A Ready-to-Use Grading Tool for Facial Palsy Examiners—Automated Grading System in Facial Palsy Patients Made Easy
Abstract
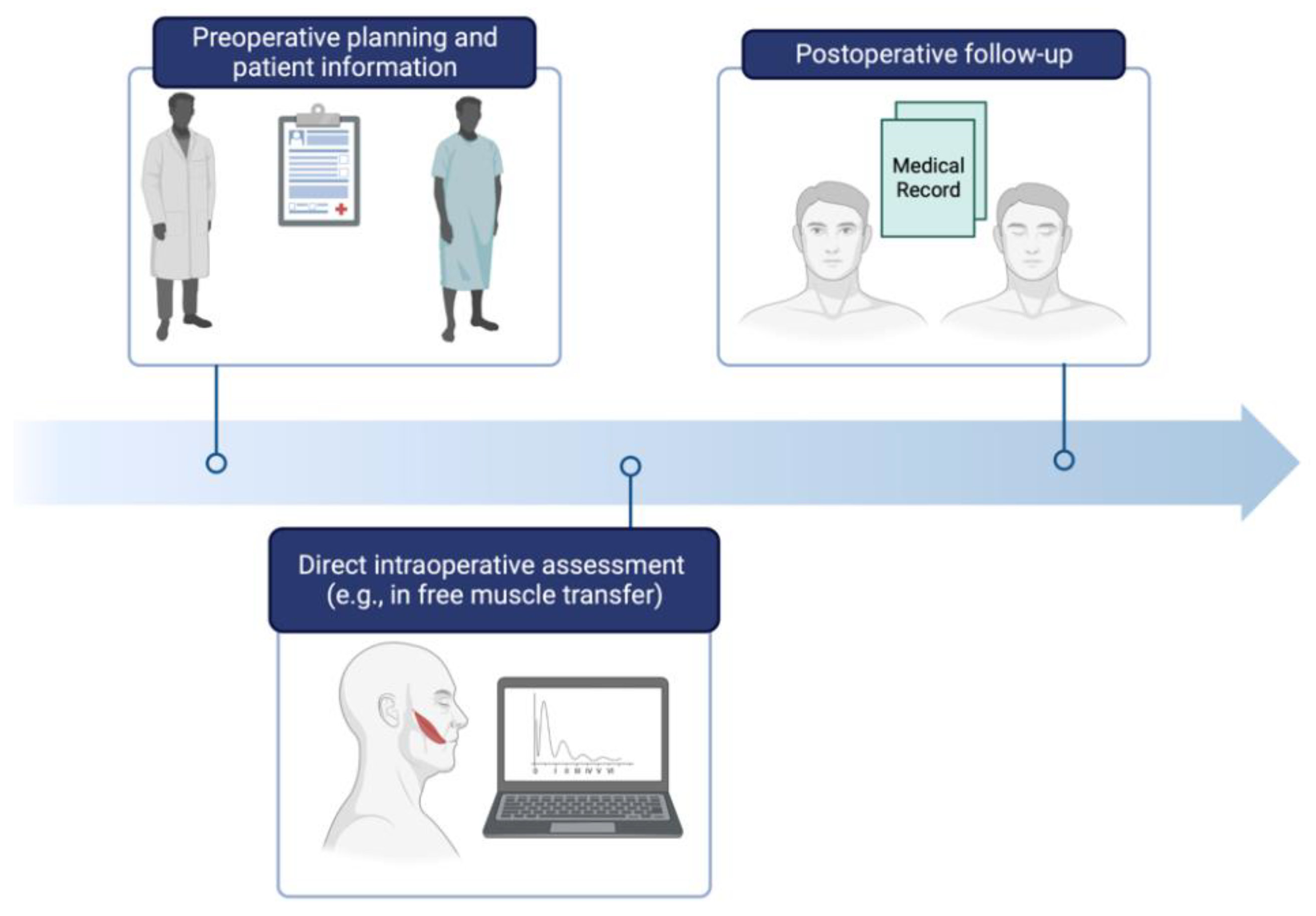
1. Introduction
2. Materials and Methods
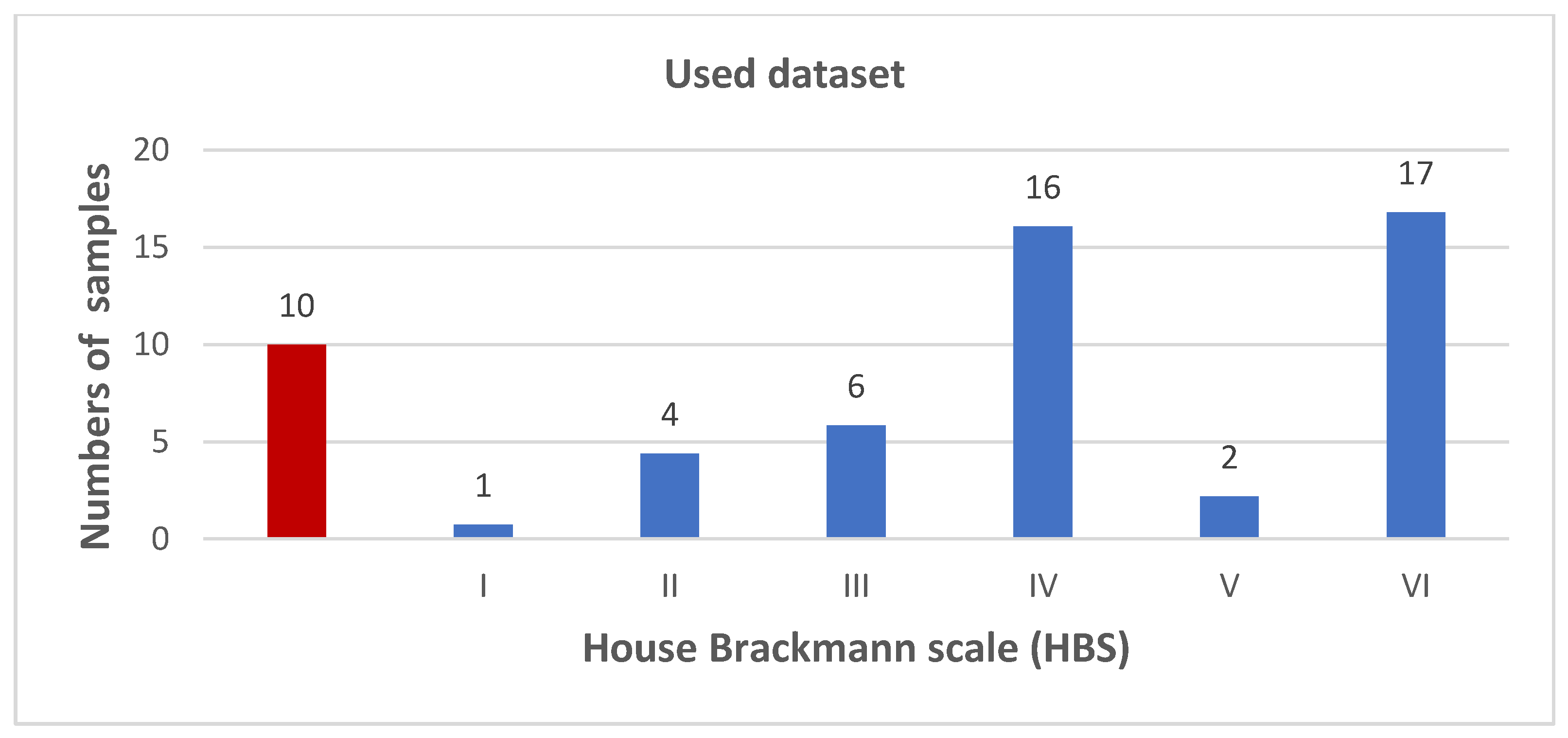
2.1. Data Acquisition from Facial Patients
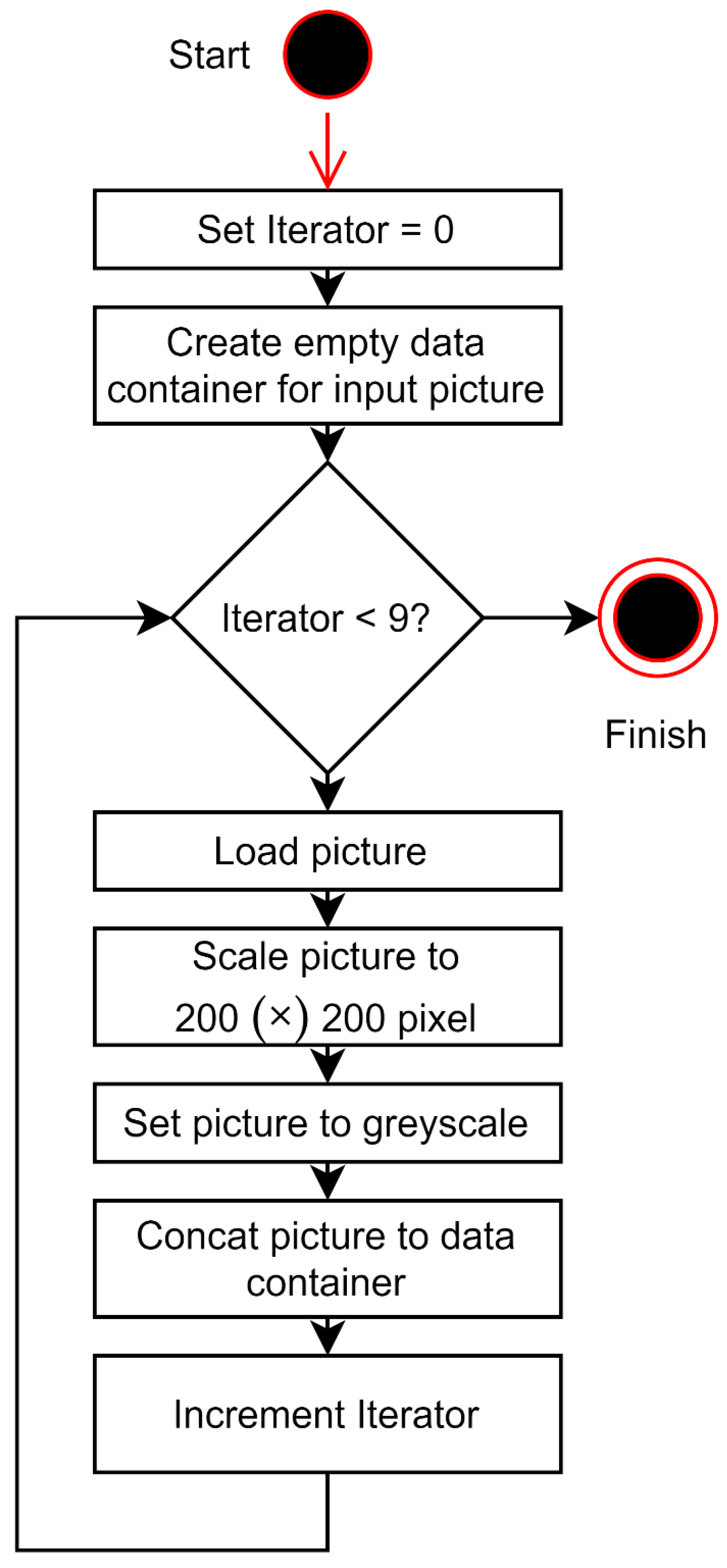
2.2. Facial Palsy Image Segmentation
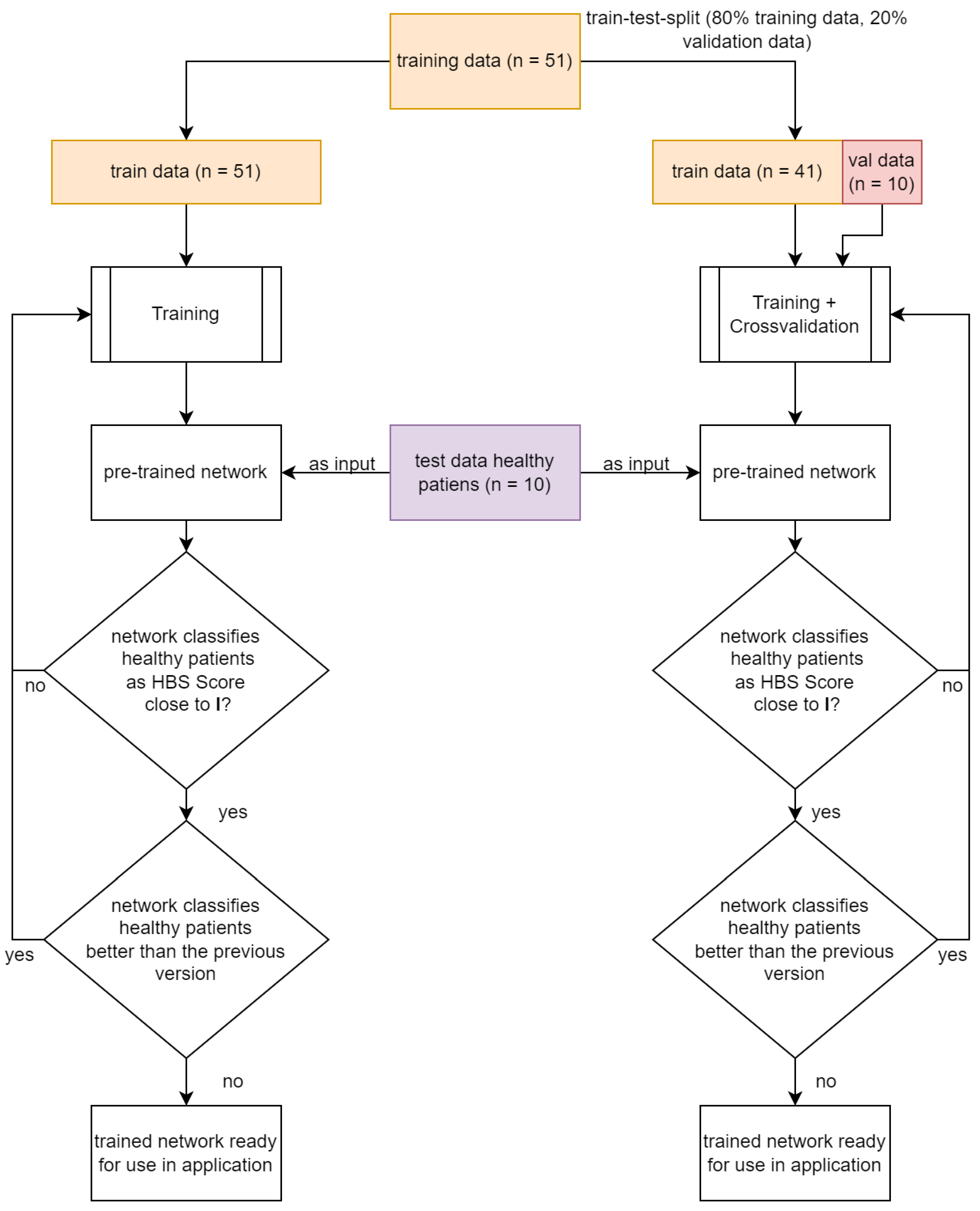
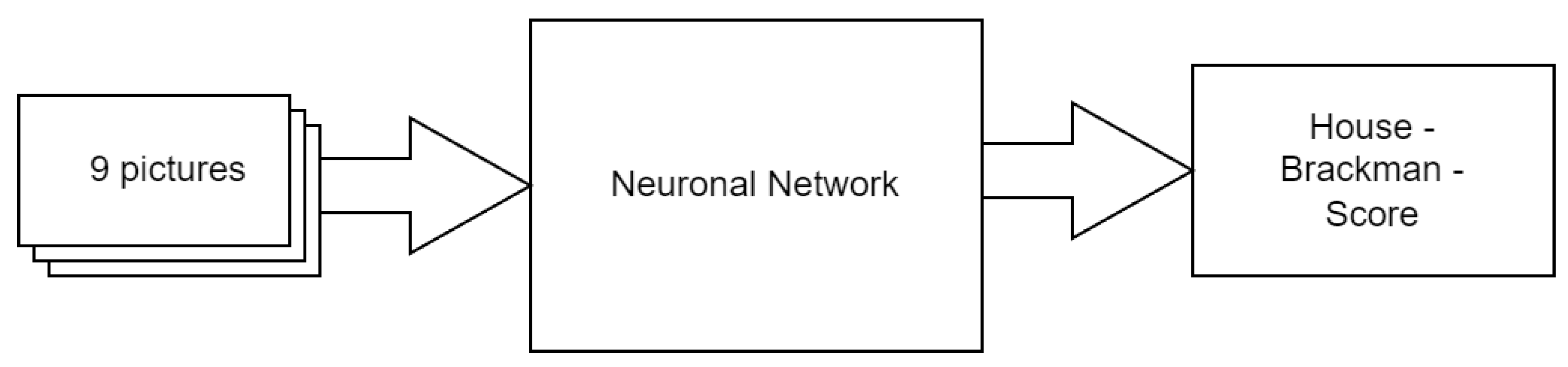
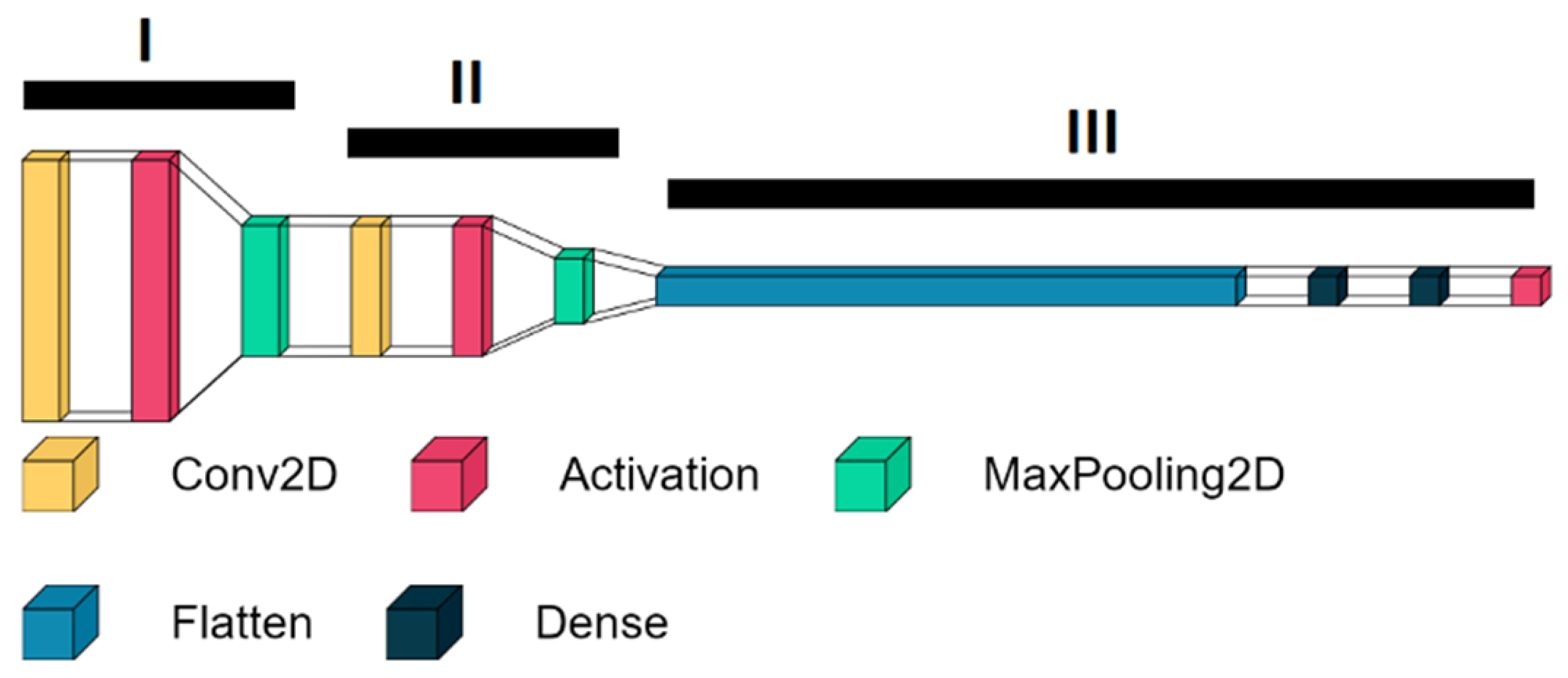
2.3. Structure of the HBS Score Classifier
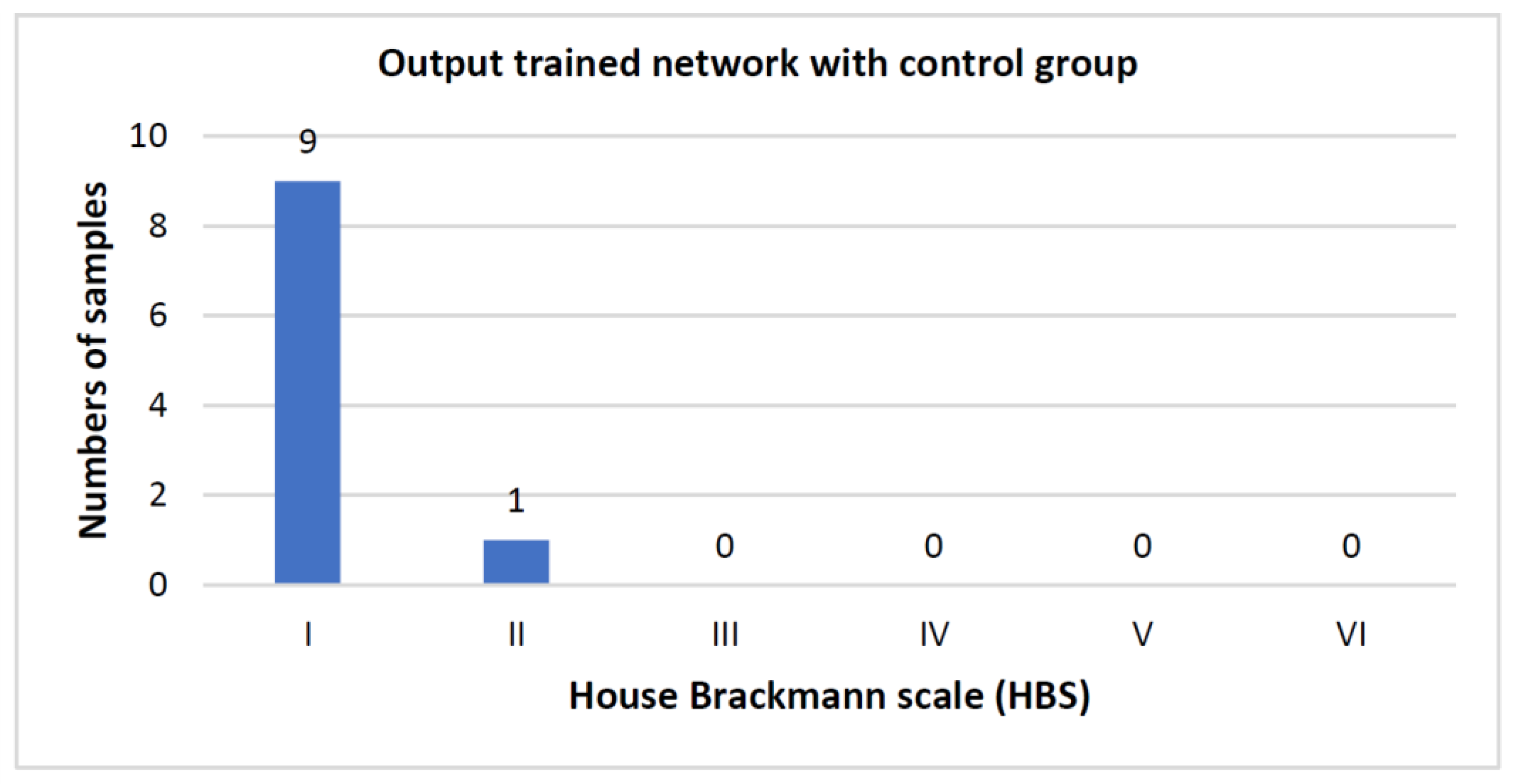
3. Results
Number of Training Runs Determines Prediction Accuracy
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jowett, N. A General Approach to Facial Palsy. Otolaryngol. Clin. N. Am. 2018, 51, 1019–1031. [Google Scholar] [CrossRef]
- Teresa, M.O.; Jowett, N.; Hadlock, T.A. Facial Palsy: Diagnostic and Therapeutic Management. Otolaryngol. Clin. N. Am. 2018, 51, xvii–xviii. [Google Scholar]
- Owusu, J.A.; Stewart, C.M.; Boahene, K. Facial Nerve Paralysis. Med. Clin. N. Am. 2018, 102, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- McCaul, J.A.; Cascarini, L.; Godden, D.; Coombes, D.; Brennan, P.A.; Kerawala, C.J. Evidence based management of Bell’s palsy. Br. J. Oral Maxillofac. Surg. 2014, 52, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Kosins, A.M.; Hurvitz, K.A.; Evans, G.R.; Wirth, G.A. Facial paralysis for the plastic surgeon. Can. J. Plast. Surg. 2007, 15, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Yanagihara, N. Incidence of Bell’s palsy. Ann. Otol. Rhinol. Laryngol. Suppl. 1988, 137, 3–4. [Google Scholar] [CrossRef]
- Bleicher, J.N.; Hamiel, S.; Gengler, J.S.; Antimarino, J. A survey of facial paralysis: Etiology and incidence. Ear Nose Throat J. 1996, 75, 355–358. [Google Scholar] [CrossRef]
- Heckmann, J.G.; Urban, P.P.; Pitz, S.; Guntinas-Lichius, O.; Gágyor, I. The Diagnosis and Treatment of Idiopathic Facial Paresis (Bell’s Palsy). Dtsch. Arztebl. Int. 2019, 116, 692–702. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, L.; Luo, T.; Wu, F.; Zhao, B.; Li, X. The etiology of Bell’s palsy: A review. J. Neurol. 2020, 267, 1896–1905. [Google Scholar] [CrossRef]
- Roob, G.; Fazekas, F.; Hartung, H.P. Peripheral facial palsy: Etiology, diagnosis and treatment. Eur. Neurol. 1999, 41, 3–9. [Google Scholar] [CrossRef]
- Tiemstra, J.D.; Khatkhate, N. Bell’s palsy: Diagnosis and management. Am. Fam. Physician 2007, 76, 997–1002. [Google Scholar] [PubMed]
- Abraham-Inpijn, L.; Devriese, P.P.; Hart, A.A. Predisposing factors in Bell’s palsy: A clinical study with reference to diabetes mellitus, hypertension, clotting mechanism and lipid disturbance. Clin. Otolaryngol. Allied. Sci. 1982, 7, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Greco, D.; Gambina, F.; Pisciotta, M.; Abrignani, M.; Maggio, F. Clinical characteristics and associated comorbidities in diabetic patients with cranial nerve palsies. J. Endocrinol. Investig. 2012, 35, 146–149. [Google Scholar]
- Liston, S.L.; Kleid, M.S. Histopathology of Bell’s palsy. Laryngoscope 1989, 99, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.P.; Chen, Y.T.; Fuh, J.L.; Tang, C.H.; Wang, S.J. Increased risk of Bell palsy in patients with migraine: A nationwide cohort study. Neurology 2015, 84, 116–124. [Google Scholar] [CrossRef]
- Hohman, M.H.; Hadlock, T.A. Etiology, diagnosis, and management of facial palsy: 2000 patients at a facial nerve center. Laryngoscope 2014, 124, E283–E293. [Google Scholar] [CrossRef]
- Azizzadeh, B.; Irvine, L.E.; Diels, J.; Slattery, W.H.; Massry, G.G.; Larian, B.; Riedler, K.L.; Peng, G.L. Modified Selective Neurectomy for the Treatment of Post-Facial Paralysis Synkinesis. Plast. Reconstr. Surg. 2019, 143, 1483–1496. [Google Scholar] [CrossRef]
- Biglioli, F.; Kutanovaite, O.; Rabbiosi, D.; Colletti, G.; Mohammed, M.; Saibene, A.M.; Cupello, S.; Privitera, A.; Battista, V.M.; Lozza, A.; et al. Surgical treatment of synkinesis between smiling and eyelid closure. J. Craniomaxillofac Surg. 2017, 45, 1996–2001. [Google Scholar] [CrossRef]
- Kehrer, A.; Engelmann, S.; Ruewe, M.; Geis, C.; Taeger, C.; Kehrer, M.; Prantl, L.; Tamm, E.; Bleys, R.R.L.A.W.; Mandlik, V. Anatomical study of the zygomatic and buccal branches of the facial nerve: Application to facial reanimation procedures. Clin. Anat. 2019, 32, 480–488. [Google Scholar] [CrossRef]
- Kehrer, A.; Engelmann, S.; Bauer, R.; Taeger, C.; Grechenig, S.; Kehrer, M.; Prantl, L.; Tamm, E.R.; Bleys, R.R.L.A.W.; Mandlik, V. The nerve supply of zygomaticus major: Variability and distinguishing zygomatic from buccal facial nerve branches. Clin. Anat. 2018, 31, 560–565. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, E.; Barbaix, E. Anatomic research on the perioral muscles, functional matrix of the maxillary and mandibular bones. Surg. Radiol. Anat. 2006, 28, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, S.; Ruewe, M.; Geis, S.; Taeger, C.D.; Kehrer, M.; Tamm, E.R.; Bleys, R.L.A.W.; Zeman, F.; Prantl, L.; Kehrer, A. Rapid and Precise Semi-Automatic Axon Quantification in Human Peripheral Nerves. Sci. Rep. 2020, 10, 1935. [Google Scholar] [CrossRef] [PubMed]
- Mandlik, V.; Ruewe, M.; Engelmann, S.; Geis, S.; Taeger, C.; Kehrer, M.; Tamm, E.R.; Bleys, R.; Prantl, L.; Kehrer, A. Significance of the Marginal Mandibular Branch in Relation to Facial Palsy Reconstruction: Assessment of Microanatomy and Macroanatomy Including Axonal Load in 96 Facial Halves. Ann. Plast. Surg. 2019, 83, e43–e49. [Google Scholar] [CrossRef] [PubMed]
- Toulgoat, F.; Sarrazin, J.; Benoudiba, F.; Pereon, Y.; Auffray-Calvier, E.; Daumas-Duport, B.; Lintia-Gaultier, A.; Desal, H. Facial nerve: From anatomy to pathology. Diagn. Interv. Imaging 2013, 94, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Natghian, H.; Fransen, J.; Rozen, S.M.; Rodriguez-Lorenzo, A. Qualitative and Quantitative Analysis of Smile Excursion in Facial Reanimation: A Systematic Review and Meta-analysis of 1- versus 2-stage Procedures. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1621. [Google Scholar] [CrossRef]
- Dobel, C.; Miltner, W.H.; Witte, O.W.; Volk, G.F.; Guntinas-Lichius, O. Emotional impact of facial palsy. Laryngorhinootologie 2013, 92, 9–23. [Google Scholar]
- Tseng, C.C.; Hu, L.Y.; Liu, M.E.; Yang, A.C.; Shen, C.C.; Tsai, S.J. Bidirectional association between Bell’s palsy and anxiety disorders: A nationwide population-based retrospective cohort study. J. Affect. Disord. 2017, 215, 269–273. [Google Scholar] [CrossRef]
- Chang, Y.-S.; Choi, J.E.; Kim, S.W.; Baek, S.-Y.; Cho, Y.-S. Prevalence and associated factors of facial palsy and lifestyle characteristics: Data from the Korean National Health and Nutrition Examination Survey 2010–2012. BMJ Open 2016, 6, e012628. [Google Scholar] [CrossRef]
- Hotton, M.; Huggons, E.; Hamlet, C.; Shore, D.; Johnson, D.; Norris, J.H.; Kilcoyne, S.; Dalton, L. The psychosocial impact of facial palsy: A systematic review. Br. J. Health Psychol. 2020, 25, 695–727. [Google Scholar] [CrossRef]
- Coulson, S.E.; O’Dwyer, N.J.; Adams, R.D.; Croxson, G.R. Expression of emotion and quality of life after facial nerve paralysis. Otol. Neurotol. 2004, 25, 1014–1019. [Google Scholar] [CrossRef]
- Ramsey, M.J.; DerSimonian, R.; Holtel, M.R.; Burgess, L.P. Corticosteroid treatment for idiopathic facial nerve paralysis: A meta-analysis. Laryngoscope 2000, 110, 335–341. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Madhok, V.B.; Gagyor, I.; Daly, F.; Somasundara, D.; Sullivan, M.; Gammie, F.; Sullivan, F. Corticosteroids for Bell’s palsy (idiopathic facial paralysis). Cochrane Database Syst. Rev. 2016, 7, Cd001942. [Google Scholar] [CrossRef] [PubMed]
- Quant, E.C.; Jeste, S.S.; Muni, R.H.; Cape, A.V.; Bhussar, M.K.; Peleg, A.Y. The benefits of steroids versus steroids plus antivirals for treatment of Bell’s palsy: A meta-analysis. BMJ 2009, 339, b3354. [Google Scholar] [CrossRef]
- Klebuc, M. The evolving role of the masseter-to-facial (V-VII) nerve transfer for rehabilitation of the paralyzed face. Ann. Chir. Plast. Esthet. 2015, 60, 436–441. [Google Scholar] [CrossRef]
- Klebuc, M.J.A.; (Labio-mental Synkinetic Dysfunction Weill Cornell School of Medicine, New York, NY, USA). Personal communication, 2020.
- Boahene, K.O.; Owusu, J.; Ishii, L.; Ishii, M.; Desai, S.; Kim, I.; Kim, L.; Byrne, P. The Multivector Gracilis Free Functional Muscle Flap for Facial Reanimation. JAMA Facial Plast. Surg. 2018, 20, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Reitzen, S.D.; Babb, J.S.; Lalwani, A.K. Significance and reliability of the House-Brackmann grading system for regional facial nerve function. Otolaryngol. Head Neck Surg. 2009, 140, 154–158. [Google Scholar] [CrossRef]
- Fattah, A.Y.; Gurusinghe, A.D.R.; Gavilan, J.; Hadlock, T.A.; Marcus, J.R.; Marres, H.; Nduka, C.C.; Slattery, W.H.; Snyder-Warwick, A.K.; Sir Charles Bell Society. Facial nerve grading instruments: Systematic review of the literature and suggestion for uniformity. Plast. Reconstr. Surg. 2015, 135, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.Z.; Oh, M.C.; Safaee, M.; Kaur, G.; Parsa, A.T. Neuroanatomical correlation of the House-Brackmann grading system in the microsurgical treatment of vestibular schwannoma. Neurosurg. Focus 2012, 33, E7. [Google Scholar] [CrossRef] [PubMed]
- House, J.W.; Brackmann, D.E. Facial nerve grading system. Otolaryngol. Head Neck Surg. 1985, 93, 146–147. [Google Scholar] [CrossRef]
- Dusseldorp, J.R.; van Veen, M.M.; Mohan, S.; Hadlock, T.A. Outcome Tracking in Facial Palsy. Otolaryngol. Clin. N. Am. 2018, 51, 1033–1050. [Google Scholar] [CrossRef]
- Banks, C.A.; Jowett, N.; Azizzadeh, B.; Beurskens, C.; Bhama, P.; Borschel, G.; Coombs, C.; Coulson, S.; Croxon, G.; Diels, J.; et al. Worldwide Testing of the eFACE Facial Nerve Clinician-Graded Scale. Plast. Reconstr. Surg. 2017, 139, 491e–498e. [Google Scholar] [CrossRef]
- Schaede, R.A.; Volk, G.F.; Modersohn, L.; Barth, J.M.; Denzler, J.; Guntinas-Lichius, O. Patienten-Instruktionsvideo mit synchroner Videoaufnahme von Gesichtsbewegungen bei Fazialisparese [Video Instruction for Synchronous Video Recording of Mimic Movement of Patients with Facial Palsy]. Laryngorhinootologie 2017, 96, 844–849. [Google Scholar]
- Santosa, K.B.; Fattah, A.; Gavilán, J.; Hadlock, T.A. Snyder-Warwick AK. Photographic Standards for Patients With Facial Palsy and Recommendations by Members of the Sir Charles Bell Society. JAMA Facial Plast. Surg. 2017, 19, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Perkel, J.M. Programming: Pick up Python. Nature 2015, 518, 125–126. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, R.; Nishio, M.; Do, R.K.G.; Togashi, K. Convolutional neural networks: An overview and application in radiology. Insights Imaging 2018, 9, 611–629. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Paniagua, V.; Segura-Bedmar, I. Evaluation of pooling operations in convolutional architectures for drug-drug interaction extraction. BMC Bioinform. 2018, 19 (Suppl. S8), 209. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.V.E.; Martuscelli, O.J.D.; Vieira, A.C.; Vieira, C.F. Prevalence of Burnout among Plastic Surgeons and Residents in Plastic Surgery: A Systematic Literature Review and Meta-analysis. Plast. Reconstr. Surg. Glob. Open 2018, 6, e1854. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Jayanti, M.K.; Taylor, A.; Williams, T.E.; Tiwari, P. The impending shortage and cost of training the future plastic surgical workforce. Ann. Plast. Surg. 2014, 72, 200–203. [Google Scholar] [CrossRef]
- Bauder, A.R.; Sarik, J.R.; Butler, P.D.; Noone, R.B.; Fischer, J.P.; Serletti, J.M.; Kanchwala, S.K.; Kovach, S.J.; Fox, J.P. Geographic Variation in Access to Plastic Surgeons. Ann. Plast. Surg. 2016, 76, 238–243. [Google Scholar] [CrossRef]
- Jarvis, T.; Thornburg, D.; Rebecca, A.M.; Teven, C.M. Artificial Intelligence in Plastic Surgery: Current Applications, Future Directions, and Ethical Implications. Plast. Reconstr. Surg. Glob. Open 2020, 8, e3200. [Google Scholar] [CrossRef]
- Sauer, M.; Guntinas-Lichius, O.; Volk, G.F. Ultrasound echomyography of facial muscles in diagnosis and follow-up of facial palsy in children. Eur. J. Paediatr. Neurol. 2016, 20, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Volk, G.F.; Wystub, N.; Pohlmann, M.; Finkensieper, M.; Chalmers, H.J.; Guntinas-Lichius, O. Quantitative ultrasonography of facial muscles. Muscle Nerve 2013, 47, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Kehrer, A.; Ruewe, M.; Klebuc, M.; Platz Batista da Silva, N.; Lonic, D.; Heidkrueger, P.; Jung, E.M.; Prantl, L.; Knoedler, L. Objectifying the Antagonistic Role of the Depressor Anguli Oris Muscle in Synkinetic Smile Formation Utilizing High-Resolution Ultrasound—A Prospective Study. Plast. Reconstr. Surg. 2022; ahead of print. [Google Scholar]
- Haase, D.; Minnigerode, L.; Volk, G.F.; Denzler, J.; Guntinas-Lichius, O. Automated and objective action coding of facial expressions in patients with acute facial palsy. Eur. Arch. Oto-Rhino-Laryngol. 2015, 272, 1259–1267. [Google Scholar] [CrossRef]
- Meier-Gallati, V.; Scriba, H.; Fisch, U.P. Objective scaling of facial nerve function based on area analysis (OSCAR). Otolaryngol. Head Neck Surg. 1998, 118, 545–550. [Google Scholar]
- Shinkunas, L.A.; Klipowicz, C.J.; Carlisle, E.M. Shared decision making in surgery: A scoping review of patient and surgeon preferences. BMC Med. Inform. Decis. Mak. 2020, 20, 190. [Google Scholar] [CrossRef] [PubMed]
- Morrell, D.; Evans, M.; Morris, R.; Roland, M. The “five minute” consultation: Effect of time constraint on clinical content and patient satisfaction. Br. Med. J. (Clin. Res. Ed) 1986, 292, 870–873. [Google Scholar] [CrossRef] [PubMed]
- IsHak, W.W.; Lederer, S.; Mandili, C.; Nikravesh, R.; Seligman, L.; Vasa, M.; Ogunyemi, D.; Bernstein, C.A. Burnout during residency training: A literature review. J. Grad. Med. Educ. 2009, 1, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Edú-Valsania, S.; Laguía, A.; Moriano, J.A. Burnout: A Review of Theory and Measurement. Int. J. Environ. Res. Public Health 2022, 19, 1780. [Google Scholar] [CrossRef]
- Olivetto, M.; Sarhan, F.-R.; Ben Mansour, K.; Marie, J.-P.; Marin, F.; Dakpé, S. Quantitative Analysis of Facial Palsy Based on 3D Motion Capture (SiMoVi—FaceMoCap Project). Arch. Phys. Med. Rehabil. 2019, 100, e112. [Google Scholar] [CrossRef]
- Su, Z.; Liang, B.; Shi, F.; Gelfond, J.; Šegalo, S.; Wang, J.; Jia, P.; Hao, X. Deep learning-based facial image analysis in medical research: A systematic review protocol. BMJ Open 2021, 11, e047549. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Wang, L.; Gupta, S.; Goli, H.; Padmanabhan, P.; Gulyás, B. 3D Deep Learning on Medical Images: A Review. Sensors 2020, 20, 5097. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, S.-H.; Zhang, Y.-D. Detection of abnormal brain in MRI via improved AlexNet and ELM optimized by chaotic bat algorithm. Neural Comput. Appl. 2021, 33, 10799–10811. [Google Scholar] [CrossRef]
- Pérez, E.; Sanchez, S. Deep Learning Transfer with AlexNet for chest X-ray COVID-19 recognition. IEEE Access 2020, 100, 4336. [Google Scholar]
- Storey, G.; Jiang, R.; Keogh, S.; Bouridane, A.; Li, C.-T. 3DPalsyNet: A Facial Palsy Grading and Motion Recognition Framework Using Fully 3D Convolutional Neural Networks. IEEE Access 2019, 7, 121655–121664. [Google Scholar] [CrossRef]
- Jiang, C.; Wu, J.; Zhong, W.; Wei, M.; Tong, J.; Yu, H.; Wang, L. Automatic Facial Paralysis Assessment via Computational Image Analysis. J. Healthc. Eng. 2020, 2020, 2398542. [Google Scholar] [CrossRef] [PubMed]
- Raj, A.; Mothes, O.; Sickert, S.; Volk, G.F.; Guntinas-Lichius, O.; Denzler, J. Automatic and Objective Facial Palsy Grading Index Prediction Using Deep Feature Regression. In Medical Image Understanding and Analysis, Proceedings of the MIUA 2020 Communications in Computer and Information, Oxford, UK, 15–17 July 2020; SciencePapież, B., Namburete, A., Yaqub, M., Noble, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; Volume 1248. [Google Scholar]
- Zhao, Y.; Feng, G.; Wu, H.; Aodeng, S.; Tian, X.; Volk, G.F.; Guntinas-Lichius, O.; Gao, Z. Prognostic value of a three-dimensional dynamic quantitative analysis system to measure facial motion in acute facial paralysis patients. Head Face Med. 2020, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- Overschmidt, B.; Qureshi, A.A.; Parikh, R.P.; Yan, Y.; Tenenbaum, M.M.; Myckatyn, T.M. A prospective evaluation of three-dimensional image simulation: Patient-reported outcomes and mammometrics in primary breast augmentation. Plast. Reconstr. Surg. 2018, 142, 133e–144e. [Google Scholar] [CrossRef]
- Wesselius, T.S.; Verhulst, A.C.; Vreeken, R.D.; Xi, T.; Maal, T.J.; Ulrich, D.J. Accuracy of three software applications for breast volume calculations from three-dimensional surface images. Plast. Reconstr. Surg. 2018, 142, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pérez, R.; Bajorath, J. Interpretation of Compound Activity Predictions from Complex Machine Learning Models Using Local Approximations and Shapley Values. J. Med. Chem. 2020, 63, 8761–8777. [Google Scholar] [CrossRef]
- Guntinas-Lichius, O.; Denzler, J. Automatic and objective facial palsy grading index prediction using deep feature regression. In Medical Image Understanding and Analysis, Proceedings of the 24th Annual Conference, Geneva, Switzerland, 15 November 2022; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Ross, B.R.; Fradet, G.; Nedzelski, J.M. Development of a sensitive clinical facial grading system. Eur. Arch. Otorhinolaryngol. 1996, 114, 380–386. [Google Scholar]
- Mothes, O.; Modersohn, L.; Volk, G.F.; Klingner, C.; Witte, O.W.; Schlattmann, P.; Denzler, J.; Guntinas-Lichius, O. Automated objective and marker-free facial grading using photographs of patients with facial palsy. Eur. Arch. Otorhinolaryngol. 2019, 276, 3335–3343. [Google Scholar] [CrossRef] [PubMed]
- Fattah, A.Y.; Gavilan, J.; Hadlock, T.A.; Marcus, J.R.; Marres, H.; Nduka, C.; Slattery, W.H.; Snyder-Warwick, A.K. Survey of methods of facial palsy documentation in use by members of the Sir Charles Bell Society. Laryngoscope 2014, 124, 2247–2251. [Google Scholar] [CrossRef] [PubMed]
- Gaber, A.; Taher, M.F.; Wahed, M.A. Quantifying facial paralysis using the Kinect v2. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 2497–2501. [Google Scholar]
- Guo, Z.; Shen, M.; Duan, L.; Zhou, Y.; Xiang, J.; Ding, H.; Chen, S.; Deussen, O.; Dan, G. Deep assessment process: Objective assessment process for unilateral peripheral facial paralysis via deep convolutional neural network. In Proceedings of the 2017 IEEE 14th International Symposium on Biomedical Imaging (ISBI 2017), Melbourne, Australia, 18–21 April 2017; pp. 135–138. [Google Scholar] [CrossRef]
- Miller, M.Q.; Hadlock, T.A.; Fortier, E.; Guarin, D.L. The Auto-eFACE: Machine Learning-Enhanced Program Yields Automated Facial Palsy Assessment Tool. Plast. Reconstr. Surg. 2021, 147, 467–474. [Google Scholar] [CrossRef]
- Guarin, D.L.; Yunusova, Y.; Taati, B.; Dusseldorp, J.R.; Mohan, S.; Tavares, J.; van Veen, M.M.; Fortier, E.; Hadlock, T.A.; Jowett, N. Toward an Automatic System for Computer-Aided Assessment in Facial Palsy. Facial Plast. Surg. Aesthet. Med. 2020, 22, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Banks, C.A.; Bhama, P.K.; Park, J.; Hadlock, C.R.; Hadlock, T.A. Clinician-Graded Electronic Facial Paralysis Assessment: The eFACE. Plast. Reconstr. Surg. 2015, 136, 223e–230e. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knoedler, L.; Miragall, M.; Kauke-Navarro, M.; Obed, D.; Bauer, M.; Tißler, P.; Prantl, L.; Machens, H.-G.; Broer, P.N.; Baecher, H.; et al. A Ready-to-Use Grading Tool for Facial Palsy Examiners—Automated Grading System in Facial Palsy Patients Made Easy. J. Pers. Med. 2022, 12, 1739. https://doi.org/10.3390/jpm12101739
Knoedler L, Miragall M, Kauke-Navarro M, Obed D, Bauer M, Tißler P, Prantl L, Machens H-G, Broer PN, Baecher H, et al. A Ready-to-Use Grading Tool for Facial Palsy Examiners—Automated Grading System in Facial Palsy Patients Made Easy. Journal of Personalized Medicine. 2022; 12(10):1739. https://doi.org/10.3390/jpm12101739
Chicago/Turabian StyleKnoedler, Leonard, Maximilian Miragall, Martin Kauke-Navarro, Doha Obed, Maximilian Bauer, Patrick Tißler, Lukas Prantl, Hans-Guenther Machens, Peter Niclas Broer, Helena Baecher, and et al. 2022. "A Ready-to-Use Grading Tool for Facial Palsy Examiners—Automated Grading System in Facial Palsy Patients Made Easy" Journal of Personalized Medicine 12, no. 10: 1739. https://doi.org/10.3390/jpm12101739
APA StyleKnoedler, L., Miragall, M., Kauke-Navarro, M., Obed, D., Bauer, M., Tißler, P., Prantl, L., Machens, H.-G., Broer, P. N., Baecher, H., Panayi, A. C., Knoedler, S., & Kehrer, A. (2022). A Ready-to-Use Grading Tool for Facial Palsy Examiners—Automated Grading System in Facial Palsy Patients Made Easy. Journal of Personalized Medicine, 12(10), 1739. https://doi.org/10.3390/jpm12101739








