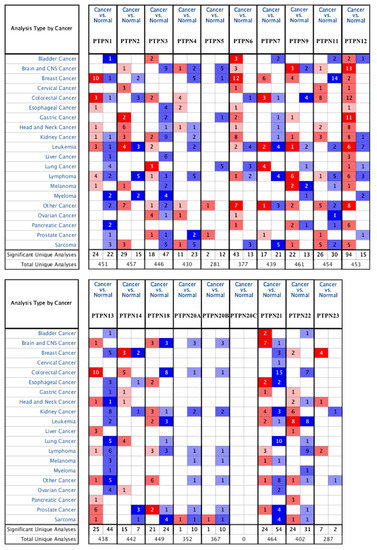
Figure 1.
Transcription levels of protein tyrosine phosphatase non-receptor type (PTPN) family genes in different types of cancers. The thresholds of the p value and fold change were as follows: p = 0.01, fold change = 1.5, gene rank = 10%.
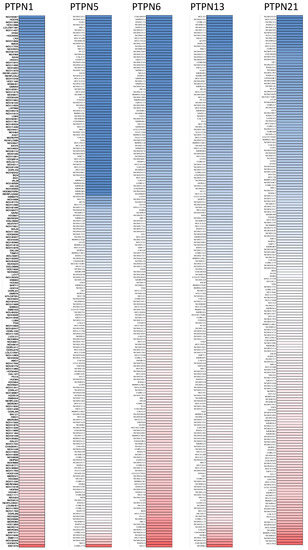
Figure 2.
Gene expression levels of protein tyrosine phosphatase non-receptor type (PTPN) family members in lung cancer cell lines (from the CCLE database). The blue blocks indicate underexpression, whereas red blocks represent overexpression.
3.2. Prognostic Values of PTPN Family Members in LUAD Patients
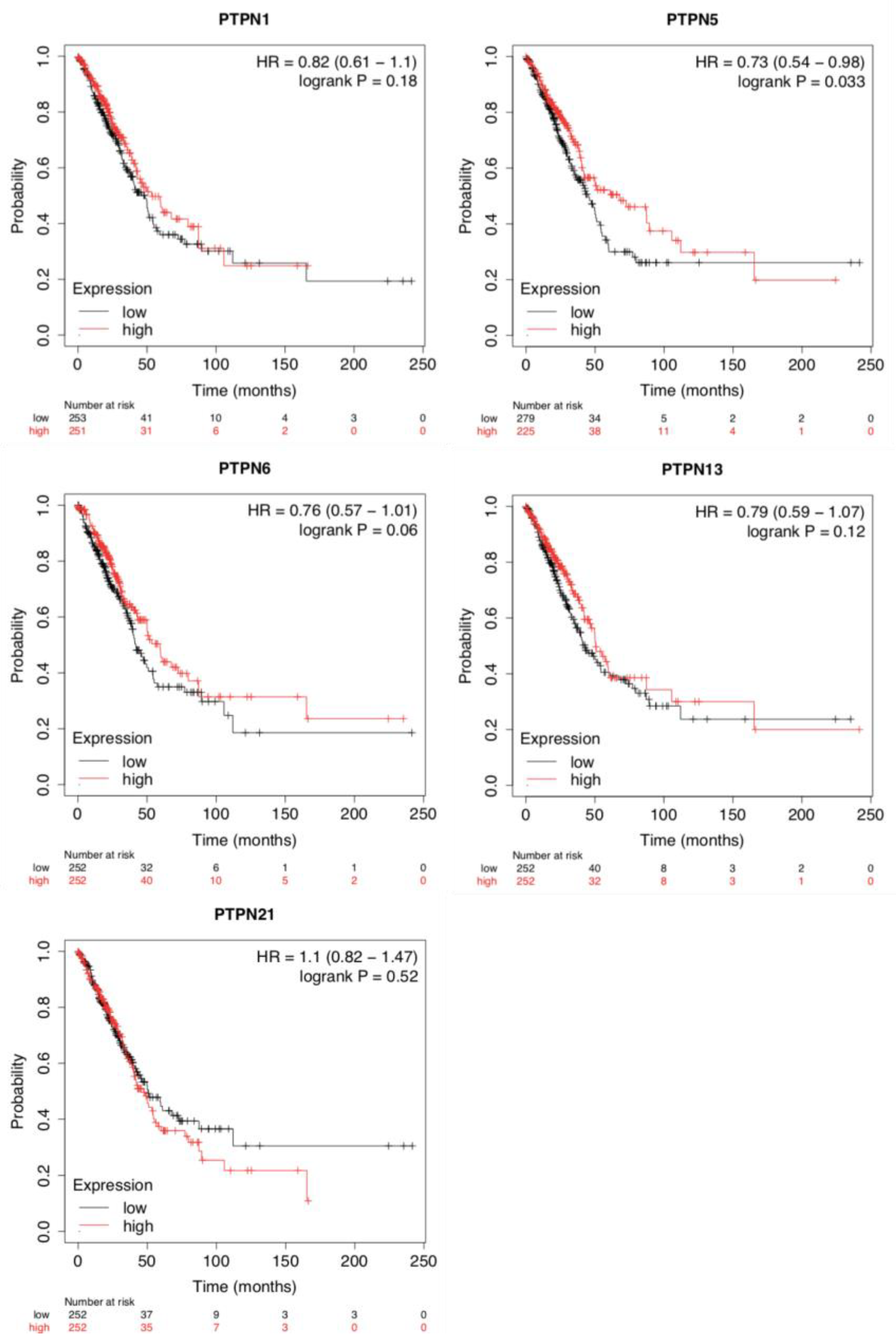
To evaluate the impacts of PTPNs at different expression levels on the progression of LUAD, we assessed correlations between these PTPN members and clinical outcomes using a KM plotter analysis (Figure 3). Survival curves revealed that LUAD patients with high transcription levels of PTPN1, PTPN5, PTPN6, PTPN13, and PTPN21 were associated with longer OS. Results indicated that high PTPN1/5/6/13/21 mRNA expression levels were related to a better prognosis of LUAD. Details of other PTPN genes are illustrated in Supplementary Table S2.
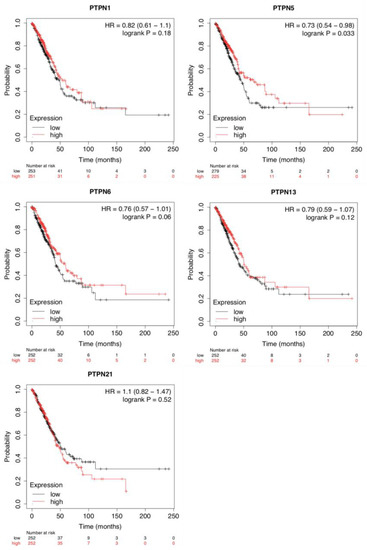
Figure 3.
Prognostic values of different expressed protein tyrosine phosphatase non-receptor type (PTPN) family members in overall survival of lung adenocarcinoma (LUAD) patients (Kaplan–Meier plotter).
3.3. Relationships between PTPN Genes and Clinicopathological Parameters of LUAD Patients
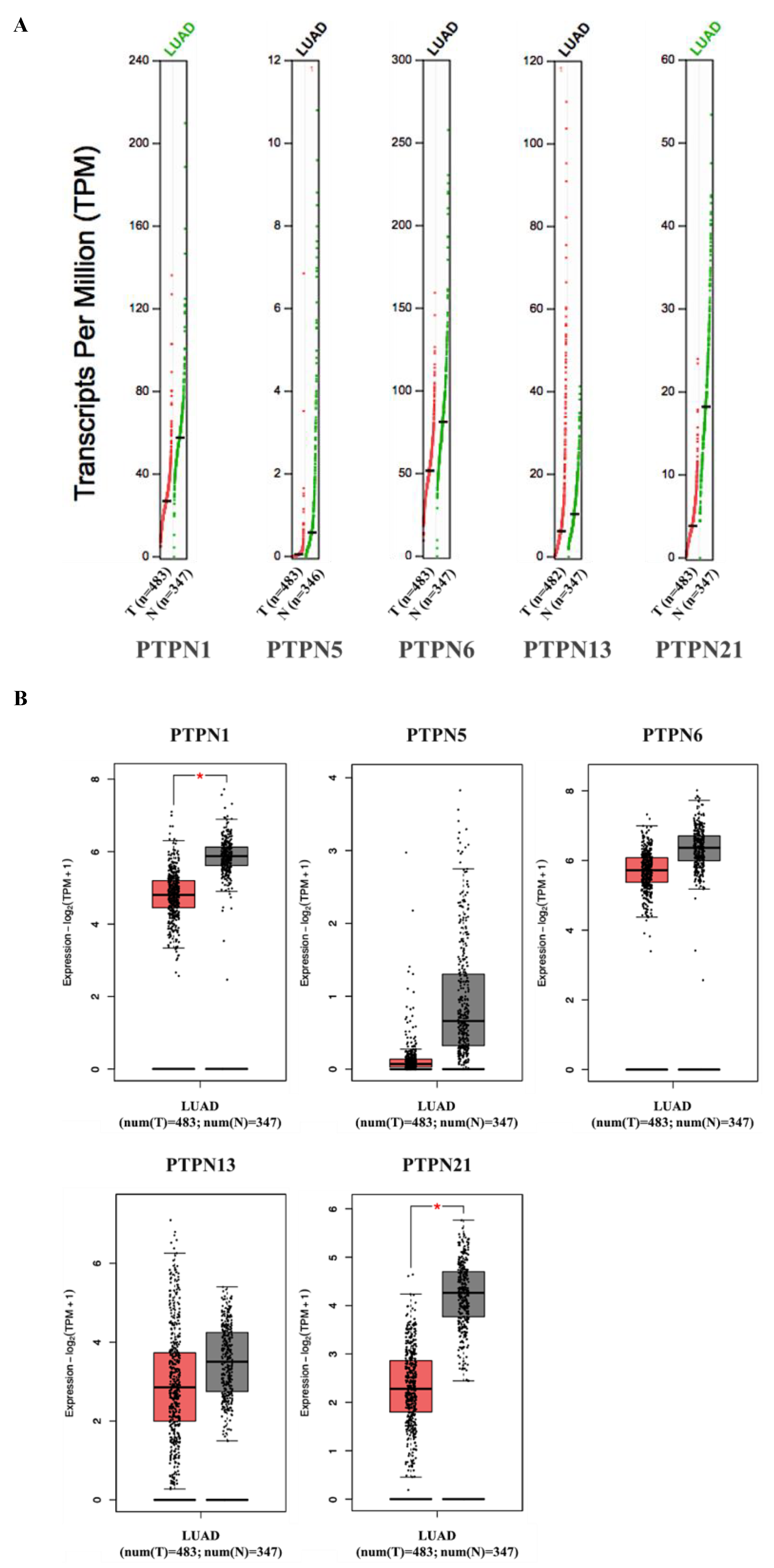
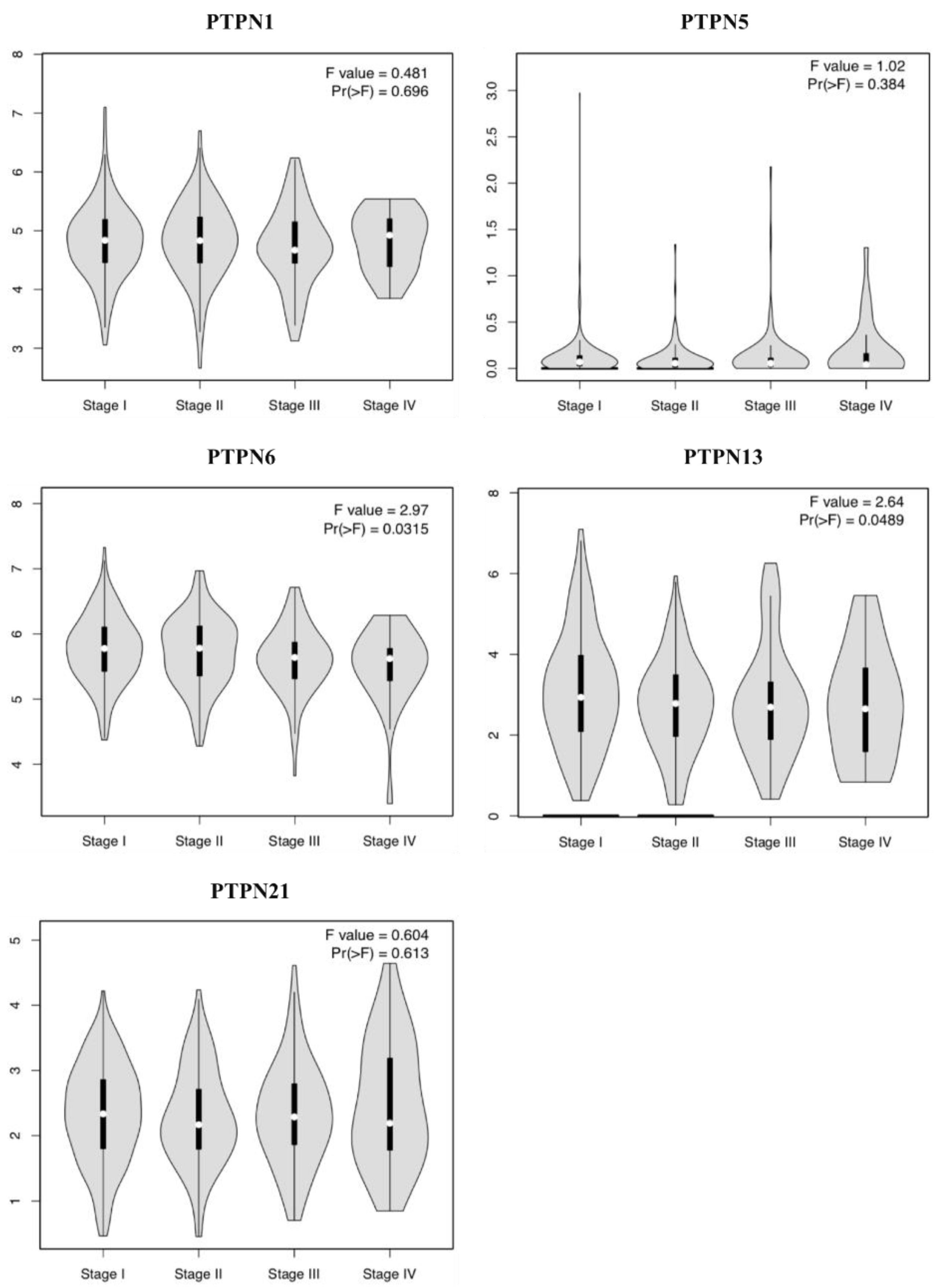
Furthermore, we compared transcriptional levels of PTPNs between LUAD and normal samples by interrogating the GEPIA dataset (Figure 4). We found that transcription levels of PTPN1 and PTPN21 in LUAD tissues were significantly lower than those in normal tissues, while transcription levels of PTPN5, PTPN6, and PTPN13 did not significantly differ between LUAD and normal tissues. Additionally, we also evaluated correlations between PTPN gene expression levels and the pathological stage of LUAD patients, and we discovered that expression levels of PTPN6 and PTPN13 were significantly correlated with the tumor stage of LUAD patients, while expression levels of PTPN1, PTPN5, and PTPN21 were not (Figure 5). According to the above results, it was confirmed that PTPN1, PTPN5, PTPN6, PTPN13, and PTPN21 were associated with the clinicopathological features of LUAD.
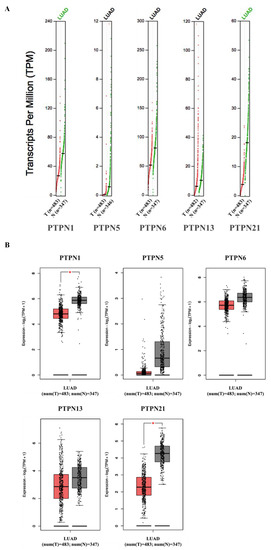
Figure 4.
(A,B) Expressions of PTPN members in LUAD patients and normal tissue via the GEPIA 2 platform. The q-value cut-off was set to 0.01. The red star in the pictures indicates a significant difference between LUAD and normal tissues.
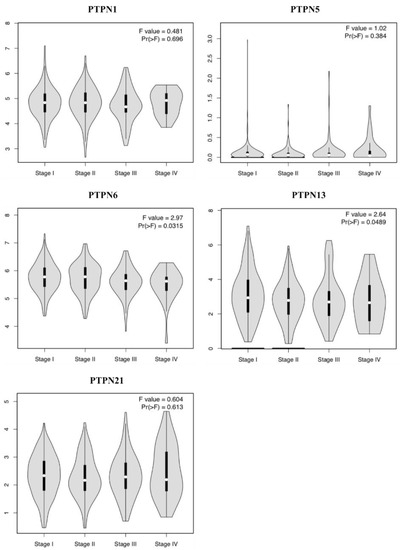
Figure 5.
Correlations between expressions of protein tyrosine phosphatase non-receptor type (PTPN) family members and pathological stage in patients with lung adenocarcinoma (LUAD) via the GEPIA database.
3.4. Genetic Alterations, Co-Expression, and Functional Enrichment Analyses of PTPNs in LUAD Patients
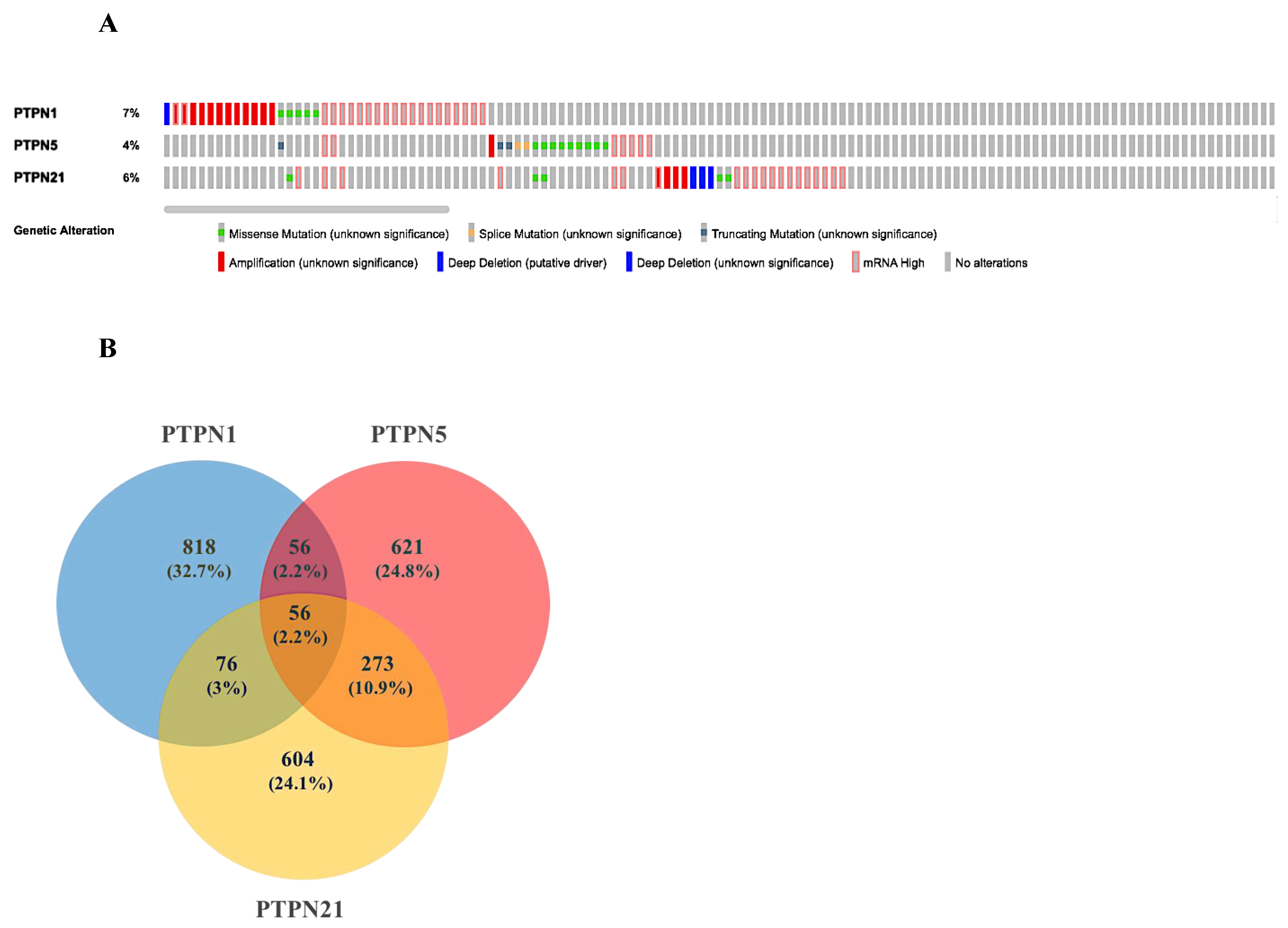
Based on the above results, we selected PTPN1, PTPN5, and PTPN21 to further conduct a comprehensive analysis of the molecular characteristics. First, we used the cBioPortal online tool for TCGA LUAD cohort to analyze genetic alterations of these PTPN genes. Results showed that genetic alterations of PTPNs had different types, including missense mutations, splice mutations, truncating mutations, deep deletions, high mRNA expression, and amplifications. The genetic variation rate of PTPN1 among 503 cases was 7%, which was the most frequently altered gene in these PTPN members, which included missense mutations, deep deletions, high mRNA expression, and amplifications. Others with high rates were PTPN5 (4%) and PTPN21 (6%) (Figure 6A).
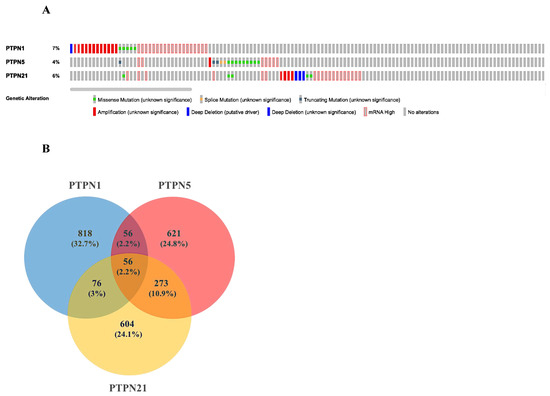
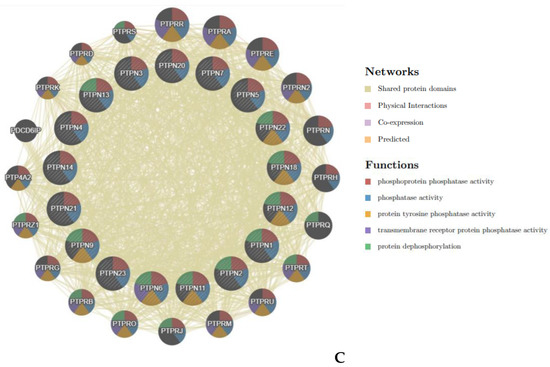
Figure 6.
Genetic alterations, co-expression, and functional enrichment analysis of protein tyrosine phosphatase non-receptor type (PTPN) family members in lung adenocarcinoma (LUAD) patients. (A) Genetic alterations in PTPN family members in LUAD (from cBioPortal). (B,C) Common genes among PTPN1, PTPN5, and PTPN21 (from Venny 2.0).
Next, we input the genetic data of PTPNs from cBioPortal into Venny 2.0 and calculated the overlap rate of each group. Results revealed that there were 56 genes in common among PTPN1, PTPN5, and PTPN21 (Figure 6B). Moreover, we analyzed the Gene Ontology (GO) in DAVID to identify enriched biological roles of PTPNs and predict their functions. The GO analysis was grouped into three aspects, including molecular functions (MFs), cellular components (CCs), and biological processes (BPs) (Supplementary Table S3C–F). We discovered that the BPs of PTPN1, PTPN5, and PTPN21 were significantly highly enriched in positive regulation of GTPase activity, regulation of small GTPase-mediated signal transduction, response to mechanical stimuli, vasculogenesis, response to estrogen, organ morphogenesis, regulation of stress fiber assembly, mitogen-activated protein kinase (MAPK) cascade, cell migration involved in sprouting angiogenesis, and positive regulation of angiogenesis. These items may be related to the regulation of cell differentiation and cell migration of LUAD. In the CC group, the most significantly highly enriched items included cytoplasm, focal adhesion, intracellular, cytoskeleton, extracellular matrix, cell cortex, lamellipodium, transforming growth factor beta receptor homodimeric complex, and nuclear outer membrane. As for MFs, changes were mainly enriched in GTPase activator activity, heparin binding, protein binding, calcium ion binding, and lipid binding.
3.5. Gene-Gene Interaction (GGI) Networks of PTPNs in LUAD
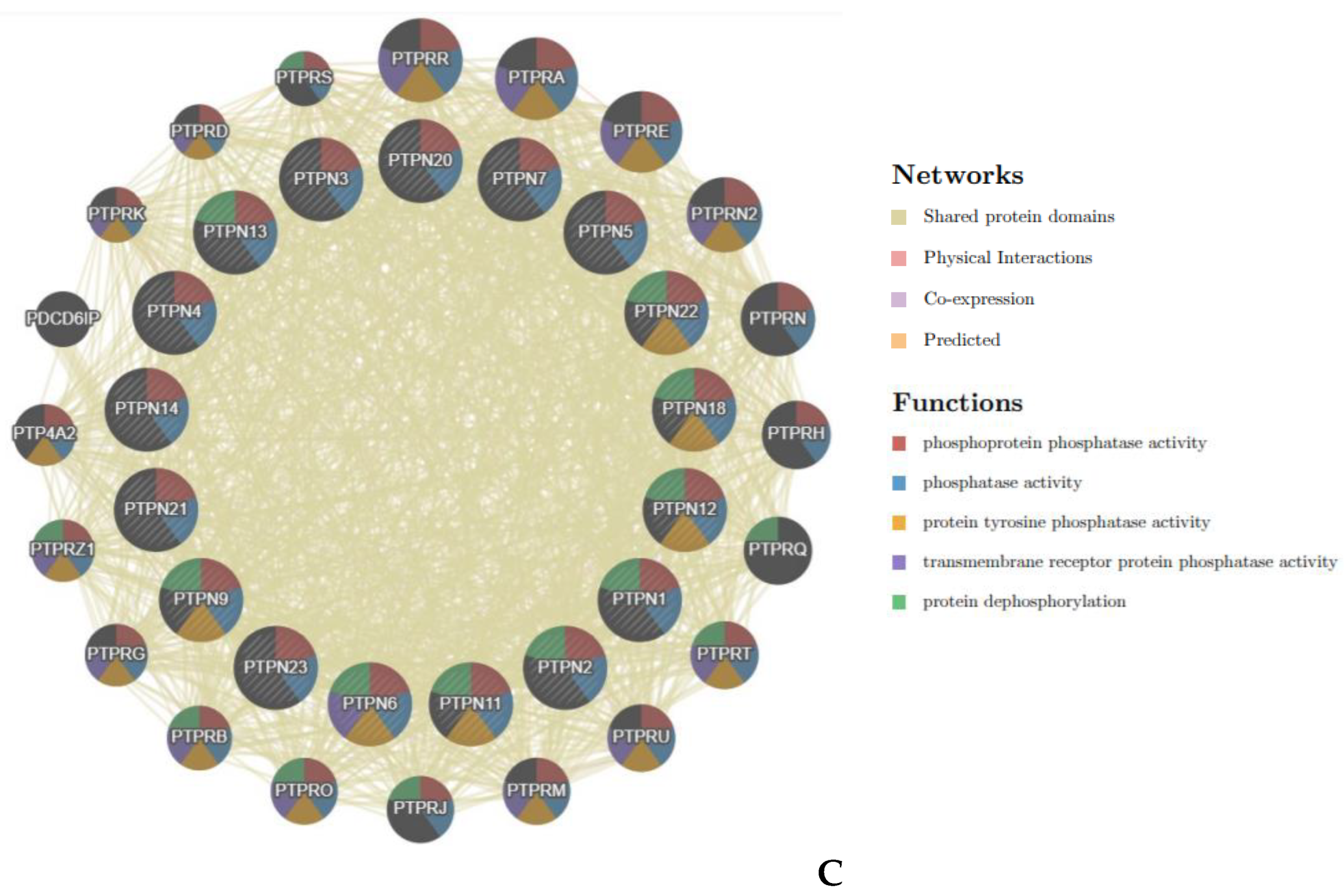
Next, we used the GeneMANIA database to construct GGI networks of PTPN1, PTPN5, and PTPN21, and further analyzed their functions. As shown in Figure 6A–C, each group had 20 nodes, which, respectively represented genes that were strongly related to PTPN1, PTPN5, and PTPN21 in terms of physical interactions, co-expression, predictions, co-localization, pathways, genetic interactions, and shared protein domains. The top five genes significantly associated with PTPN1 were NOX4 (NADPH oxidase 4), CTH (cystathionine gamma-lyase), JAK2 (Janus kinase 2), TRPV6 (transient receptor potential), and FCGR2A (Fc fragment of IgG receptor II-a). A further functional analysis showed that these genes were involved in the JAK-STAT pathway, and it was indicated that PTPN1 was related to cell proliferation and cell differentiation (Figure 6A). In addition, Figure 6B shows that the main genes associated with PTPN5 included CAPN1 (calpain 1), PTPRN (protein tyrosine phosphatase receptor type N), GRIN1 (glutamate ionotropic receptor NMDA type subunit 1), GRIN2B (glutamate ionotropic receptor NMDA type subunit 2B), and MAPK3 (mitogen-activated protein kinase 3). From results of the functional analysis, we discovered that PTPN5 was related to the transmission process of protein kinase signaling (Figure 6B). Moreover, Figure 6C also reveals that KIF1C (kinesin family member 1C), SRC (SRC proto-oncogene, non-receptor tyrosine kinase), NRG3 (neuregulin 3), BMX (BMX non-receptor tyrosine kinase), and PARD6B (par-6 family cell polarity regulator beta) were genes highly associated with PTPN21. According to the functional analysis, we observed that PTPN21 was primarily correlated with the activity of protein tyrosine kinase and cell migration (Figure 6C).
3.6. GGI Networks of PTPNs in LUAD
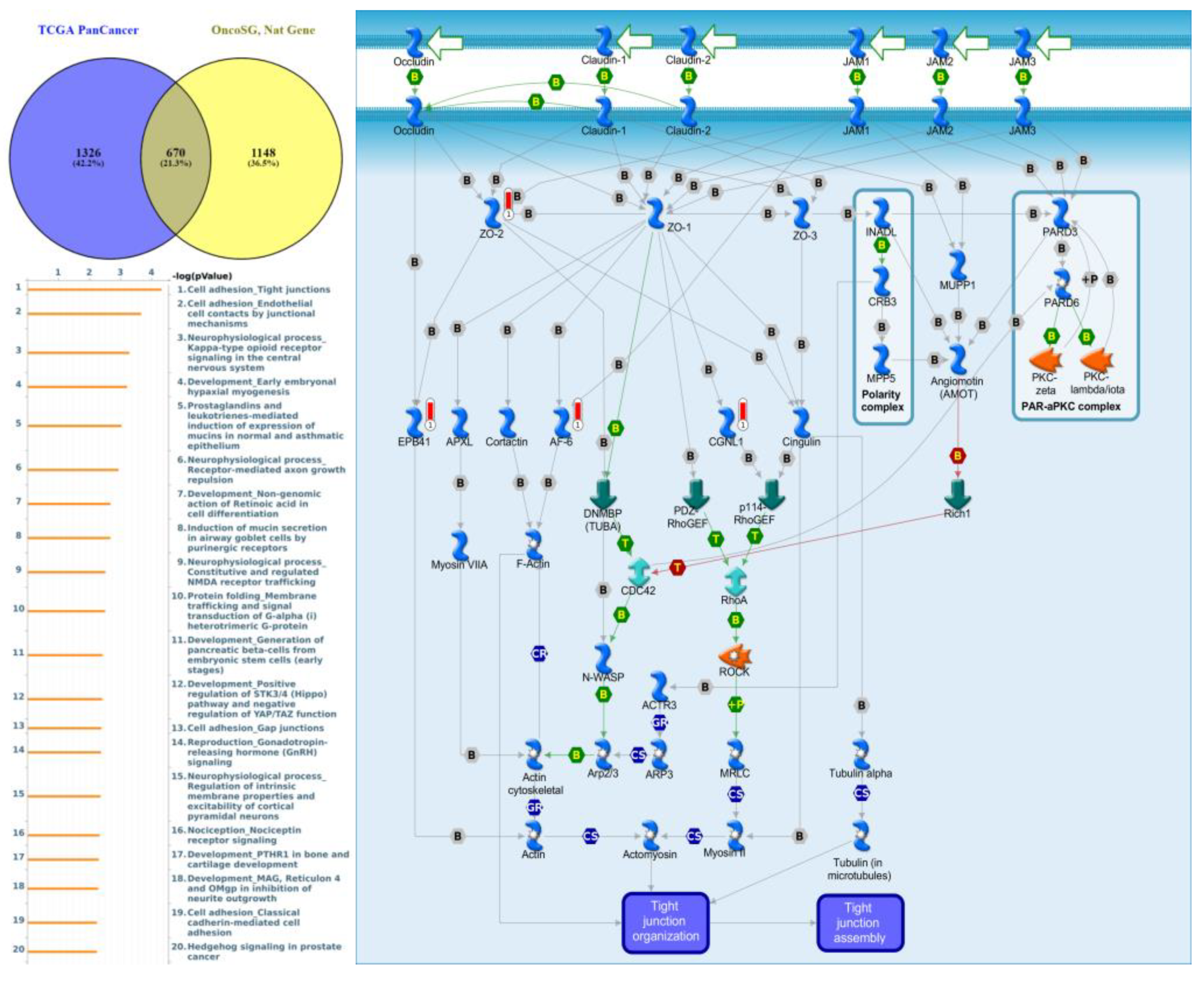
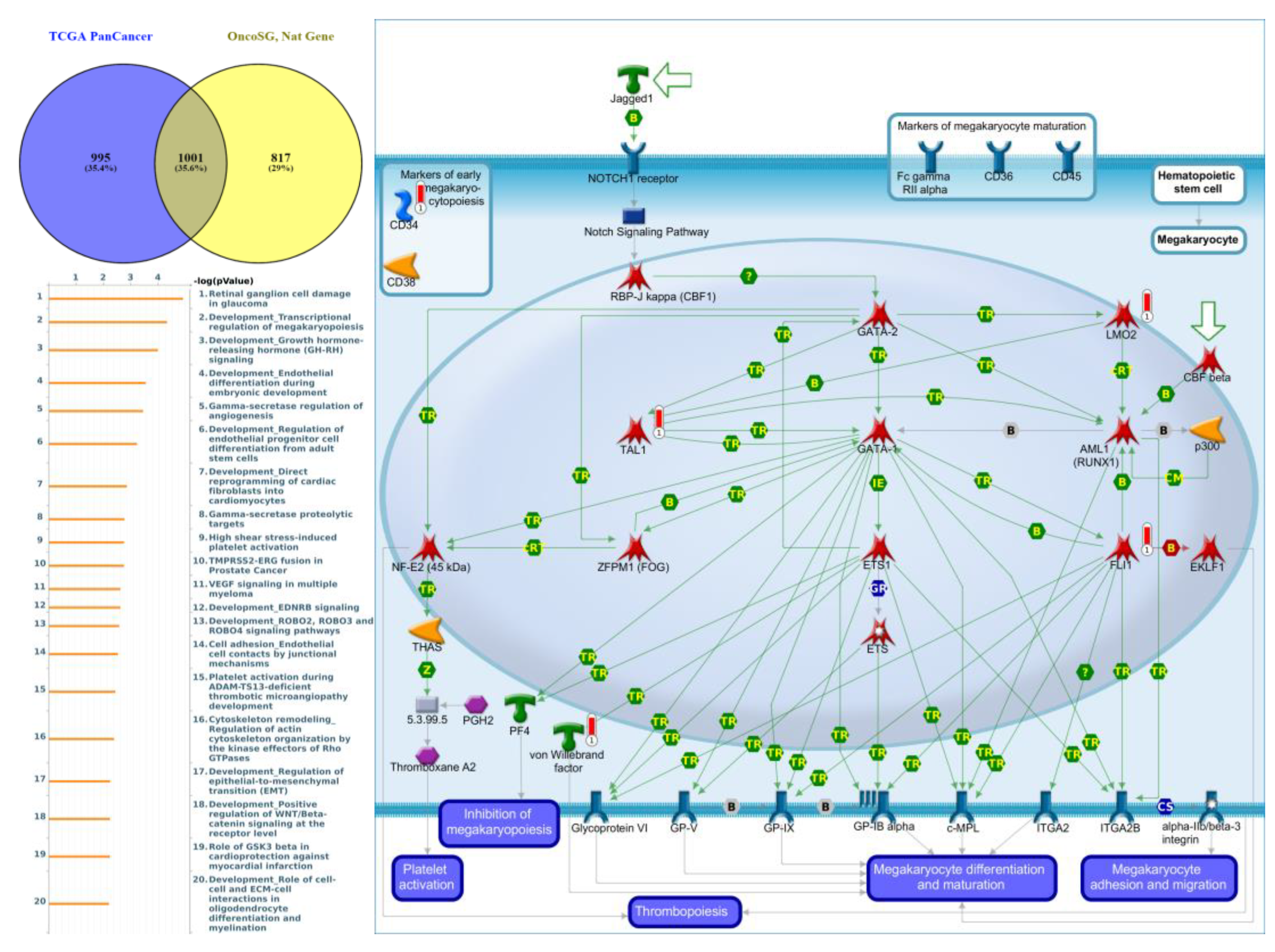
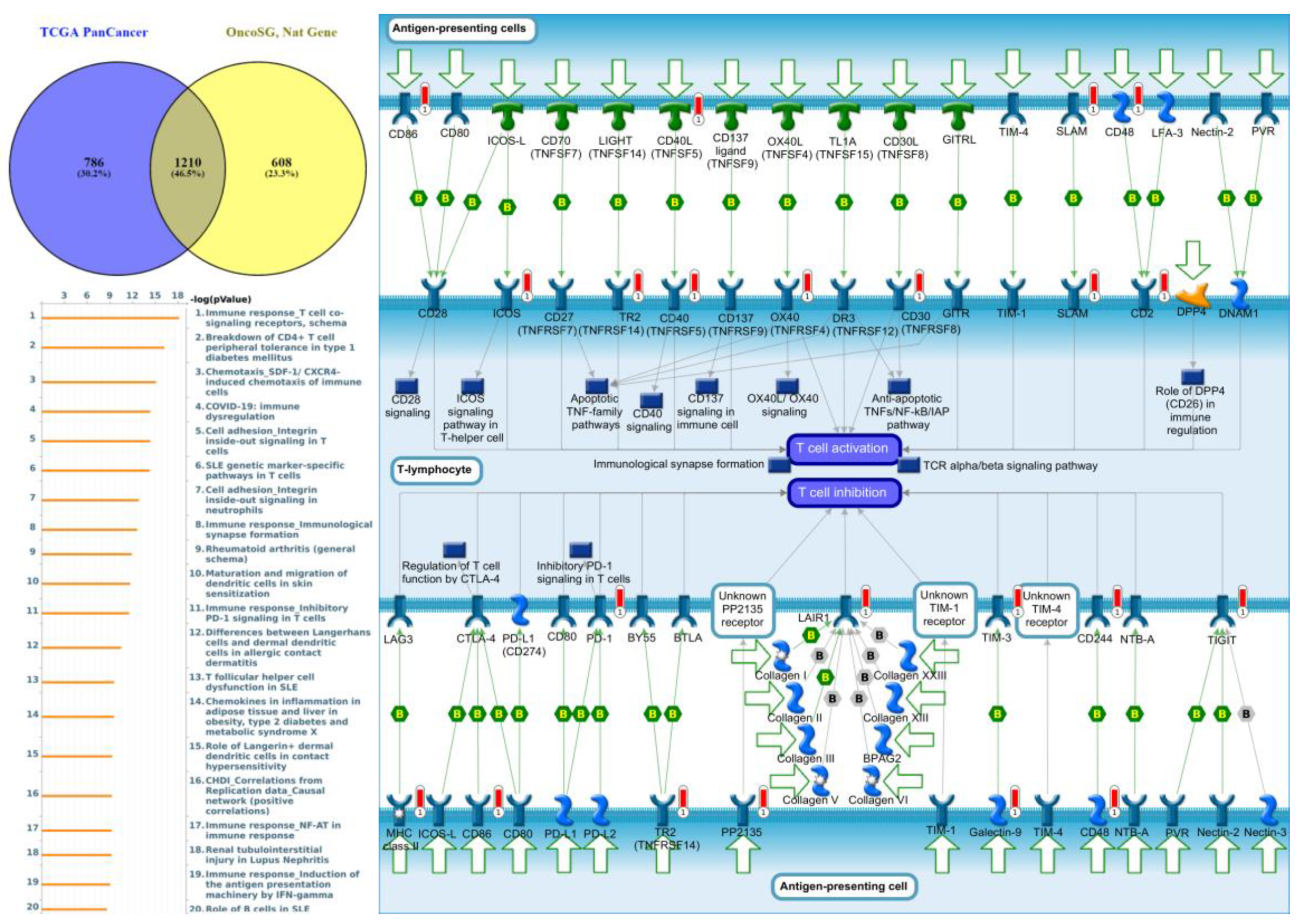
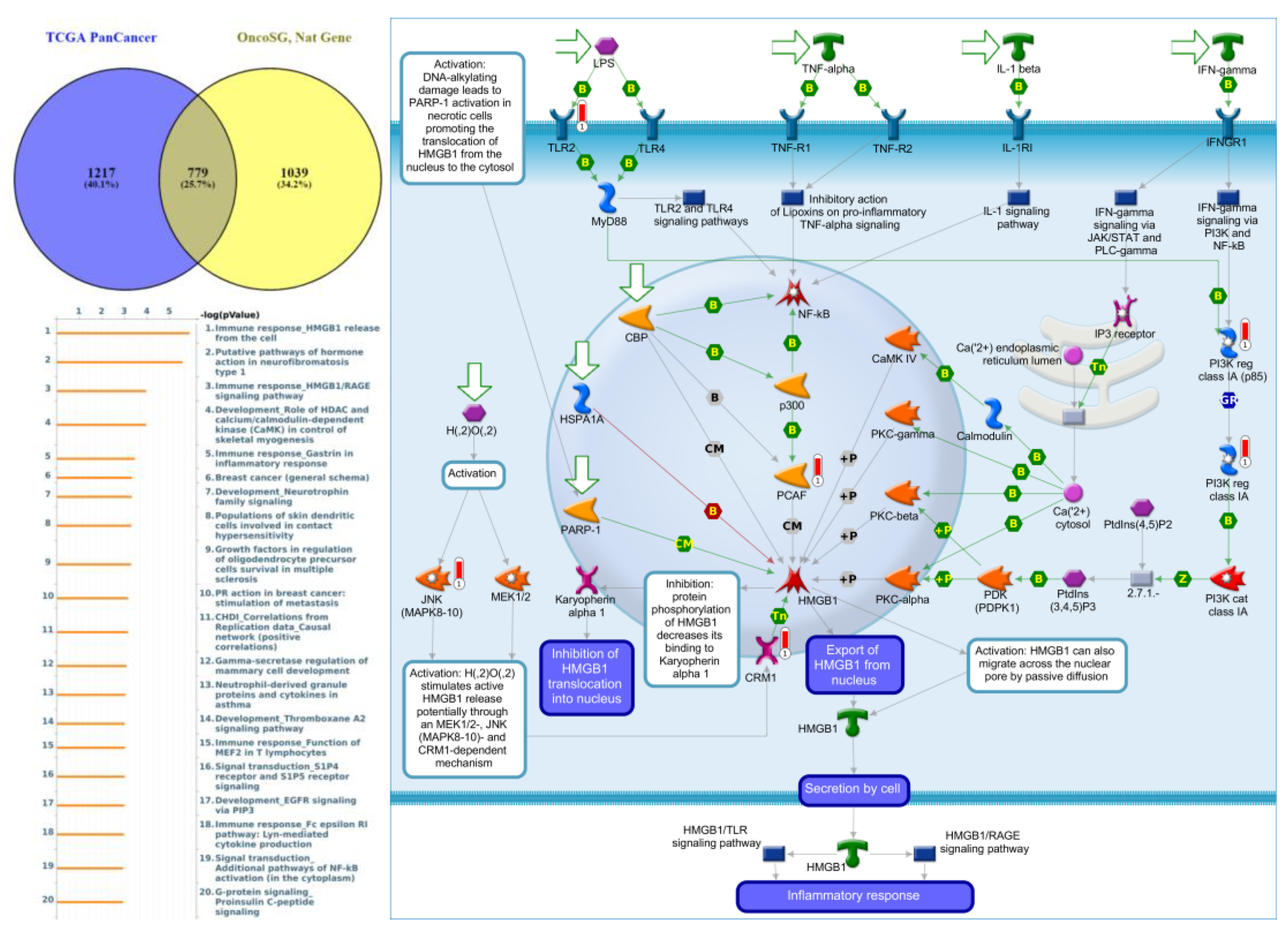
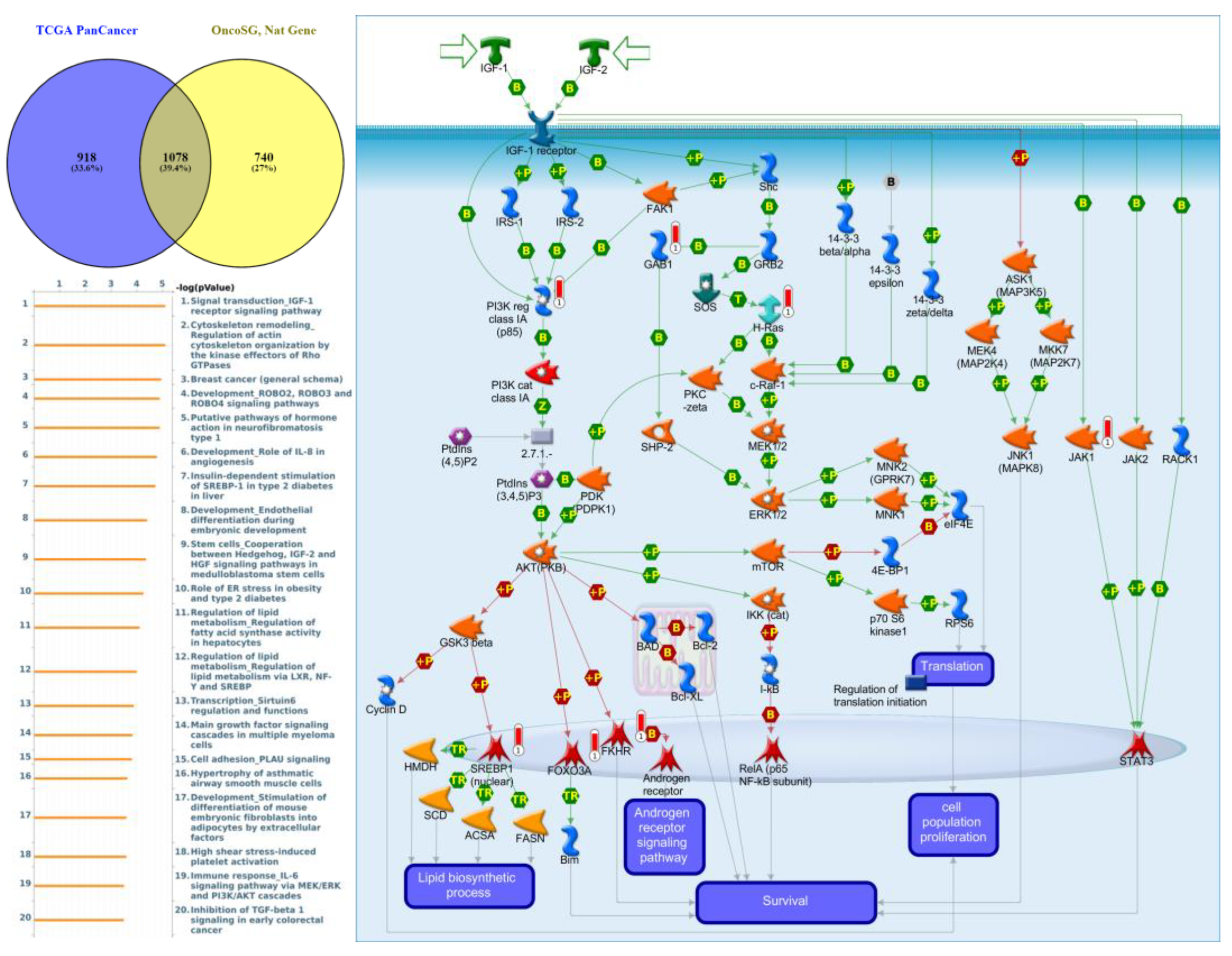
Genes co-expressed with PTPN3 were correlated with “Cell adhesion_Tight junctions”, “Cell adhesion_Endothelial cell contacts by junctional mechanisms”, and “Neurophysiological process_Kappa-type opioid receptor signaling in the central nervous system” (Figure 7, Supplementary Table S4). Genes co-expressed with PTPN5 were correlated with “Retinal ganglion cell damage in glaucoma”, “Development_Transcriptional regulation of megakaryopoiesis”, and “Development_Growth hormone-releasing hormone (GH-RH) signaling” (Figure 8, Supplementary Table S5). Genes co-expressed with PTPN6 were correlated with “Immune response_T cell co-signaling receptors, schema”, “Breakdown of CD4+ T cell peripheral tolerance in type 1 diabetes mellitus”, and “Chemotaxis_SDF-1/CXCR4-induced chemotaxis of immune cells” (Figure 9, Supplementary Table S6). Genes co-expressed with PTPN13 were correlated with “Immune response_HMGB1 release from the cell”, “Putative pathways of hormone action in neurofibromatosis type 1”, and “Immune response_HMGB1/RAGE signaling pathway” (Figure 10, Supplementary Table S7). Genes co-expressed with PTPN21 were correlated with “Signal transduction_IGF-1 receptor signaling pathway”, “Cytoskeleton remodeling_Regulation of actin cytoskeleton organization by the kinase effectors of Rho GTPases”, and “Breast cancer (general schema)” (Figure 11, Supplementary Table S8). Meanwhile, we also use Human Protein Atlas database to detect the protein expression of PTPN family members (Supplementary Figure S1), and validate above data via cBioPortal (Supplementary Figure S2) and GSEA as well as KEGG database (Supplementary Figure S3).
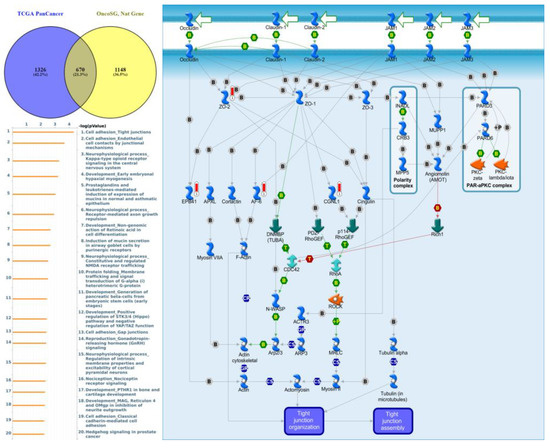
Figure 7.
Expression of the protein tyrosine phosphatase non-receptor type 3 (PTPN3) signaling pathway in lung cancer (from Metacore). The functional analysis of “Cell adhesion_Tight junctions” was correlated with lung cancer development.
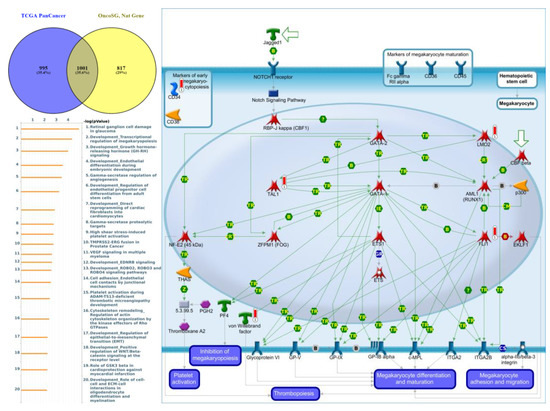
Figure 8.
Expression of the protein tyrosine phosphatase non-receptor type 5 (PTPN5) signaling pathway in lung cancer (from Metacore). The functional analysis of “Development_Transcriptional regulation of megakaryopoiesis” was correlated with lung cancer development.
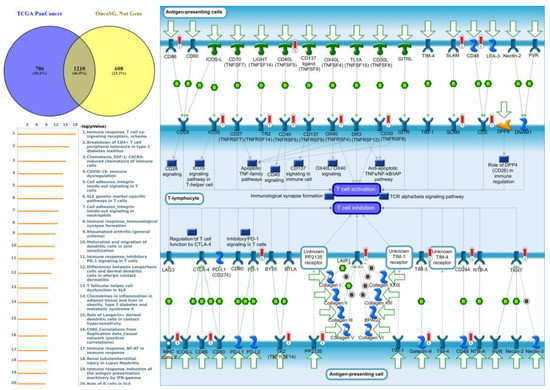
Figure 9.
Expression of the protein tyrosine phosphatase non-receptor type 6 (PTPN6) signaling pathway in lung cancer (from Metacore). The functional analysis of “Immune response_T cell co-signaling receptors, schema” was correlated with lung cancer development.
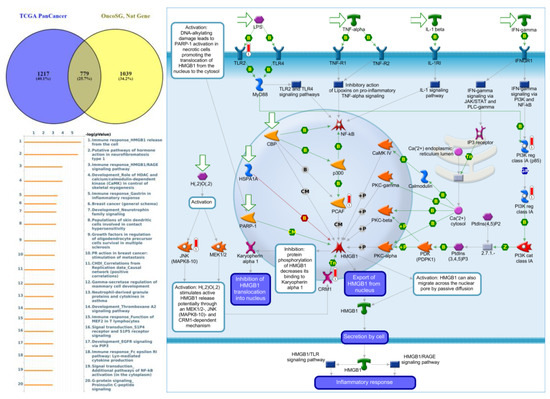
Figure 10.
Expression of the protein tyrosine phosphatase non-receptor type 13 (PTPN13) signaling pathway in lung cancer (from Metacore). The functional analysis of “Immune response_HMGB1 release from the cell” was correlated with lung cancer development.
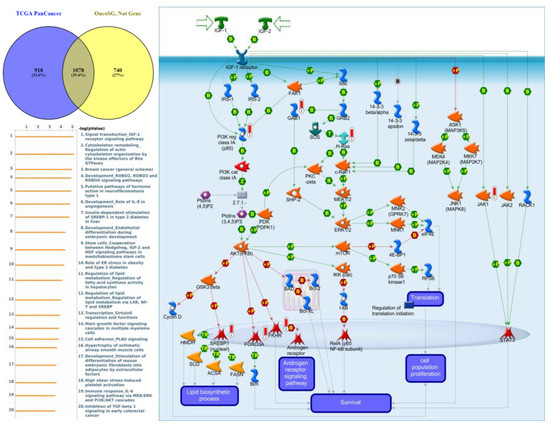
Figure 11.
Expression of the protein tyrosine phosphatase non-receptor type 21 (PTPN21) signaling pathway in lung cancer (from Metacore). The functional analysis of “Signal transduction_IGF-1 receptor signaling pathway” was correlated with lung cancer development.
4. Discussion
Lung cancer is composed of SCLC and NSCLC. NSCLC comprises around 85% of lung cancer cases, and it is further classified into three subtypes, squamous-cell carcinoma, adenocarcinoma, and large-cell carcinoma. LUAD is the main subtype of NSCLC, accounting for 40% of all cases of lung cancer. Therefore, it is especially important to explore effective biomarkers and exploit novel therapeutic developments [41,42,43,44,45]. By applying advances in high-throughput screening for cancer transcriptome profiling [46,47,48,49], alterations of transcriptome patterns of encoded PTPN gene families have been discovered to be significantly associated with several types of malignancies. However, since previous research has yet to elaborate on the roles of PTPN family genes in LUAD, this study may serve as the first and foremost work to specifically examine roles of PTPN individuals in this disease, prior to giving a more extensive and incisive understanding about potential therapeutic and prognostic values in view of the benefits they offer to LUAD patients.
A previous study demonstrated that PPDPF induces hyperactive STAT3 by interfering with STAT3-PTPN1 interactions in LUAD [50]. The diversity of PTPN2’s effects in different types of tumor makes it a potential target for tumor immunotherapy [51]. PTPN3 is a potential target for new cancer immunotherapy that has a dual effect of T cell activation [52]. MiR-375 directly interfered with the expression of PTPN4, which in turn stabilized phosphorylated STAT3 in castration-resistant prostate cancer [53]. Increased expression of PTPN5 was seen in canine oral tumor groups [54]. STAT3 Lys140 hypermethylation caused by a Jmjd1c deletion inhibited interaction with the Ptpn6 phosphatase [55]. PTPN7 is amplified in myeloid malignancies and deleted in lymphoproliferative disorders [56]. miR-126-3p mediated the inhibitory effect of exosomes on A549 cells by negative regulation of PTPN9 in NSCLC cells [57]. Concomitant deletion of Ptpn6 and Ptpn11 in T cells failed to improve anticancer responses [58]. The miR-940/PTPN12 axis could be a potential drug target to treat esophageal squamous cell carcinoma [59]. miR-200b, ZEB2, and PTPN13 are downregulated in colorectal carcinoma with serosal invasion [60]. PTPN14 loss-of-function variants were associated with high risks of cervical cancer and an early age at diagnosis [61]. Nuclear import of PTPN18 inhibited breast cancer metastasis mediated by MVP and importin β2 [62]. PTPN20 was associated with a worse OS of digestive tract cancers [63]. PTPN21 was upregulated in glioma tissues, and a high PTPN21 level predicted poor survival rates in glioma patients [64]. PTPN22 negatively modulates platelet function and thrombus formation [65]. PTPN23 was recently associated with several human epithelial cancers [66].
Our bioinformatics analysis indicated that PTPN gene expressions were found to be involve in tumor multi-stage progression. According to various database analyses, PTPN3, PTPN7, PTPN12, and PTPN14 were expressed at higher levels, while expressions of PTPN1, PTPN5, PTPN6, PTPN9, PTPN12, PTPN13, PTPN18, PTPN21, and PTPN22 were downregulated in lung cancer. Survival curves revealed that LUAD patients with high transcription levels of PTPN1, PTPN5, PTPN6, PTPN13, and PTPN21 were associated with longer OS. These data suggest that PTPN1, PTPN5, PTPN6, PTPN13, and PTPN21 are associated with clinicopathological features of LUAD.
Meanwhile, GO indicated that genes co-expressed with PTPN1, PTPN5, and PTPN21 were significantly highly enriched in the positive regulation of GTPase activity, regulation of small GTPase-mediated signal transduction, response to mechanical stimuli, vasculogenesis, response to estrogen, organ morphogenesis, regulation of stress fiber assembly, MAPK cascade, cell migration involved in sprouting angiogenesis, and positive regulation of angiogenesis. The MetaCore analysis of lung cancer patients also suggested that PTPN3 signaling was correlated with “Cell adhesion_Tight junctions”, PTPN5 signaling was correlated with “Development_Transcriptional regulation of megakaryopoiesis”, PTPN6 signaling was correlated with “Development_Transcriptional regulation of megakaryopoiesis”, PTPN13 signaling was correlated with “Immune response_HMGB1 release from the cell”, and PTPN21 signaling was correlated with “Signal transduction_IGF-1 receptor signaling pathway”.
These results were consistent with previous findings. However, there are a few limitations of our research. The data we analyzed in this study were derived from high throughput databases. Therefore, further cellular studies are required to verify our findings and investigate possible mechanisms, molecular interactions, and clinical applications of various PTPN family genes involved in cancer development.
5. Conclusions
As previous research was yet to elaborate the roles of PTPN family genes in LUAD, this study may serve as the first and foremost work that specifically examined roles of PTPN individuals in this disease, prior to giving a more extensive and incisive understanding of potential therapeutic and prognostic values in view of the benefits they offer to LUAD patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jpm12121947/s1. Figure S1. Protein expression levels of members of the protein tyrosine phosphatase non-receptor type (PTPN) family in lung cancer specimens from the Human Protein Atlas; Figure S2. Correlations between different protein tyrosine phosphatase non-receptor type (PTPN) family members in lung cancer (from cBioPortal); insignificant correlations are marked by crosses; Figure S3. Hallmark and KEGG signaling pathway analysis of protein tyrosine phosphatase non-receptor type (PTPN) family members in lung cancer via the GSEA databse; Table S1 Significant changes in expressions of protein tyrosine phosphatase non-receptor type (PTPN) family members at the transcription level between different types of lung cancer (from the ONCOMINE database); Table S2 Prognostic roles of protein tyrosine phosphatase non-receptor type (PTPN) family members in lung adenocarcinoma (LUAD); Table S3A-D. (C) GO analysis of the protein tyrosine phosphatase non-receptor type (PTPN) family, (D) PTPN1, (E) PTPN5, (F) PTPN21. The top 20 enriched classifications for biological processes, cellular components, and molecular functions are shown (from DAVID); Table S4: Pathway analysis of genes coexpressed with PTPN3 (protein tyrosine phosphatase non-receptor type 3) (from public lung cancer databases using the MetaCore database (with p < 0.05 set as the cutoff value); Table S5: Pathway analysis of genes coexpressed with PTPN5 (protein tyrosine phosphatase non-receptor type 5) from public lung cancer databases using the MetaCore database (with p < 0.05 set as the cutoff value); Table S6: Pathway analysis of genes coexpressed with PTPN6 (protein tyrosine phosphatase non-receptor type 6) from public lung cancer databases using the MetaCore database (with p < 0.05 set as the cutoff value); Table S7: Pathway analysis of genes coexpressed with PTPN13 (protein tyrosine phosphatase non-receptor type 13) from public lung cancer databases using the MetaCore database (with p < 0.05 set as the cutoff value); Table S8: Pathway analysis of genes coexpressed with PTPN21 (protein tyrosine phosphatase non-receptor type 21) from public lung cancer databases using the MetaCore database (with p < 0.05 set as the cutoff value).
Author Contributions
C.-C.W., W.-J.S., G.A., H.D.K.T., D.T.M.X., S.-T.C., C.-F.S., J.-Z.J. and Z.S. performed the bioinformatics analysis, conceived the project, and wrote the manuscript. C.-Y.W. and W.-J.W. performed the data analysis and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from the Ministry of Science and Technology (MOST) of Taiwan (MOST-110-2320-B-039-068 to W.-J.W. and 109-2320-B-038-009-MY2 to C.-Y.W.), National Science and Technology Council of Taiwan (NSTC-111-2314-B-182A-151 to C.-C.W.), Kaohsiung Chang Gung Memorial Hospital (CMRPG8E1661-3, CMRPG8F1441, CMRPG8F1351, CMRPG8H1201, CORPG8F1491-3, CMRPG8K0481-2, and CMRPG8L0521 to C-C.W.), China Medical University (CMU110-MF-47 to W.-J.W.), Taipei Medical University (TMU-108-AE1-B16 to C.-Y.W.), and the TMU Research Center of Cancer Translational Medicine from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan (DP2-111-21121-01-N-10 and DP2-111-21121-01-C-01-01).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors truly appreciate the professional English editing by Daniel P. Chamberlin from the Office of Research and Development at Taipei Medical University. The authors acknowledge the statistical/computational/technical support of the Clinical Data Center, Office of Data Science, Taipei Medical University, Taiwan.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non-small cell lung cancer: Epidemiology, screening, diagnosis, and treatment. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1623–1640. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Cordero, R.; Devine, W.P. Targeted therapy and checkpoint immunotherapy in lung cancer. Surg. Pathol. Clin. 2020, 13, 17–33. [Google Scholar] [CrossRef]
- Chung, C.C.; Huang, T.Y.; Chu, H.R.; De Luca, R.; Candelotti, E.; Huang, C.H.; Yang, Y.S.H.; Incerpi, S.; Pedersen, J.Z.; Lin, C.Y.; et al. Heteronemin and tetrac derivatives suppress non-small cell lung cancer growth via ERK1/2 inhibition. Food Chem. Toxicol. 2022, 161, 112850. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, S.H.; Chen, W.T.; Chung, C.L.; Chou, Y.T.; Lin, S.E.; Hong, S.Y.; Chang, J.H.; Chang, T.H.; Chien, L.N. Comparative survival analysis of platinum-based adjuvant chemotherapy for early-stage squamous cell carcinoma and adenocarcinoma of the lung. Cancer Med. 2022, 11, 2067–2078. [Google Scholar] [CrossRef] [PubMed]
- Kuo, K.T.; Lin, C.H.; Wang, C.H.; Pikatan, N.W.; Yadav, V.K.; Fong, I.H.; Yeh, C.T.; Lee, W.H.; Huang, W.C. HNMT Upregulation Induces Cancer Stem Cell Formation and Confers Protection against Oxidative Stress through Interaction with HER2 in Non-Small-Cell Lung Cancer. Int. J. Mol. Sci. 2022, 23, 1663. [Google Scholar] [CrossRef] [PubMed]
- Tseng, P.-C.; Chen, C.-L.; Lee, K.-Y.; Feng, P.-H.; Wang, Y.-C.; Satria, R.D.; Lin, C.-F. Epithelial-to-mesenchymal transition hinders interferon-γ-dependent immunosurveillance in lung cancer cells. Cancer Lett. 2022, 539, 215712. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Lu, Y.H.; Huang, Y.L.; Huang, S.L.; Chuang, H.C. Air Pollution Effects to the Subtype and Severity of Lung Cancers. Front. Med. 2022, 9, 835026. [Google Scholar] [CrossRef] [PubMed]
- Labbe, D.P.; Hardy, S.; Tremblay, M.L. Protein tyrosine phosphatases in cancer: Friends and foes! Prog. Mol. Biol. Transl. Sci. 2012, 106, 253–306. [Google Scholar] [PubMed]
- Ruckert, M.T.; de Andrade, P.V.; Santos, V.S.; Silveira, V.S. Protein tyrosine phosphatases: Promising targets in pancreatic ductal adenocarcinoma. Cell. Mol. Life Sci. 2019, 76, 2571–2592. [Google Scholar] [CrossRef]
- Zhao, S.; Sedwick, D.; Wang, Z. Genetic alterations of protein tyrosine phosphatases in human cancers. Oncogene 2015, 34, 3885–3894. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Sasin, J.; Bottini, N.; Friedberg, I.; Friedberg, I.; Osterman, A.; Godzik, A.; Hunter, T.; Dixon, J.; Mustelin, T. Protein tyrosine phosphatases in the human genome. Cell 2004, 117, 699–711. [Google Scholar] [CrossRef]
- Yuan, B.; Liu, J.; Cao, J.; Yu, Y.; Zhang, H.; Wang, F.; Zhu, Y.; Xiao, M.; Liu, S.; Ye, Y. PTPN 3 acts as a tumor suppressor and boosts TGF-β signaling independent of its phosphatase activity. EMBO J. 2019, 38, e99945. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, Y.; Liu, H.; Liu, Z.; Mills, C.; Han, Y.; Hung, R.J.; Brhane, Y.; McLaughlin, J.; Brennan, P. Genetic variants of PTPN2 are associated with lung cancer risk: A re-analysis of eight GWASs in the TRICL-ILCCO consortium. Sci. Rep. 2017, 7, 825. [Google Scholar] [CrossRef]
- Zhang, B.D.; Li, Y.R.; Ding, L.D.; Wang, Y.Y.; Liu, H.Y.; Jia, B.Q. Loss of PTPN4 activates STAT3 to promote the tumor growth in rectal cancer. Cancer Sci. 2019, 110, 2258. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Fan, Y.; Gao, Z.; Sun, X.; Zhang, H.; Wang, Z.; Cui, Y.; Song, W.; Wang, Z.; Zhang, F. SHP2 promotes proliferation of breast cancer cells through regulating Cyclin D1 stability via the PI3K/AKT/GSK3β signaling pathway. Cancer Biol. Med. 2020, 17, 707. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for functional genomics data sets-update. Nucleic Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef]
- Lin, J.C.; Liu, T.P.; Yang, P.M. CDKN2A-Inactivated Pancreatic Ductal Adenocarcinoma Exhibits Therapeutic Sensitivity to Paclitaxel: A Bioinformatics Study. J. Clin. Med. 2020, 9, 4019. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Wang, P.W.; Huang, C.H.; Yang, P.M.; Pan, T.L. Characterizing the Relapse Potential in Different Luminal Subtypes of Breast Cancers with Functional Proteomics. Int. J. Mol. Sci. 2020, 21, 6077. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.W.; Hsieh, Y.Y.; Yang, P.M. Bioinformatics Data Mining Repurposes the JAK2 (Janus Kinase 2) Inhibitor Fedratinib for Treating Pancreatic Ductal Adenocarcinoma by Reversing the KRAS (Kirsten Rat Sarcoma 2 Viral Oncogene Homolog)-Driven Gene Signature. J. Pers. Med. 2020, 10, 130. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.M.; Hsieh, Y.Y.; Du, J.L.; Yen, S.C.; Hung, C.F. Sequential Interferon β-Cisplatin Treatment Enhances the Surface Exposure of Calreticulin in Cancer Cells via an Interferon Regulatory Factor 1-Dependent Manner. Biomolecules 2020, 10, 643. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.M.; Lin, L.S.; Liu, T.P. Sorafenib Inhibits Ribonucleotide Reductase Regulatory Subunit M2 (RRM2) in Hepatocellular Carcinoma Cells. Biomolecules 2020, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.; Varambally, S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef]
- Ta, H.D.K.; Minh Xuan, D.T.; Tang, W.C.; Anuraga, G.; Ni, Y.C.; Pan, S.R.; Wu, Y.F.; Fitriani, F.; Putri Hermanto, E.M.; Athoillah, M.; et al. Novel Insights into the Prognosis and Immunological Value of the SLC35A (Solute Carrier 35A) Family Genes in Human Breast Cancer. Biomedicines 2021, 9, 1804. [Google Scholar] [CrossRef]
- Chiao, C.C.; Liu, Y.H.; Phan, N.N.; An Ton, N.T.; Ta, H.D.K.; Anuraga, G.; Minh Xuan, D.T.; Fitriani, F.; Putri Hermanto, E.M.; Athoillah, M.; et al. Prognostic and Genomic Analysis of Proteasome 20S Subunit Alpha (PSMA) Family Members in Breast Cancer. Diagnostics 2021, 11, 2220. [Google Scholar] [CrossRef]
- Xuan, D.T.M.; Wu, C.C.; Kao, T.J.; Ta, H.D.K.; Anuraga, G.; Andriani, V.; Athoillah, M.; Chiao, C.C.; Wu, Y.F.; Lee, K.H.; et al. Prognostic and immune infiltration signatures of proteasome 26S subunit, non-ATPase (PSMD) family genes in breast cancer patients. Aging (Albany NY) 2021, 13, 24882–24913. [Google Scholar] [CrossRef]
- Chou, C.W.; Hsieh, Y.H.; Ku, S.C.; Shen, W.J.; Anuraga, G.; Khoa Ta, H.D.; Lee, K.H.; Lee, Y.C.; Lin, C.H.; Wang, C.Y.; et al. Potential Prognostic Biomarkers of OSBPL Family Genes in Patients with Pancreatic Ductal Adenocarcinoma. Biomedicines 2021, 9, 1601. [Google Scholar] [CrossRef]
- Barretina, J.; Caponigro, G.; Stransky, N.; Venkatesan, K.; Margolin, A.A.; Kim, S.; Wilson, C.J.; Lehár, J.; Kryukov, G.V.; Sonkin, D.; et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 2012, 483, 603–607. [Google Scholar] [CrossRef]
- Anuraga, G.; Wang, W.J.; Phan, N.N.; An Ton, N.T.; Ta, H.D.K.; Berenice Prayugo, F.; Minh Xuan, D.T.; Ku, S.C.; Wu, Y.F.; Andriani, V.; et al. Potential Prognostic Biomarkers of NIMA (Never in Mitosis, Gene A)-Related Kinase (NEK) Family Members in Breast Cancer. J. Pers. Med. 2021, 11, 1089. [Google Scholar] [CrossRef]
- Shahi, P.; Wang, C.Y.; Lawson, D.A.; Slorach, E.M.; Lu, A.; Yu, Y.; Lai, M.D.; Gonzalez Velozo, H.; Werb, Z. ZNF503/Zpo2 drives aggressive breast cancer progression by down-regulation of GATA3 expression. Proc. Natl. Acad. Sci. USA 2017, 114, 3169–3174. [Google Scholar] [CrossRef]
- Shahi, P.; Slorach, E.M.; Wang, C.Y.; Chou, J.; Lu, A.; Ruderisch, A.; Werb, Z. The Transcriptional Repressor ZNF503/Zeppo2 Promotes Mammary Epithelial Cell Proliferation and Enhances Cell Invasion. J. Biol. Chem. 2015, 290, 3803–3813. [Google Scholar] [CrossRef] [PubMed]
- Xuan, D.T.M.; Yeh, I.J.; Wu, C.C.; Su, C.Y.; Liu, H.L.; Chiao, C.C.; Ku, S.C.; Jiang, J.Z.; Sun, Z.; Ta, H.D.K.; et al. Comparison of Transcriptomic Signatures between Monkeypox-Infected Monkey and Human Cell Lines. J. Immunol. Res. 2022, 2022, 3883822. [Google Scholar] [CrossRef] [PubMed]
- Kao, T.J.; Wu, C.C.; Phan, N.N.; Liu, Y.H.; Ta, H.D.K.; Anuraga, G.; Wu, Y.F.; Lee, K.H.; Chuang, J.Y.; Wang, C.Y. Prognoses and genomic analyses of proteasome 26S subunit, ATPase (PSMC) family genes in clinical breast cancer. Aging (Albany NY) 2021, 13, 17970. [Google Scholar] [CrossRef]
- Wang, C.Y.; Chiao, C.C.; Phan, N.N.; Li, C.Y.; Sun, Z.D.; Jiang, J.Z.; Hung, J.H.; Chen, Y.L.; Yen, M.C.; Weng, T.Y.; et al. Gene signatures and potential therapeutic targets of amino acid metabolism in estrogen receptor-positive breast cancer. Am. J. Cancer Res. 2020, 10, 95–113. [Google Scholar] [PubMed]
- Wu, Y.H.; Yeh, I.J.; Phan, N.N.; Yen, M.C.; Hung, J.H.; Chiao, C.C.; Chen, C.F.; Sun, Z.; Hsu, H.P.; Wang, C.Y.; et al. Gene signatures and potential therapeutic targets of Middle East respiratory syndrome coronavirus (MERS-CoV)-infected human lung adenocarcinoma epithelial cells. J. Microbiol. Immunol. Infect. 2021, 54, 845–857. [Google Scholar] [CrossRef]
- Liu, H.L.; Yeh, I.J.; Phan, N.N.; Wu, Y.H.; Yen, M.C.; Hung, J.H.; Chiao, C.C.; Chen, C.F.; Sun, Z.; Jiang, J.Z.; et al. Gene signatures of SARS-CoV/SARS-CoV-2-infected ferret lungs in short- and long-term models. Infect. Genet. Evol. 2020, 85, 104438. [Google Scholar] [CrossRef]
- Huang, T.H.; Mokgautsi, N.; Huang, Y.J.; Wu, A.T.H.; Huang, H.S. Comprehensive Omics Analysis of a Novel Small-Molecule Inhibitor of Chemoresistant Oncogenic Signatures in Colorectal Cancer Cell with Antitumor Effects. Cells 2021, 10, 1970. [Google Scholar] [CrossRef]
- Yadav, V.K.; Huang, Y.J.; George, T.A.; Wei, P.L.; Sumitra, M.R.; Ho, C.L.; Chang, T.H.; Wu, A.T.H.; Huang, H.S. Preclinical Evaluation of the Novel Small-Molecule MSI-N1014 for Treating Drug-Resistant Colon Cancer via the LGR5/β-catenin/miR-142-3p Network and Reducing Cancer-Associated Fibroblast Transformation. Cancers 2020, 12, 1590. [Google Scholar] [CrossRef]
- Lawal, B.; Wang, Y.C.; Wu, A.T.H.; Huang, H.S. Pro-Oncogenic c-Met/EGFR, Biomarker Signatures of the Tumor Microenvironment are Clinical and Therapy Response Prognosticators in Colorectal Cancer, and Therapeutic Targets of 3-Phenyl-2H-benzo[e][1,3]-Oxazine-2,4(3H)-Dione Derivatives. Front. Pharmacol. 2021, 12, 691234. [Google Scholar] [CrossRef]
- Thorat, M.A.; Balasubramanian, R. Breast cancer prevention in high-risk women. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 65, 18–31. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lee, C.H.; Chuang, Y.H.; Lee, J.Y.; Chiu, Y.Y.; Wu Lee, Y.H.; Jong, Y.J.; Hwang, J.K.; Huang, S.H.; Chen, L.C.; et al. Membrane protein-regulated networks across human cancers. Nat. Commun. 2019, 10, 3131. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.T.; Huang, C.S.; Tu, C.C.; Liu, C.Y.; Huang, C.J.; Ho, Y.S.; Tu, S.H.; Tseng, L.M.; Huang, C.C. Multi-gene signature of microcalcification and risk prediction among Taiwanese breast cancer. Sci. Rep. 2020, 10, 18276. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Liao, Y.C.; Ho, Y.S.; Chen, L.C.; Chang, H.W.; Cheng, T.C.; Liu, D.; Lee, W.R.; Shen, S.C.; Wu, C.H.; et al. The α9 Nicotinic Acetylcholine Receptor Mediates Nicotine-Induced PD-L1 Expression and Regulates Melanoma Cell Proliferation and Migration. Cancers 2019, 11, 1991. [Google Scholar] [CrossRef]
- Lee, K.L.; Kuo, Y.C.; Ho, Y.S.; Huang, Y.H. Triple-Negative Breast Cancer: Current Understanding and Future Therapeutic Breakthrough Targeting Cancer Stemness. Cancers 2019, 11, 1334. [Google Scholar] [CrossRef]
- Lawal, B.; Liu, Y.L.; Mokgautsi, N.; Khedkar, H.; Sumitra, M.R.; Wu, A.T.H.; Huang, H.S. Pharmacoinformatics and Preclinical Studies of NSC765690 and NSC765599, Potential STAT3/CDK2/4/6 Inhibitors with Antitumor Activities against NCI60 Human Tumor Cell Lines. Biomedicines 2021, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Lawal, B.; Lee, C.Y.; Mokgautsi, N.; Sumitra, M.R.; Khedkar, H.; Wu, A.T.H.; Huang, H.S. mTOR/EGFR/iNOS/MAP2K1/FGFR/TGFB1 Are Druggable Candidates for N-(2,4-Difluorophenyl)-2′,4′-Difluoro-4-Hydroxybiphenyl-3-Carboxamide (NSC765598), With Consequent Anticancer Implications. Front. Oncol. 2021, 11, 656738. [Google Scholar] [CrossRef]
- Lawal, B.; Kuo, Y.C.; Sumitra, M.R.; Wu, A.T.H.; Huang, H.S. In vivo Pharmacokinetic and Anticancer Studies of HH-N25, a Selective Inhibitor of Topoisomerase I, and Hormonal Signaling for Treating Breast Cancer. J. Inflamm. Res. 2021, 14, 4901–4913. [Google Scholar] [CrossRef] [PubMed]
- Lawal, B.; Kuo, Y.C.; Wu, A.T.H.; Huang, H.S. BC-N102 suppress breast cancer tumorigenesis by interfering with cell cycle regulatory proteins and hormonal signaling, and induction of time-course arrest of cell cycle at G1/G0 phase. Int. J. Biol. Sci. 2021, 17, 3224–3238. [Google Scholar] [CrossRef]
- Zheng, Q.W.; Ni, Q.Z.; Zhu, B.; Liang, X.; Ma, N.; Wang, Y.K.; Xu, S.; Cao, H.J.; Xia, J.; Zhang, F.K.; et al. PPDPF promotes lung adenocarcinoma progression via inhibiting apoptosis and NK cell-mediated cytotoxicity through STAT3. Oncogene 2022, 41, 4244–4256. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Lan, J.; Tang, J.; Luo, N. PTPN2 in the Immunity and Tumor Immunotherapy: A Concise Review. Int. J. Mol. Sci. 2022, 23, 10025. [Google Scholar] [CrossRef]
- Koga, S.; Onishi, H.; Masuda, S.; Fujimura, A.; Ichimiya, S.; Nakayama, K.; Imaizumi, A.; Nishiyama, K.; Kojima, M.; Miyoshi, K.; et al. PTPN3 is a potential target for a new cancer immunotherapy that has a dual effect of T cell activation and direct cancer inhibition in lung neuroendocrine tumor. Transl. Oncol. 2021, 14, 101152. [Google Scholar] [CrossRef] [PubMed]
- Gan, J.; Liu, S.; Zhang, Y.; He, L.; Bai, L.; Liao, R.; Zhao, J.; Guo, M.; Jiang, W.; Li, J.; et al. MicroRNA-375 is a therapeutic target for castration-resistant prostate cancer through the PTPN4/STAT3 axis. Exp. Mol. Med. 2022, 54, 1290–1305. [Google Scholar] [CrossRef] [PubMed]
- Ploypetch, S.; Roytrakul, S.; Phaonakrop, N.; Kittisenachai, S.; Leetanasaksakul, K.; Pisamai, S.; Kalpravidh, C.; Rungsipipat, A.; Suriyaphol, G. In-gel digestion coupled with mass spectrometry (GeLC-MS/MS)-based salivary proteomic profiling of canine oral tumors. BMC Vet. Res. 2020, 16, 335. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Yang, X.; Wu, S.; Ding, X.; Zhu, H.; Long, X.; Wang, Y.; Zhai, S.; Chen, Y.; Che, N.; et al. Jmjd1c demethylates STAT3 to restrain plasma cell differentiation and rheumatoid arthritis. Nat. Immunol. 2022, 23, 1342–1354. [Google Scholar] [CrossRef] [PubMed]
- Jeeves, M.; McClelland, D.M.; Barr, A.J.; Overduin, M. Sequence-specific 1H, 13C and 15N backbone resonance assignments of the 34 kDa catalytic domain of human PTPN7. Biomol. NMR Assign. 2008, 2, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ding, C.; Yang, X.; Zhao, J. BMSCs-Derived Exosomal MiR-126-3p Inhibits the Viability of NSCLC Cells by Targeting PTPN9. J. BU ON Off. J. Balk. Union Oncol. 2021, 26, 1832–1841. [Google Scholar]
- Ventura, P.M.O.; Gakovic, M.; Fischer, B.A.; Spinelli, L.; Rota, G.; Pathak, S.; Khameneh, H.J.; Zenobi, A.; Thomson, S.; Birchmeier, W.; et al. Concomitant deletion of Ptpn6 and Ptpn11 in T cells fails to improve anticancer responses. EMBO Rep. 2022, 23, e55399. [Google Scholar] [CrossRef]
- Niu, Y.; Guo, Y.; Li, Y.; Shen, S.; Liang, J.; Guo, W.; Dong, Z. LncRNA GATA2-AS1 suppresses esophageal squamous cell carcinoma progression via the mir-940/PTPN12 axis. Exp. Cell. Res. 2022, 416, 113130. [Google Scholar] [CrossRef]
- Ranković, B.; Boštjančič, E.; Zidar, N.; Žlajpah, M.; Jeruc, J. miR-200b, ZEB2 and PTPN13 Are Downregulated in Colorectal Carcinoma with Serosal Invasion. Biomedicines 2022, 10, 2149. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, S.; Rheinstein, P.H. PTPN14 Mutations and Cervical Cancer. Cancer Diagn. Progn. 2021, 1, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ba, X.; Zhang, X.; Zhang, N.; Wang, G.; Bai, B.; Li, T.; Zhao, J.; Zhao, Y.; Yu, Y.; et al. Nuclear import of PTPN18 inhibits breast cancer metastasis mediated by MVP and importin β2. Cell Death Dis. 2022, 13, 720. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, X.; Yuan, Y.; Jing, J.J. The expression patterns and the diagnostic/prognostic roles of PTPN family members in digestive tract cancers. Cancer Cell Int. 2020, 20, 238. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Q.; Liu, B.C.; Jiang, X.B.; Gao, W.W.; Rong, B.; Wei, Y.; Wang, F.L.; Zhao, H.K.; Zhang, L. Inhibition of PTPN21 has antitumor effects in glioma by restraining the EGFR/PI3K/AKT pathway. Toxicol. Appl. Pharmacol. 2022, 451, 116180. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, G.; Ding, Y.; Gui, X.; Tong, H.; Xu, X.; Zhang, S.; Sun, Z.; Ju, W.; Li, Y.; et al. Protein tyrosine phosphatase PTPN22 negatively modulates platelet function and thrombus formation. Blood 2022, 140, 1038–1051. [Google Scholar] [CrossRef] [PubMed]
- van der Lely, L.; Häfliger, J.; Montalban-Arques, A.; Bäbler, K.; Schwarzfischer, M.; Sabev, M.; Gottier, C.; Lang, S.; Scharl, M.; Spalinger, M.R. Loss of PTPN23 Promotes Proliferation and Epithelial-to-Mesenchymal Transition in Human Intestinal Cancer Cells. Inflamm. Intest. Dis. 2019, 4, 161–173. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).