Potential Plasma Metabolite Biomarkers of Diabetic Nephropathy: Untargeted Metabolomics Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Blood Sample Collection and Processing
2.3. Mass Spectrometry Analysis
2.4. Mass Spectra Processing
2.5. Data Analysis and Statistics
2.6. Metabolite Annotation
3. Results
3.1. Cohort Characteristics
3.2. Mass Spectrometry Data Analysis of Plasma in T1D Patients with or without DN
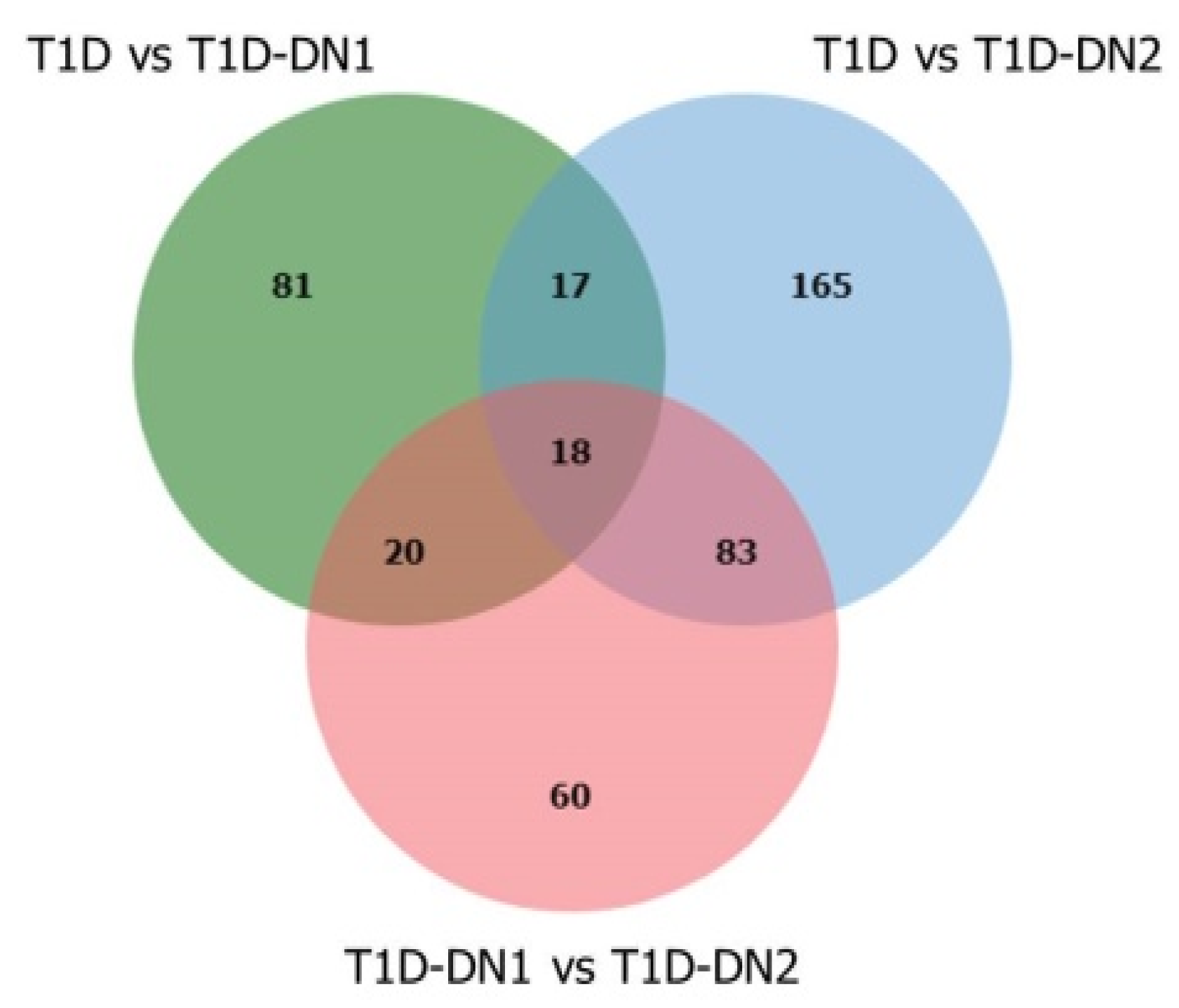
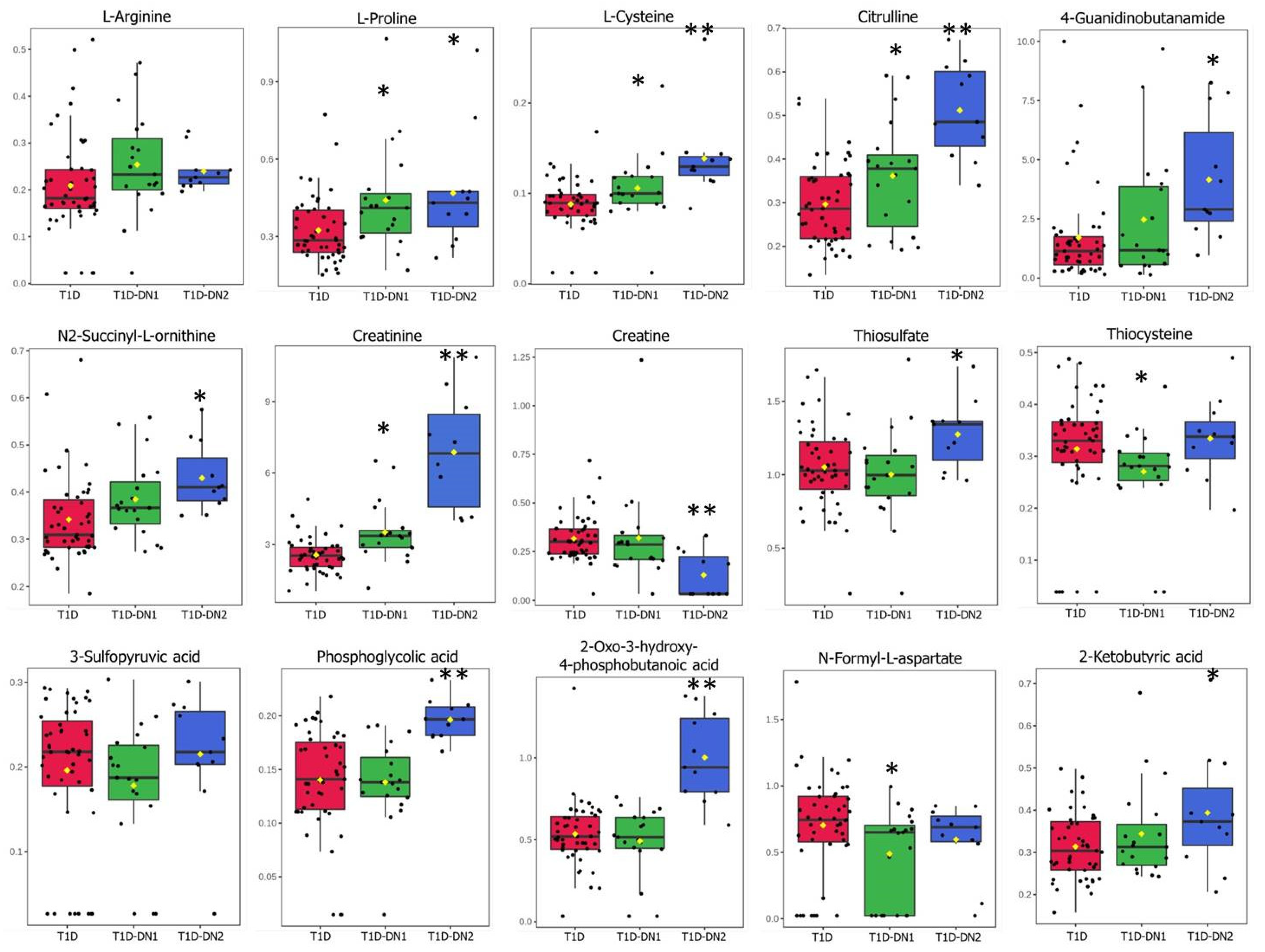
3.3. Metabolites and Metabolite Pathways Dysregulated during DN
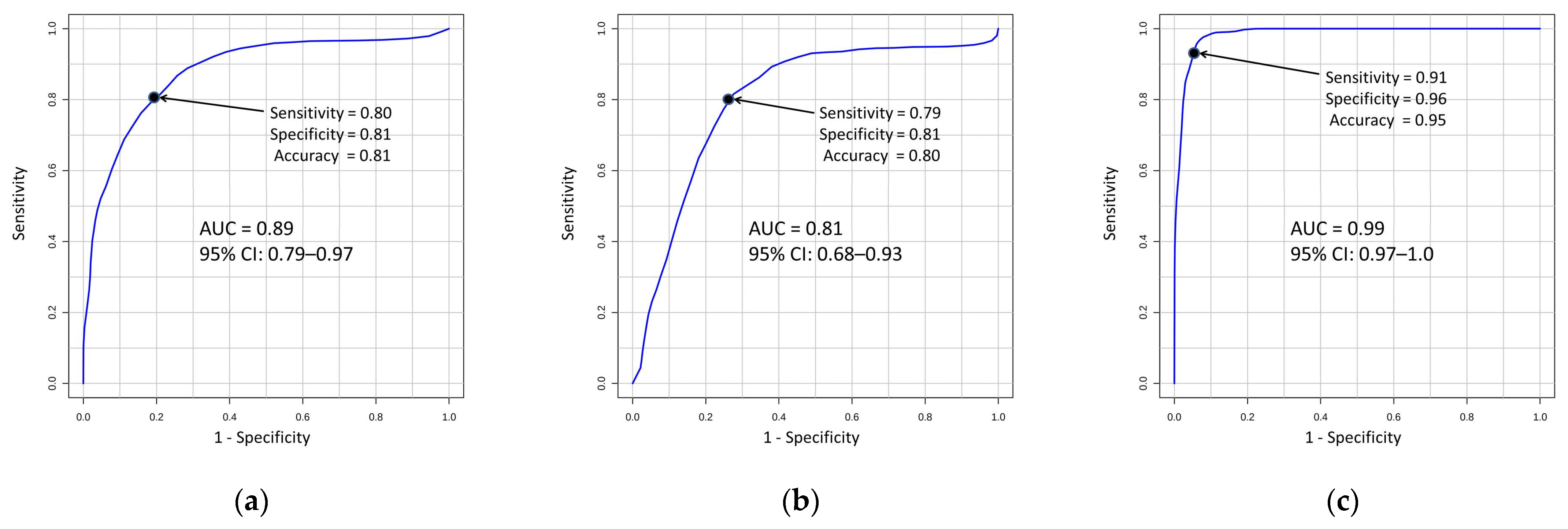
3.4. Metabolite Signatures for DN Diagnosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pérez-Sáez, M.J.; Pascual, J. Kidney Transplantation in the Diabetic Patient. J. Clin. Med. 2015, 4, 1269. [Google Scholar] [CrossRef] [PubMed]
- Himmelfarb, J.; Vanholder, R.; Mehrotra, R.; Tonelli, M. The current and future landscape of dialysis. Nat. Rev. Nephrol. 2020, 16, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO). CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Levey, A.S.; Becker, C.; Inker, L.A. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: A systematic review. JAMA 2015, 313, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO). Diabetes Work Group KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020, 98, S1–S115. [Google Scholar] [CrossRef]
- Caramori, M.L.; Fioretto, P.; Mauer, M. The need for early predictors of diabetic nephropathy risk: Is albumin excretion rate sufficient? Diabetes 2000, 49, 1399–1408. [Google Scholar] [CrossRef]
- Warram, J.H.; Scott, L.J.; Hanna, L.S.; Wantman, M.; Cohen, S.E.; Laffel, L.M.B.; Ryan, L.; Krolewski, A.S. Progression of microalbuminuria to proteinuria in type 1 diabetes: Nonlinear relationship with hyperglycemia. Diabetes 2000, 49, 94–100. [Google Scholar] [CrossRef]
- Krolewski, A.S. Progressive renal decline: The new paradigm of diabetic nephropathy in type 1 diabetes. Diabetes Care 2015, 38, 954–962. [Google Scholar] [CrossRef]
- Warram, J.H.; Gearin, G.; Laffel, L.; Krolewski, A.S. Effect of duration of type I diabetes on the prevalence of stages of diabetic nephropathy defined by urinary albumin/creatinine ratio. J. Am. Soc. Nephrol. 1996, 7, 930–937. [Google Scholar] [CrossRef]
- Luis-Lima, S.; Linares, T.H.; Henríquez-Gómez, L.; Alonso-Pescoso, R.; Jimenez, A.; López-Hijazo, A.M.; Negrín-Mena, N.; Martín, C.; Sánchez-Gallego, M.; Galindo-Hernández, S.J.; et al. The Error of Estimated GFR in Type 2 Diabetes Mellitus. J. Clin. Med. 2019, 8, 1543. [Google Scholar] [CrossRef]
- Keshvari-Shad, F.; Hajebrahimi, S.; Laguna Pes, M.P.; Mahboub-Ahari, A.; Nouri, M.; Seyednejad, F.; Yousefi, M. A Systematic Review of Screening Tests for Chronic Kidney Disease: An Accuracy Analysis. Galen Med. J. 2020, 9, e1573. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O. Metabolomics—The link between genotypes and phenotypes. Plant. Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Macel, M.; van dam, N.M.; Keurentjes, J.J.B. Metabolomics: The chemistry between ecology and genetics. Mol. Ecol. Resour. 2010, 10, 583–593. [Google Scholar] [CrossRef]
- Trifonova, O.P.; Maslov, D.L.; Balashova, E.E.; Lokhov, P.G. Mass spectrometry-based metabolomics diagnostics—Myth or reality? Expert Rev. Proteom. 2021, 18, 7–12. [Google Scholar] [CrossRef]
- Lindon, J.C.; Nicholson, J.K. The emergent role of metabolic phenotyping in dynamic patient stratification. Expert Opin. Drug Metab. Toxicol. 2014, 10, 915–919. [Google Scholar] [CrossRef]
- Haukka, J.K.; Sandholm, N.; Forsblom, C.; Cobb, J.E.; Groop, P.H.; Ferrannini, E. Metabolomic Profile Predicts Development of Microalbuminuria in Individuals with Type 1 Diabetes. Sci. Rep. 2018, 8, 13853. [Google Scholar] [CrossRef] [PubMed]
- Roointan, A.; Gheisari, Y.; Hudkins, K.L.; Gholaminejad, A. Non-invasive metabolic biomarkers for early diagnosis of diabetic nephropathy: Meta-analysis of profiling metabolomics studies. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2253–2272. [Google Scholar] [CrossRef]
- Cordero-Pérez, P.; Sánchez-Martínez, C.; García-Hernández, P.A.; Saucedo, A.L. Metabolomics of the diabetic nephropathy: Behind the fingerprint of development and progression indicators. Nefrología (Engl. Ed.) 2020, 40, 585–596. [Google Scholar] [CrossRef]
- Baker, M. In biomarkers we trust? Nat. Biotechnol. 2005, 23, 297–304. [Google Scholar] [CrossRef]
- Haijes, H.A.; Willemsen, M.; van der Ham, M.; Gerrits, J.; Pras-Raves, M.L.; Prinsen, H.C.M.T.; van Hasselt, P.M.; de Sain-Van der Velden, M.G.M.; Verhoeven-Duif, N.M.; Jans, J.J.M. Direct infusion based metabolomics identifies metabolic disease in patients’ dried blood spots and plasma. Metabolites 2019, 9, 12. [Google Scholar] [CrossRef]
- Barrett, D. Advances in metabolic profiling. Bioanalysis 2012, 4, 643–644. [Google Scholar] [CrossRef] [PubMed]
- Nilavan, E.; Sundar, S.; Shenbagamoorthy, M.; Narayanan, H.; Nandagopal, B.; Srinivasan, R. Identification of biomarkers for early diagnosis of diabetic nephropathy disease using direct flow through mass spectrometry. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 2073–2078. [Google Scholar] [CrossRef] [PubMed]
- Sarvin, B.; Lagziel, S.; Sarvin, N.; Mukha, D.; Kumar, P.; Aizenshtein, E.; Shlomi, T. Fast and sensitive flow-injection mass spectrometry metabolomics by analyzing sample-specific ion distributions. Nat. Commun. 2020, 11, 3186. [Google Scholar] [CrossRef] [PubMed]
- Lokhov, P.G.; Balashova, E.E.; Trifonova, O.P.; Maslov, D.L.; Ponomarenko, E.A.; Archakov, A.I. Mass spectrometry-based metabolomics analysis of obese patients’ blood plasma. Int. J. Mol. Sci. 2020, 21, 568. [Google Scholar] [CrossRef]
- Lokhov, P.G.; Kharybin, O.N.; Archakov, A.I. Diagnosis of lung cancer based on direct-infusion electrospray mass spectrometry of blood plasma metabolites. Int. J. Mass Spectrom. 2012, 309, 200–205. [Google Scholar] [CrossRef]
- MetaboAnalyst. Available online: https://www.metaboanalyst.ca/MetaboAnalyst/home.xhtml (accessed on 8 June 2022).
- KEGG: Kyoto Encyclopedia of Genes and Genomes. Available online: https://www.genome.jp/kegg/ (accessed on 8 June 2022).
- Trevethan, R. Sensitivity, Specificity, and Predictive Values: Foundations, Pliabilities, and Pitfalls in Research and Practice. Front. Public Health 2017, 5, 307. [Google Scholar] [CrossRef]
- Human Metabolome Database. Available online: http://www.hmdb.ca (accessed on 8 June 2022).
- METLIN. Available online: http://metlin.scripps.edu/ (accessed on 8 June 2022).
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis: Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Lokhov, P.G.; Trifonova, O.P.; Maslov, D.L.; Balashova, E.E.; Archakov, A.I.; Shestakova, E.A.; Shestakova, M.V.; Dedov, I.I. Diagnosing impaired glucose tolerance using direct infusion mass spectrometry of blood plasma. PLoS ONE 2014, 9, e105343. [Google Scholar] [CrossRef]
- Hocher, B.; Adamski, J. Metabolomics for clinical use and research in chronic kidney disease. Nat. Rev. Nephrol. 2017, 13, 269–284. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, Q.; Lu, L.; Wang, J.; Liu, D.; Liu, Z. Metabolomic Profiling of Amino Acids in Human Plasma Distinguishes Diabetic Kidney Disease From Type 2 Diabetes Mellitus. Front. Med. 2021, 8, 2342. [Google Scholar] [CrossRef]
- Benito, S.; Sánchez, A.; Unceta, N.; Andrade, F.; Aldámiz-Echevarria, L.; Goicolea, M.A.; Barrio, R.J. LC-QTOF-MS-based targeted metabolomics of arginine-creatine metabolic pathway-related compounds in plasma: Application to identify potential biomarkers in pediatric chronic kidney disease. Anal. Bioanal. Chem. 2016, 408, 747–760. [Google Scholar] [CrossRef] [PubMed]
- Waikar, S.S.; Betensky, R.A.; Bonventre, J.V. Creatinine as the gold standard for kidney injury biomarker studies? Nephrol. Dial. Transplant. 2009, 24, 3263–3265. [Google Scholar] [CrossRef] [PubMed]
- Pundir, C.S.; Jakhar, S.; Narwal, V. Determination of urea with special emphasis on biosensors: A review. Biosens. Bioelectron. 2019, 123, 36–50. [Google Scholar] [CrossRef]
- Shao, M.; Lu, H.; Yang, M.; Liu, Y.; Yin, P.; Li, G.; Wang, Y.; Chen, L.; Chen, Q.; Zhao, C.; et al. Serum and urine metabolomics reveal potential biomarkers of T2DM patients with nephropathy. Ann. Transl. Med. 2020, 8, 199. [Google Scholar] [CrossRef] [PubMed]
- Razi, F.; Nasli-Esfahani, E.; Bandarian, F. Association of serum uric acid with nephropathy in Iranian type 2 diabetic patients. J. Diabetes Metab. Disord. 2018, 17, 71. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Zampino, M.; Moaddel, R.; Chen, T.K.; Tian, Q.; Ferrucci, L.; Semba, R.D. Plasma metabolites associated with chronic kidney disease and renal function in adults from the Baltimore Longitudinal Study of Aging. Metabolomics 2021, 17, 9. [Google Scholar] [CrossRef]
- Yang, J.; Liu, D.; Liu, Z. Integration of Metabolomics and Proteomics in Exploring the Endothelial Dysfunction Mechanism Induced by Serum Exosomes From Diabetic Retinopathy and Diabetic Nephropathy Patients. Front. Endocrinol. 2022, 13, 277. [Google Scholar] [CrossRef]
- Vanholder, R.; De Smet, R.; Glorieux, G.; Argilés, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; De Deyn, P.P.; Deppisch, R.; et al. Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int. 2003, 63, 1934–1943. [Google Scholar] [CrossRef]
- Duranton, F.; Cohen, G.; De Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argiles, A. Normal and pathologic concentrations of uremic toxins. J. Am. Soc. Nephrol. 2012, 23, 1258–1270. [Google Scholar] [CrossRef]
- Niwa, T. Update of uremic toxin research by mass spectrometry. Mass Spectrom. Rev. 2011, 30, 510–521. [Google Scholar] [CrossRef]
- Sun, H.; Frassetto, L.A.; Huang, Y.; Benet, L.Z. Hepatic clearance, but not gut availability, of erythromycin is altered in patients with end-stage renal disease. Clin. Pharmacol. Ther. 2010, 87, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Koppe, L.; Fouque, D.; Soulage, C.O. Metabolic Abnormalities in Diabetes and Kidney Disease: Role of Uremic Toxins. Curr. Diabetes Rep. 2018, 18, 97. [Google Scholar] [CrossRef] [PubMed]
- Giandalia, A.; Giuffrida, A.E.; Gembillo, G.; Cucinotta, D.; Squadrito, G.; Santoro, D.; Russo, G.T. Gender Differences in Diabetic Kidney Disease: Focus on Hormonal, Genetic and Clinical Factors. Int. J. Mol. Sci. 2021, 22, 5808. [Google Scholar] [CrossRef] [PubMed]
- Piani, F.; Melena, I.; Tommerdahl, K.L.; Nokoff, N.; Nelson, R.G.; Pavkov, M.E.; van Raalte, D.H.; Cherney, D.Z.; Johnson, R.J.; Nadeau, K.J.; et al. Sex-related differences in diabetic kidney disease: A review on the mechanisms and potential therapeutic implications. J. Diabetes Complicat. 2021, 35, 107841. [Google Scholar] [CrossRef]
- Yu, M.K.; Lyles, C.R.; Bent-Shaw, L.A.; Young, B.A. Risk factor, age and sex differences in chronic kidney disease prevalence in a diabetic cohort: The pathways study. Am. J. Nephrol. 2012, 36, 245–251. [Google Scholar] [CrossRef]
- Möllsten, A.; Svensson, M.; Waernbaum, I.; Berhan, Y.; Schön, S.; Nyström, L.; Arnqvist, H.J.; Dahlquist, G. Cumulative risk, age at onset, and sex-specific differences for developing end-stage renal disease in young patients with type 1 diabetes: A nationwide population-based cohort study. Diabetes 2010, 59, 1803–1808. [Google Scholar] [CrossRef]
- Harjutsalo, V.; Maric, C.; Forsblom, C.; Thorn, L.; Wadén, J.; Groop, P.H. Sex-related differences in the long-term risk of microvascular complications by age at onset of type 1 diabetes. Diabetologia 2011, 54, 1992–1999. [Google Scholar] [CrossRef]
- Sharma, K.; Karl, B.; Mathew, A.V.; Gangoiti, J.A.; Wassel, C.L.; Saito, R.; Pu, M.; Sharma, S.; You, Y.H.; Wang, L.; et al. Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J. Am. Soc. Nephrol. 2013, 24, 1901–1912. [Google Scholar] [CrossRef]
- Hirayama, A.; Nakashima, E.; Sugimoto, M.; Akiyama, S.I.; Sato, W.; Maruyama, S.; Matsuo, S.; Tomita, M.; Yuzawa, Y.; Soga, T. Metabolic profiling reveals new serum biomarkers for differentiating diabetic nephropathy. Anal. Bioanal. Chem. 2012, 404, 3101–3109. [Google Scholar] [CrossRef]
- Ibarra-González, I.; Cruz-Bautista, I.; Bello-Chavolla, O.Y.; Vela-Amieva, M.; Pallares-Méndez, R.; Ruiz de Santiago Y Nevarez, D.; Salas-Tapia, M.F.; Rosas-Flota, X.; González-Acevedo, M.; Palacios-Peñaloza, A.; et al. Optimization of kidney dysfunction prediction in diabetic kidney disease using targeted metabolomics. Acta Diabetol. 2018, 55, 1151–1161. [Google Scholar] [CrossRef]
| T1D n = 50 | T1D-DN1 n = 19 | T1D-DN2 n = 11 | p-Value | |
|---|---|---|---|---|
| Age (years) | 31.7 ± 10 | 47.7 ± 13.8 | 36.1 ± 9.6 | <0.001 |
| Sex (M/F) | 26/24 | 6/13 | 3/8 | |
| BMI (kg/m2) | 24.5 ± 5 | 26.3 ± 4.4 | 23.4 ± 5.5 | 0.2213 |
| Years since T1D diagnosed | 14.0 ± 8.5 | 27.5 ± 10.4 | 25.0 ± 7 | <0.001 |
| Systolic blood pressure (mmHg) | 121.4 ± 10.7 | 128.0 ± 12.5 | 133.2 ± 27.2 | 0.02863 |
| Diastolic blood pressure (mmHg) | 76.5 ± 8.8 | 79.7 ± 9.2 | 79.1 ± 11.4 | 0.38071 |
| HbA1c (%) | 8.3 ± 1.5 | 8.8 ± 1.3 | 8.5 ± 1.4 | 0.44263 |
| Fasting Glucose (mmol/L) | 8.2 ± 1.5 | 7.9 ± 0.9 | 8.5 ± 1.2 | 0.43648 |
| Creatinine (µmol/L) | 70.6 ± 9.5 | 87.0 ± 11.4 | 196.1 ± 75.0 | <0.001 |
| Urea (mmol/L) | 4.6 ± 1.1 | 6.0 ± 1.8 | 11.2 ± 4.6 | <0.001 |
| Cholesterol (mmol/L) | 4.9 ± 1.2 | 5.1 ± 1.2 | 5.5 ± 2.0 | 0.46141 |
| HDL Cholesterol (mmol/L) | 1.4 ± 0.4 | 1.3 ± 0.5 | 1.6 ± 0.8 | 0.20182 |
| LDL Cholesterol (mmol/L) | 3.0 ± 1.1 | 3.2 ± 1.0 | 3.4 ± 1.3 | 0.43088 |
| Triglycerides (mmol/L) | 1.1 ± 0.7 | 1.2 ± 0.5 | 1.1 ± 0.5 | 0.90265 |
| eGFR (mL/min/1.73 m2) | 114.3 ± 10.1 | 77.8 ± 8.5 | 35.7 ± 14.8 | <0.001 |
| ACR (mg/mmol) | 1.2 ± 0.7 | 8.8 ± 8.0 | 34.1 ± 28.8 | 0 |
| Top Pathways | Compounds | Significant Hits | |||
|---|---|---|---|---|---|
| Hits/Total | T1D vs. T1D-DN1 | T1D vs. T1D-DN2 | T1D-DN1 vs. T1D-DN2 | Identified Metabolites | |
| Aspartate and asparagine metabolism | 20/114 | 8 | 8 | 5 | L-arginine, L-cysteine, 2-ketobutyric acid, L-proline, citrulline, N-formyl-L-aspartate, 4-guanidinobutanamide, N2-succinyl-L-ornithine |
| Arginine and proline metabolism | 12/45 | 4 | 4 | 2 | L-arginine, L-proline, citrulline, 4-guanidinobutanamide |
| Methionine and cysteine metabolism | 14/94 | 4 | 2 | 4 | L-cysteine, thiosulfate, thiocysteine, 3-sulfopyruvic acid, 5-L-glutamyl-taurine |
| Alanine and aspartate metabolism | 4/30 | 2 | 3 | 1 | L-arginine, citrulline, 2-oxosuccinamic acid |
| Urea cycle/amino group metabolism | 12/85 | 3 | 5 | 4 | L-arginine, L-proline, creatine, citrulline, creatinine |
| Glycine, serine, alanine, and threonine metabolism | 17/88 | 2 | 5 | 4 | L-arginine, creatine, phosphoglycolic acid, L-2-amino-3-oxobutanoic acid, 2-oxo-3-hydroxy-4-phosphobutanoic acid |
| T1D vs. T1D-DN1/DN2 | T1D vs. T1D-DN1 | T1D vs. T1D-DN2 | |
|---|---|---|---|
| Creatinine | + | + | + |
| L-Proline | + | + | + |
| L-Cysteine | + | + | + |
| Thiocysteine | − | − | + |
| 4-Guanidinobutanamide | − | − | + |
| Creatine | + | − | + |
| N-Formyl-L-aspartate | − | + | − |
| 1-Methylhistidine | + | + | + |
| 2-Oxo-3-hydroxy- 4-phosphobutanoic acid | − | − | + |
| L-Arginine | + | − | − |
| Citrulline | + | − | + |
| Oxalosuccinic acid | + | + | + |
| N-Acetyl-b-glucosaminylamine | + | + | − |
| N2-Succinyl-L-ornithine | + | + | + |
| 3-Carboxy-4-methyl-5-propyl- 2-furanpropionic acid | + | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trifonova, O.P.; Maslov, D.L.; Balashova, E.E.; Lichtenberg, S.; Lokhov, P.G. Potential Plasma Metabolite Biomarkers of Diabetic Nephropathy: Untargeted Metabolomics Study. J. Pers. Med. 2022, 12, 1889. https://doi.org/10.3390/jpm12111889
Trifonova OP, Maslov DL, Balashova EE, Lichtenberg S, Lokhov PG. Potential Plasma Metabolite Biomarkers of Diabetic Nephropathy: Untargeted Metabolomics Study. Journal of Personalized Medicine. 2022; 12(11):1889. https://doi.org/10.3390/jpm12111889
Chicago/Turabian StyleTrifonova, Oxana P., Dmitry L. Maslov, Elena E. Balashova, Steven Lichtenberg, and Petr G. Lokhov. 2022. "Potential Plasma Metabolite Biomarkers of Diabetic Nephropathy: Untargeted Metabolomics Study" Journal of Personalized Medicine 12, no. 11: 1889. https://doi.org/10.3390/jpm12111889
APA StyleTrifonova, O. P., Maslov, D. L., Balashova, E. E., Lichtenberg, S., & Lokhov, P. G. (2022). Potential Plasma Metabolite Biomarkers of Diabetic Nephropathy: Untargeted Metabolomics Study. Journal of Personalized Medicine, 12(11), 1889. https://doi.org/10.3390/jpm12111889










