Fractional Exhaled Nitric Oxide (FeNO) in Patients with Stable Chronic Obstructive Pulmonary Disease: Short-Term Variability and Potential Clinical Implications
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Study Procedures
2.3. Statistical Analyses
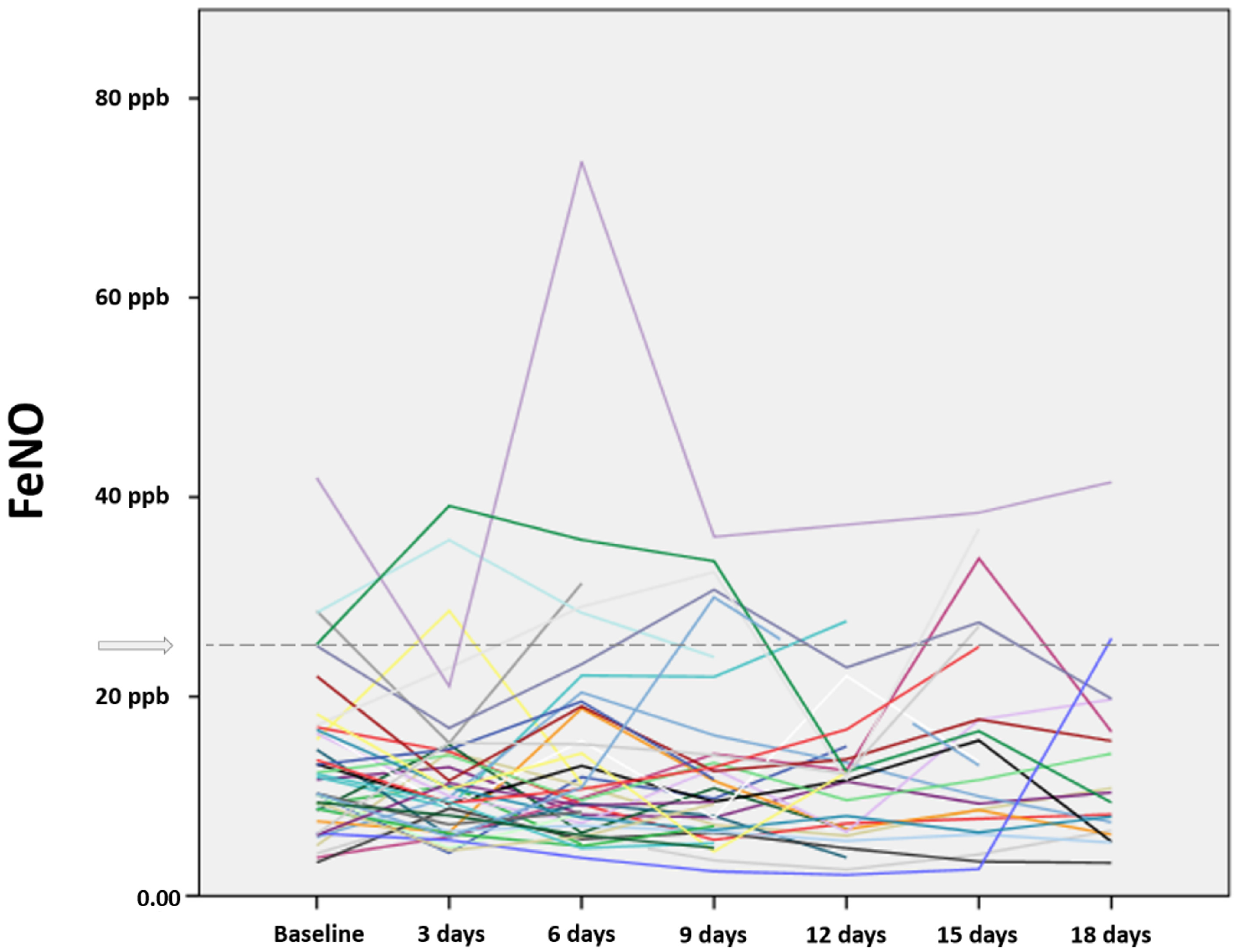
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adeloye, D.; Song, P.; Zhu, Y.; Campbell, H.; Sheikh, A.; Rudan, I. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: A systematic review and modelling analysis. Lancet Respir. Med. 2022, 10, 447–458. [Google Scholar] [CrossRef]
- Vestbo, J.; Hurd, S.S.; Agustí, A.G.; Jones, P.W.; Vogelmeier, C.; Anzueto, A.; Barnes, P.J.; Fabbri, L.M.; Martinez, F.J.; Nishimura, M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2013, 187, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Divo, M.J.; Casanova, C.; Marin, J.M.; Pinto-Plata, V.M.; De-Torres, J.P.; Zulueta, J.J.; Cabrera, C.; Zagaceta, J.; Sanchez-Salcedo, P.; Berto, J.; et al. COPD comorbidities network. Eur. Respir. J. 2015, 46, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Angchaisuksiri, P.; Bassand, J.; Camm, A.J.; Dominguez, H.; Illingworth, L.; Gibbs, H.; Goldhaber, S.Z.; Goto, S.; Jing, Z.-C.; et al. Management and 1-Year Outcomes of Patients with Newly Diagnosed Atrial Fibrillation and Chronic Kidney Disease: Results From the Prospective GARFIELD-AF Registry. J. Am. Heart Assoc. 2019, 8, e010510. [Google Scholar] [CrossRef] [PubMed]
- Ter Beek, L.; Van Der Vaart, H.; Wempe, J.B.; Krijnen, W.P.; Roodenburg, J.L.; Van Der Schans, C.; Jager-Wittenaar, H. Coexistence of malnutrition, frailty, physical frailty and disability in patients with COPD starting a pulmonary rehabilitation program. Clin. Nutr. 2020, 39, 2557–2563. [Google Scholar] [CrossRef]
- Yohannes, A.M. Disability in Patients With COPD. Chest 2014, 145, 200–202. [Google Scholar] [CrossRef]
- Ambrosino, P.; Lupoli, R.; Iervolino, S.; De Felice, A.; Pappone, N.; Storino, A.; Di Minno, M.N.D. Clinical assessment of endothelial function in patients with chronic obstructive pulmonary disease: A systematic review with meta-analysis. Intern. Emerg. Med. 2017, 12, 877–885. [Google Scholar] [CrossRef]
- Poulsen, P.B.; Brogaard, S.L.; Nielsen, M.B.D.; Nielsen, L.U.; Albretsen, T.M.; Bundgaard, M.; Anker, N.; Gustavsen, K.; Lindkvist, R.-M.; Skjoldan, A.; et al. Health care and social care costs of pneumonia in Denmark: A register-based study of all citizens and patients with COPD in three municipalities. Int. J. Chronic Obstr. Pulm. Dis. 2015, 10, 2303–2309. [Google Scholar] [CrossRef][Green Version]
- Negro, R.D.; Tognella, S.; Tosatto, R.; Dionisi, M.; Turco, P.; Donner, C. Costs of chronic obstructive pulmonary disease (COPD) in Italy: The SIRIO study (Social Impact of Respiratory Integrated Outcomes). Respir. Med. 2008, 102, 92–101. [Google Scholar] [CrossRef]
- Merino, M.; Villoro, R.; Hidalgo-Vega, Á.; Carmona, C. Social economic costs of COPD in Extremadura (Spain): An observational study. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 2501–2514. [Google Scholar] [CrossRef]
- Barbosa, M.; Andrade, R.; de Melo, C.A.; Torres, R. Community-Based Pulmonary Rehabilitation Programs in Individuals With COPD. Respir. Care 2022, 67, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Berriet, A.-C.; Beaumont, M.; Peran, L.; Le Ber, C.; Couturaud, F. Effects of pulmonary rehabilitation on fear of falling in Chronic Obstructive Pulmonary Disease (COPD) patients: An observational study. Respir. Med. Res. 2022, 82, 100932. [Google Scholar] [CrossRef] [PubMed]
- Zampogna, E.; Ferriero, G.; Visca, D.; Patrini, M.; Negrini, S.; Arienti, C. An Overview of Cochrane Systematic Reviews for pulmonary rehabilitation interventions in people with COPD: A mapping synthesis. Panminerva Med. 2022; Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Larsson, K. Aspects on pathophysiological mechanisms in COPD. J. Intern. Med. 2007, 262, 311–340. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, A.I.; Wedzicha, J.A. Definition, Causes, Pathogenesis, and Consequences of Chronic Obstructive Pulmonary Disease Exacerbations. Clin. Chest Med. 2020, 41, 421–438. [Google Scholar] [CrossRef]
- Barnes, P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016, 138, 16–27. [Google Scholar] [CrossRef]
- Fernandes, L.; Rane, S.; Mandrekar, S.; Mesquita, A.M. Eosinophilic Airway Inflammation in Patients with Stable Biomass Smoke- versus Tobacco Smoke-Associated Chronic Obstructive Pulmonary Disease. J. Health Pollut. 2019, 9, 191209. [Google Scholar] [CrossRef]
- Oishi, K.; Matsunaga, K.; Shirai, T.; Hirai, K.; Gon, Y. Role of Type2 Inflammatory Biomarkers in Chronic Obstructive Pulmonary Disease. J. Clin. Med. 2020, 9, 2670. [Google Scholar] [CrossRef]
- Taylor, D.R.; Pijnenburg, M.W.; Smith, A.D.; Jongste, J.C.D. Exhaled nitric oxide measurements: Clinical application and interpretation. Thorax 2006, 61, 817–827. [Google Scholar] [CrossRef]
- Jacinto, T.; Malinovschi, A.; Janson, C.; Fonseca, J.; Alving, K. Differential effect of cigarette smoke exposure on exhaled nitric oxide and blood eosinophils in healthy and asthmatic individuals. J. Breath Res. 2017, 11, 036006. [Google Scholar] [CrossRef]
- Barnes, P.J.; Chowdhury, B.; Kharitonov, S.A.; Magnussen, H.; Page, C.P.; Postma, D.; Saetta, M. Pulmonary Biomarkers in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2006, 174, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.W.; Chang, A.B.; Yang, I. Clinical utility of exhaled nitric oxide fraction in the management of asthma and COPD. Breathe 2019, 15, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Maziak, W.; Loukides, S.; Culpitt, S.; Sullivan, P.; Kharitonov, S.A.; Barnes, P.J. Exhaled Nitric Oxide in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 1998, 157, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, M.T.R.; Moragues, M.A.J.; González, R.F.; Rodríguez, V.; Andrés, E.A.; Camino, J.P.Z.; Pareja, R.R.; De La Torre, A.E. Value of Exhaled Nitric Oxide (FeNO) And Eosinophilia During the Exacerbations of Chronic Obstructive Pulmonary Disease Requiring Hospital Admission. COPD J. Chronic Obstr. Pulm. Dis. 2018, 15, 369–376. [Google Scholar] [CrossRef]
- Lu, Z.; Huang, W.; Wang, L.; Xu, N.; Ding, Q.; Cao, C. Exhaled nitric oxide in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 2695–2705. [Google Scholar] [CrossRef]
- Rouhos, A.; Kainu, A.; Piirilä, P.; Sarna, S.; Lindqvist, A.; Karjalainen, J.; Sovijärvi, A.R.A. Repeatability of exhaled nitric oxide measurements in patients with COPD. Clin. Physiol. Funct. Imaging 2010, 31, 26–31. [Google Scholar] [CrossRef]
- de Laurentiis, G.; Maniscalco, M.; Cianciulli, F.; Stanziola, A.; Marsico, S.; Lundberg, J.O.; Weitzberg, E.; Sofia, M. Exhaled nitric oxide monitoring in COPD using a portable analyzer. Pulm. Pharmacol. Ther. 2008, 21, 689–693. [Google Scholar] [CrossRef]
- Maniscalco, M.; Di Mauro, V.; Farinaro, E.; Carratù, L.; Sofia, M. Transient Decrease of Exhaled Nitric Oxide after Acute Exposure to Passive Smoke in Healthy Subjects. Arch. Environ. Health Int. J. 2002, 57, 437–440. [Google Scholar] [CrossRef]
- Montella, S.; Alving, K.; Maniscalco, M.; Sofia, M.; De Stefano, S.; Raia, V.; Santamaria, F. Measurement of nasal nitric oxide by hand-held and stationary devices. Eur. J. Clin. Investig. 2011, 41, 1063–1070. [Google Scholar] [CrossRef]
- Qaseem, A.; Wilt, T.J.; Weinberger, S.E.; Hanania, N.A.; Criner, G.; Van Der Molen, T.; Marciniuk, D.D.; Denberg, T.; Schünemann, H.; Wedzicha, W.; et al. Diagnosis and Management of Stable Chronic Obstructive Pulmonary Disease: A Clinical Practice Guideline Update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann. Intern. Med. 2011, 155, 179–191. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Tammeling, G.J.; Cotes, J.E.; Pedersen, O.F.; Peslin, R.; Yernault, J.C. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur. Respir. J. Suppl. 1993, 16, 5–40. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; Van Der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Wanger, J.; Clausen, J.L.; Coates, A.; Pedersen, O.F.; Brusasco, V.; Burgos, F.; Casaburi, R.; Crapo, R.; Enright, P.; Van Der Grinten, C.P.M.; et al. Standardisation of the measurement of lung volumes. Eur. Respir. J. 2005, 26, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Hernandes, N.A.; Wouters, E.F.M.; Meijer, K.; Annegarn, J.; Pitta, F.; Spruit, M.A. Reproducibility of 6-minute walking test in patients with COPD. Eur. Respir. J. 2010, 38, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Molino, A.; Fuschillo, S.; Mosella, M.; Accardo, M.; Guida, P.; Motta, A.; Maniscalco, M. Comparison of three different exhaled nitric oxide analyzers in chronic respiratory disorders. J. Breath Res. 2019, 13, 021002. [Google Scholar] [CrossRef]
- Dweik, R.A.; Boggs, P.B.; Erzurum, S.C.; Irvin, C.G.; Leigh, M.W.; Lundberg, J.O.; Olin, A.-C.; Plummer, A.L.; Taylor, D.R. American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. An Official ATS Clinical Practice Guideline: Interpretation of Exhaled Nitric Oxide Levels (FeNO) for Clinical Applications. Am. J. Respir. Crit. Care Med. 2011, 184, 602–615. [Google Scholar] [CrossRef]
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, m., and Prevention of CHRONIC obstructive Pulmonary Disease 2020 Report. Available online: http://www.goldcopd.org (accessed on 6 September 2022).
- Arron, J.R.; Izuhara, K. Asthma biomarkers: What constitutes a ‘gold standard’? Thorax 2014, 70, 105–107. [Google Scholar] [CrossRef]
- Gao, J.; Wu, F. Association between fractional exhaled nitric oxide, sputum induction and peripheral blood eosinophil in uncontrolled asthma. Allergy Asthma Clin. Immunol. 2018, 14, 21. [Google Scholar] [CrossRef]
- Heffler, E.; Carpagnano, G.E.; Favero, E.; Guida, G.; Maniscalco, M.; Motta, A.; Paoletti, G.; Rolla, G.; Baraldi, E.; Pezzella, V.; et al. Fractional Exhaled Nitric Oxide (FENO) in the management of asthma: A position paper of the Italian Respiratory Society (SIP/IRS) and Italian Society of Allergy, Asthma and Clinical Immunology (SIAAIC). Multidiscip. Respir. Med. 2020, 15, 36. [Google Scholar] [CrossRef]
- Couillard, S.; Larivée, P.; Courteau, J.; Vanasse, A. Eosinophils in COPD Exacerbations Are Associated With Increased Readmissions. Chest 2017, 151, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Patel, A.R.; Singh, S.; Singh, S.; Khawaja, I. Global Initiative for Chronic Obstructive Lung Disease: The Changes Made. Cureus 2019, 11, e4985. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative on Asthma. Available online: www.ginasthma.org (accessed on 24 June 2022).
- Yun, J.; Lamb, A.; Chase, R.; Singh, D.; Parker, M.M.; Saferali, A.; Vestbo, J.; Tal-Singer, R.; Castaldi, P.J.; Silverman, E.K.; et al. Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2018, 141, 2037–2047.e10. [Google Scholar] [CrossRef]
- Bafadhel, M.; McKenna, S.; Terry, S.; Mistry, V.; Reid, C.; Haldar, P.; McCormick, M.; Haldar, K.; Kebadze, T.; Duvoix, A.; et al. Acute Exacerbations of Chronic Obstructive Pulmonary Disease: Identification of biologic clusters and their biomarkers. Am. J. Respir. Crit. Care Med. 2011, 184, 662–671. [Google Scholar] [CrossRef]
- Siva, R.; Green, R.H.; Brightling, C.; Shelley, M.; Hargadon, B.; McKenna, S.; Monteiro, W.; Berry, M.; Parker, D.; Wardlaw, A.; et al. Eosinophilic airway inflammation and exacerbations of COPD: A randomised controlled trial. Eur. Respir. J. 2007, 29, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Fieldes, M.; Bourguignon, C.; Assou, S.; Nasri, A.; Fort, A.; Vachier, I.; De Vos, J.; Ahmed, E.; Bourdin, A. Targeted therapy in eosinophilic chronic obstructive pulmonary disease. ERJ Open Res. 2021, 7, 00437. [Google Scholar] [CrossRef]
- Mjosberg, J.M.; Trifari, S.; Crellin, N.K.; Peters, C.P.; Van Drunen, C.M.; Piet, B.; Fokkens, W.J.; Cupedo, T.; Spits, H. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat. Immunol. 2011, 12, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Tao, S.; Zhang, S.; Wang, J.; Zhang, F.; Li, F.; Ding, J. Type 2 innate lymphoid cells participate in IL-33-stimulated Th2-associated immune response in chronic obstructive pulmonary disease. Exp. Ther. Med. 2019, 18, 3109–3116. [Google Scholar] [CrossRef]
- McKenzie, A.N.J. Type-2 Innate Lymphoid Cells in Asthma and Allergy. Ann. Am. Thorac. Soc. 2014, 11, S263–S270. [Google Scholar] [CrossRef]
- Silver, J.S.; Kearley, J.; Copenhaver, A.; Sanden, C.; Mori, M.; Yu, L.; Pritchard, G.H.; Berlin, A.A.; Hunter, C.A.; Bowler, R.; et al. Erratum: Inflammatory triggers associated with exacerbations of COPD orchestrate plasticity of group 2 innate lymphoid cells in the lungs. Nat. Immunol. 2016, 17, 1005. [Google Scholar] [CrossRef]
- Karta, M.R.; Broide, D.H.; Doherty, T.A. Insights into Group 2 Innate Lymphoid Cells in Human Airway Disease. Curr. Allergy Asthma Rep. 2016, 16, 8. [Google Scholar] [CrossRef] [PubMed]
- Donovan, T.; Milan, S.J.; Wang, R.; Banchoff, E.; Bradley, P.; Crossingham, I. Anti-IL-5 therapies for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2020, 12, CD013432. [Google Scholar] [CrossRef]
- Ortega, H.G.; Yancey, S.W.; Mayer, B.; Gunsoy, N.B.; Keene, O.N.; Bleecker, E.R.; Brightling, C.E.; Pavord, I.D. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: A secondary analysis of the DREAM and MENSA studies. Lancet Respir. Med. 2016, 4, 549–556. [Google Scholar] [CrossRef]
- Bleecker, E.R.; FitzGerald, J.M.; Chanez, P.; Papi, A.; Weinstein, S.F.; Barker, P.; Sproule, S.; Gilmartin, G.; Aurivillius, M.; Werkström, V.; et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): A randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016, 388, 2115–2127. [Google Scholar] [CrossRef]
- Dasgupta, A.; Kjarsgaard, M.; Capaldi, D.; Radford, K.; Aleman, F.; Boylan, C.; Altman, L.C.; Wight, T.N.; Parraga, G.; O’Byrne, P.M.; et al. A pilot randomised clinical trial of mepolizumab in COPD with eosinophilic bronchitis. Eur. Respir. J. 2017, 49, 1602486. [Google Scholar] [CrossRef] [PubMed]
- Sciurba, F.C.; Bradford, E.S.; Pavord, I.D.; Xia, Y.; Li, W.; Shen, H.; Shahlavi-Monavvar, P.; Mobasher-Jannat, A. Mepolizumab for Eosinophilic COPD. N. Engl. J. Med. 2018, 378, 680–683. [Google Scholar] [CrossRef]
- Pavord, I.D.; Chanez, P.; Criner, G.J.; Kerstjens, H.A.; Korn, S.; Lugogo, N.; Martinot, J.-B.; Sagara, H.; Albers, F.C.; Bradford, E.S.; et al. Mepolizumab for Eosinophilic Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2017, 377, 1613–1629. [Google Scholar] [CrossRef]
- Criner, G.J.; Celli, B.R.; Brightling, C.E.; Agusti, A.; Papi, A.; Singh, D.; Sin, D.D.; Vogelmeier, C.F.; Sciurba, F.C.; Bafadhel, M.; et al. Benralizumab for the Prevention of COPD Exacerbations. N. Engl. J. Med. 2019, 381, 1023–1034. [Google Scholar] [CrossRef]
- Criner, G.J.; Celli, B.R.; Singh, D.; Agusti, A.; Papi, A.; Jison, M.; Makulova, N.; Shih, V.H.; Brooks, L.; Barker, P.; et al. Predicting response to benralizumab in chronic obstructive pulmonary disease: Analyses of GALATHEA and TERRANOVA studies. Lancet Respir. Med. 2019, 8, 158–170. [Google Scholar] [CrossRef]
- Annangi, S.; Nutalapati, S.; Sturgill, J.; Flenaugh, E.; Foreman, M. Eosinophilia and fractional exhaled nitric oxide levels in chronic obstructive lung disease. Thorax 2021, 77, 351–356. [Google Scholar] [CrossRef]
- Su, K.-C.; Ko, H.-K.; Hsiao, Y.-H.; Chou, K.-T.; Chen, Y.-W.; Yu, W.-K.; Pan, S.-W.; Feng, J.-Y.; Perng, D.-W. Fractional Exhaled Nitric Oxide Guided-Therapy in Chronic Obstructive Pulmonary Disease: A Stratified, Randomized, Controlled Trial. Arch. De Bronconeumol. 2022, 58, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.-S.; Rani, F.A.; Tan, L.-E. Response of exhaled nitric oxide to inhaled corticosteroids in patients with stable COPD: A systematic review and meta-analysis. Clin. Respir. J. 2016, 12, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Escamilla-Gil, J.M.; Fernandez-Nieto, M.; Acevedo, N. Understanding the Cellular Sources of the Fractional Exhaled Nitric Oxide (FeNO) and Its Role as a Biomarker of Type 2 Inflammation in Asthma. BioMed Res. Int. 2022, 2022, 5753524. [Google Scholar] [CrossRef] [PubMed]
- Loewenthal, L.; Menzies-Gow, A. FeNO in Asthma. Semin. Respir. Crit. Care Med. 2022, 43, 635–645. [Google Scholar] [CrossRef]
- Mostafavi-Pour-Manshadi, S.-M.; Naderi, N.; Barrecheguren, M.; Dehghan, A.; Bourbeau, J. Investigating Fractional Exhaled Nitric Oxide in Chronic Obstructive Pulmonary Disease (COPD) and Asthma-COPD Overlap (ACO): A Scoping Review. COPD J. Chronic Obstr. Pulm. Dis. 2018, 15, 377–391. [Google Scholar] [CrossRef]
- Uppalapati, A.; Gogineni, S.; Espiritu, J.R. Association between Body Mass Index (BMI) and fraction of exhaled nitric oxide (FeNO) levels in the National Health and Nutrition Examination Survey (NHANES) 2007–2010. Obes. Res. Clin. Pract. 2016, 10, 652–658. [Google Scholar] [CrossRef]
- Maniscalco, M.; Zedda, A.; Faraone, S.; Cristiano, S.; Sofia, M.; Motta, A. Low alveolar and bronchial nitric oxide in severe uncomplicated obesity. Obes. Res. Clin. Pract. 2015, 9, 603–608. [Google Scholar] [CrossRef]
- Maniscalco, M.; de Laurentiis, G.; Zedda, A.; Faraone, S.; Giardiello, C.; Cristiano, S.; Sofia, M. Exhaled nitric oxide in severe obesity: Effect of weight loss. Respir. Physiol. Neurobiol. 2007, 156, 370–373. [Google Scholar] [CrossRef]
- Lee, H.; Shin, S.H.; Gu, S.; Zhao, D.; Kang, D.; Joi, Y.R.; Suh, G.Y.; Pastor-Barriuso, R.; Guallar, E.; Cho, J.; et al. Racial differences in comorbidity profile among patients with chronic obstructive pulmonary disease. BMC Med. 2018, 16, 178. [Google Scholar] [CrossRef]
| Variable | Overall | FeNO ≤ 25 ppb | FeNO > 25 ppb | p-Value |
|---|---|---|---|---|
| 41 | 28 | 13 | ||
| Demographic | ||||
| Age, years | 72.9 ± 7.4 | 74.2 ± 6.7 | 70.1 ± 8.2 | 0.098 |
| Males, n (%) | 36 (87.8) | 24 (85.7) | 12 (92.3) | 0.930 |
| Anthropometric | ||||
| Height, cm | 163.6 ± 5.5 | 163.6 ± 5.7 | 163.5 ± 5.3 | 0.955 |
| Weight, kg | 76.5 ± 16.6 | 80.0 ± 18.2 | 69.0 ± 8.8 | 0.013 |
| BMI, kg/m2 | 28.1 (24.1–32.2) | 29.7 ± 6.5 | 25.9 ± 3.7 | 0.026 |
| Pulmonary function tests (PFTs) | ||||
| FEV1, L | 1.17 ± 0.38 | 1.19 ± 0.33 | 1.13 ± 0.49 | 0.740 |
| FEV1, % predicted | 49.0 ± 14.4 | 51.1 ± 14.4 | 44.3 ± 13.9 | 0.161 |
| FVC, L | 2.59 ± 0.66 | 2.55 ± 0.63 | 2.66 ± 0.72 | 0.651 |
| FVC, % predicted | 79.0 (70.0–95.5) | 83.5 ±16.9 | 81.7 ± 14.3 | 0.731 |
| FEV1/FVC | 45.9 ±12.5 | 47.7 ± 12.5 | 41.9 ± 11.9 | 0.169 |
| GOLD stage | ||||
| Group C, n (%) | 9 (21.9) | 7 (25.0) | 2 (15.4) | 0.774 |
| Group D, n (%) | 32 (78.1) | 28 (75.0) | 11 (84.6) | 0.774 |
| Blood gas analysis | ||||
| SpO2, % | 94.0 (91.8–95.0) | 93.5 (91.8–95.0) | 94.0 (90.1–95.0) | 0.631 |
| PaO2, mmHg | 60.1 ± 9.1 | 59.6 ± 8.4 | 60.9 ± 10.7 | 0.775 |
| PaCO2, mmHg | 42.7 ± 4.1 | 42.7 ± 4.4 | 42.9 ± 4.0 | 0.924 |
| pH | 7.42 ± 0.04 | 7.42 ± 0.04 | 7.42 ± 0.03 | 0.887 |
| Laboratory parameters | ||||
| Leucocytes, 103/µL | 7.90 (6.35–9.40) | 8.20 (6.80–9.40) | 7.20 (5.75–9.95) | 0.468 |
| Neutrophils, 103/µL | 4.95 ± 1.92 | 4.99 ± 1.78 | 4.87 ± 2.28 | 0.859 |
| Neutrophils, % | 61.0 ± 12.5 | 61.0 ± 12.8 | 61.1 ± 12.2 | 0.985 |
| Basophils, 103/µL | 0.05 (0.01–0.08) | 0.05 (0.01–0.08) | 0.05 (0.01–0.06) | 0.497 |
| Basophils, % | 0 (0–1.00) | 0 (0–1.00) | 0 (0–1.00) | 0.879 |
| Monocytes, 103/µL | 0.68 (0.59–0.83) | 0.69 (0.59–0.83) | 0.66 (0.60–0.85) | 1.000 |
| Monocytes, % | 9.17 ± 2.93 | 8.86 ± 3.03 | 9.85 ± 2.70 | 0.304 |
| Eosinophils, 103/µL | 0.19 (0.09–0.32) | 0.18 (0.09–0.29) | 0.19 (0.11–0.40) | 0.570 |
| Eosinophils, % | 3.00 (1.00–3.50) | 3.00 (1.00–3.00) | 3.00 (1.50–4.50) | 0.497 |
| Lymphocytes, 103/µL | 1.79 (1.49–2.34) | 1.90 (1.51–2.43) | 1.70 (1.31–2.03) | 0.216 |
| Lymphocytes, % | 26.0 (18.0–33.5) | 25.0 (18.3–33.8) | 26.0 (14.5–34.0) | 0.446 |
| CRP, mg/dL | 9.8 (5.6–17.4) | 9.8 (5.6–15.6) | 11.8 (5.5–20.3) | 0.642 |
| Pharmacological therapy | ||||
| ICS, n (%) | 25 (61.0) | 16 (57.1) | 9 (69.2) | 0.693 |
| FP equivalent dose, µg/day | 1000 (500–1000) | 1000 (500–1000) | 1000 (750–1000) | 0.718 |
| LABA, n (%) | 31 (75.6) | 22 (78.6) | 9 (69.2) | 0.797 |
| LAMA, n (%) | 31 (75.6) | 21 (75.0) | 10 (76.9) | 1.000 |
| Exercise capacity | ||||
| 6MWD, meters | 175.8 ± 43.7 | 171.0 ± 47.4 | 185.8 ± 34.3 | 0.272 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrosino, P.; Fuschillo, S.; Accardo, M.; Mosella, M.; Molino, A.; Spedicato, G.A.; Motta, A.; Maniscalco, M. Fractional Exhaled Nitric Oxide (FeNO) in Patients with Stable Chronic Obstructive Pulmonary Disease: Short-Term Variability and Potential Clinical Implications. J. Pers. Med. 2022, 12, 1906. https://doi.org/10.3390/jpm12111906
Ambrosino P, Fuschillo S, Accardo M, Mosella M, Molino A, Spedicato GA, Motta A, Maniscalco M. Fractional Exhaled Nitric Oxide (FeNO) in Patients with Stable Chronic Obstructive Pulmonary Disease: Short-Term Variability and Potential Clinical Implications. Journal of Personalized Medicine. 2022; 12(11):1906. https://doi.org/10.3390/jpm12111906
Chicago/Turabian StyleAmbrosino, Pasquale, Salvatore Fuschillo, Mariasofia Accardo, Marco Mosella, Antonio Molino, Giorgio Alfredo Spedicato, Andrea Motta, and Mauro Maniscalco. 2022. "Fractional Exhaled Nitric Oxide (FeNO) in Patients with Stable Chronic Obstructive Pulmonary Disease: Short-Term Variability and Potential Clinical Implications" Journal of Personalized Medicine 12, no. 11: 1906. https://doi.org/10.3390/jpm12111906
APA StyleAmbrosino, P., Fuschillo, S., Accardo, M., Mosella, M., Molino, A., Spedicato, G. A., Motta, A., & Maniscalco, M. (2022). Fractional Exhaled Nitric Oxide (FeNO) in Patients with Stable Chronic Obstructive Pulmonary Disease: Short-Term Variability and Potential Clinical Implications. Journal of Personalized Medicine, 12(11), 1906. https://doi.org/10.3390/jpm12111906










