Exercise Stress Test Late after Arrhythmic versus Nonarrhythmic Presentation of Myocarditis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Myocarditis Diagnosis
2.3. Treatment and Follow-Up
2.4. EST
2.5. Endpoints
2.6. Statistical Analysis
3. Results
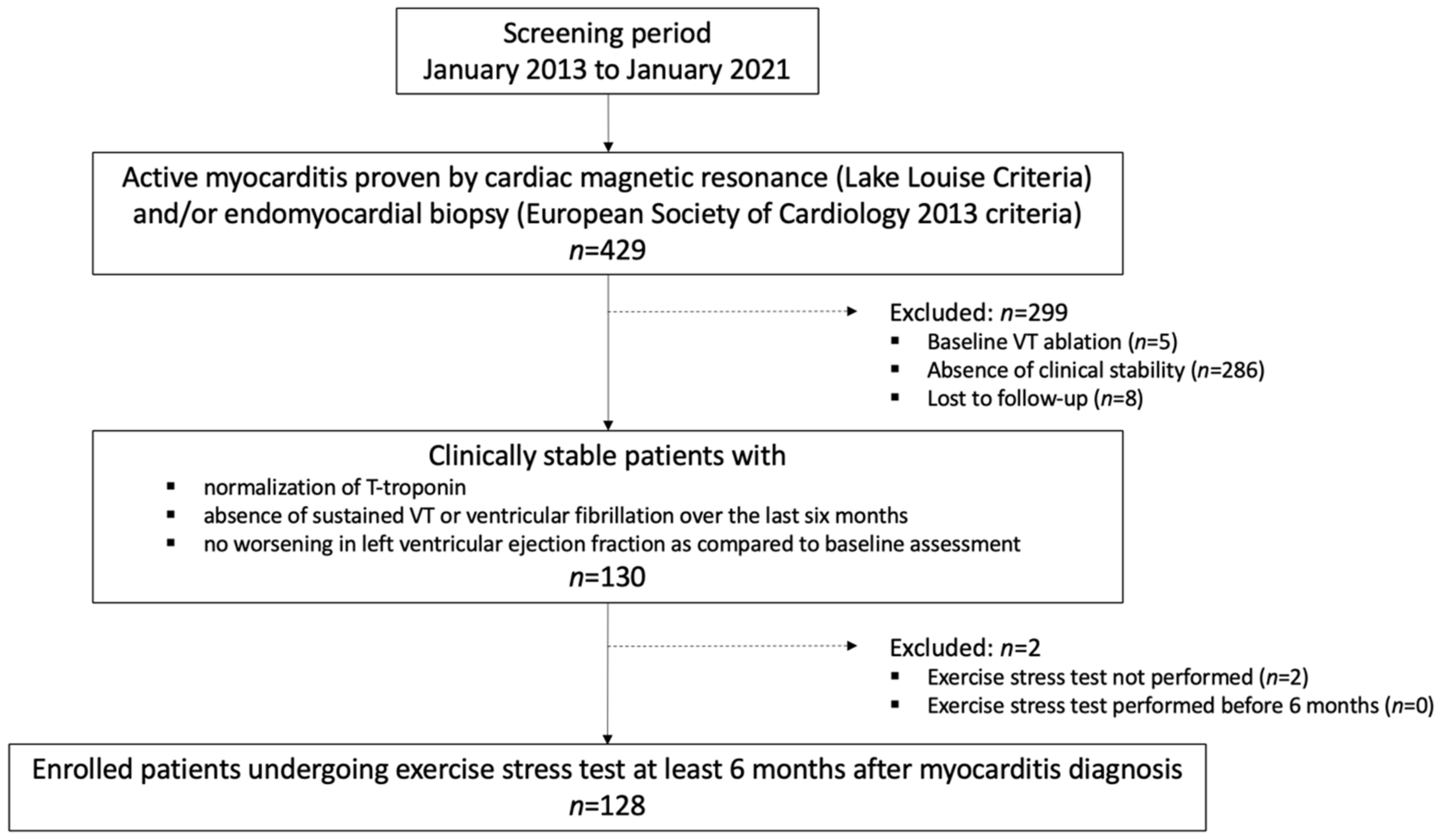
3.1. Study Population
3.2. EST Results
3.3. Outcomes
4. Discussion
4.1. Main Study Findings
4.2. EST after Myocarditis: Role of the Clinical Presentation
4.3. Significance of EST
4.4. Additional Clinical Implications of EST
4.5. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Gibbons, R.J.; Balady, G.J.; Bricker, J.T.; Chaitman, B.R.; Fletcher, G.F.; Froelicher, V.F. ACC/AHA 2002 Guideline Update for Exercise Testing: Summary Article A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). Circulation 2002, 106, 1883–1892. [Google Scholar] [CrossRef] [PubMed]
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, B.; Issa, O. Management and Treatment of Myocarditis in Athletes. Curr. Treat Options Cardiovasc. Med. 2020, 22, 65. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.-P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 2020, 42, ehaa605. [Google Scholar] [CrossRef]
- Maron, B.J.; Udelson, J.E.; Bonow, R.O.; Nishimura, R.A.; Ackerman, M.J.; Estes, N.M.; Cooper, L.; Link, M.S.; Maron, M.S. Eligibility and Disqualification Recommendations for Competitive Athletes with Cardiovascular Abnormalities: Task Force 3: Hypertrophic Cardiomyopathy, Arrhythmogenic Right Ventricular Cardiomyopathy and Other Cardiomyopathies, and Myocarditis. A Scientific Statement from the American Heart Association and American College of Cardiology. Circulation 2015, 132, e273–e280. [Google Scholar] [CrossRef]
- Modica, G.; Bianco, M.; Sollazzo, F.; Di Murro, E.; Monti, R.; Cammarano, M.; Morra, L.; Nifosì, F.M.; Gervasi, S.F.; Manes Gravina, E.; et al. Myocarditis in Athletes Recovering from COVID-19: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 4279. [Google Scholar] [CrossRef]
- Caforio, A.L.; Calabrese, F.; Angelini, A.; Tona, F.; Vinci, A.; Bottaro, S.; Ramondo, A.; Carturan, E.; Iliceto, S.; Thiene, G.; et al. A prospective study of biopsy-proven myocarditis: Prognostic relevance of clinical and aetiopathogenetic features at diagnosis. Eur. Heart J. 2007, 28, 1326–1333. [Google Scholar] [CrossRef]
- Anzini, M.; Merlo, M.; Sabbadini, G.; Barbati, G.; Finocchiaro, G.; Pinamonti, B.; Salvi, A.; Perkan, A.; Di Lenarda, A.; Bussani, R.; et al. Long-Term Evolution and Prognostic Stratification of Biopsy-Proven Active Myocarditis. Circulation 2013, 128, 2384–2394. [Google Scholar] [CrossRef]
- Ammirati, E.; Cipriani, M.; Moro, C.; Raineri, C.; Pini, D.; Sormani, P.; Mantovani, R.; Varrenti, M.; Pedrotti, P.; Conca, C.; et al. Clinical Presentation and Outcome in a Contemporary Cohort of Patients with Acute Myocarditis: Multicenter Lombardy Registry. Circulation 2018, 138, 1088–1099. [Google Scholar] [CrossRef]
- Peretto, G.; Sala, S.; Rizzo, S.; De Luca, G.; Campochiaro, C.; Sartorelli, S.; Benedetti, G.; Palmisano, A.; Esposito, A.; Tresoldi, M.; et al. Arrhythmias in myocarditis: State of the art. Heart Rhythm. 2019, 16, 793–801. [Google Scholar] [CrossRef]
- Lown, B.; Wolf, M. Approaches to sudden death from coronary heart disease. Circulation 1971, 44, 130–142. [Google Scholar] [CrossRef]
- Peretto, G.; Cappelletti, A.M.; Spoladore, R.; Slavich, M.; Rizzo, S.; Palmisano, A.; Esposito, A.; De Cobelli, F.; Margonato, A.; Basso, C.; et al. Right ventricular endomyocardial biopsy in patients with cardiac magnetic resonance showing left ventricular myocarditis. J. Cardiovasc. Med. 2021, 22, 560–566. [Google Scholar] [CrossRef]
- Friedrich, M.G.; Sechtem, U.; Schulz-Menger, J.; Holmvang, G.; Alakija, P.; Cooper, L.T.; White, J.A.; Abdel-Aty, H.; Gutberlet, M.; Prasad, S.; et al. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J. Am. Coll. Cardiol. 2009, 53, 1475–1487. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef]
- Palmisano, A.; Benedetti, G.; Faletti, R.; Rancoita, P.M.V.; Gatti, M.; Peretto, G.; Sala, S.; Boccia, E.; Francone, M.; Galea, N.; et al. Early T1 Myocardial MRI Mapping: Value in Detecting Myocardial Hyperemia in Acute Myocarditis. Radiology 2020, 295, 316–325. [Google Scholar] [CrossRef]
- Priori, S.G.; Blomström-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Van Veldhuisen, D.J. Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Europace 2015, 17, 1601–1687. [Google Scholar]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Heart Rhythm 2018, 15, e190–e252. [Google Scholar] [CrossRef]
- Peretto, G.; Sala, S.; Della Bella, P. Diagnostic and therapeutic approach to myocarditis patients presenting with arrhythmias. G. Ital. Cardiol. 2020, 21, 187–194. [Google Scholar] [CrossRef]
- De Luca, G.; Campochiaro, C.; Sartorelli, S.; Peretto, G.; Dagna, L. Therapeutic strategies for virus-negative myocarditis: A comprehensive review. Eur. J. Intern. Med. 2020, 77, 9–17. [Google Scholar] [CrossRef]
- Peretto, G.; De Luca, G.; Campochiaro, C.; Palmisano, A.; Busnardo, E.; Sartorelli, S.; Barzaghi, F.; Cicalese, M.P.; Esposito, A.; Sala, S. Telemedicine in myocarditis: Evolution of a mutidisciplinary “Disease Unit” at the time of COVID-19 pandemic. Am. Heart J. 2020, 229, 121–126. [Google Scholar] [CrossRef]
- Peretto, G.; Busnardo, E.; Ferro, P.; Palmisano, A.; Vignale, D.; Esposito, A.; De Luca, G.; Campochiaro, C.; Sartorelli, S.; De Gaspari, M.; et al. Applications of FDG-PET scan in arrhythmic myocarditis. J. Am. Coll. Cardiol. Imaging 2022. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Bires, A.M.; Lawson, D.; Wasser, T.E.; Raber-Baer, D. Comparison of Bruce treadmill exercise test protocols: Is ramped Bruce equal or superior to standard bruce in producing clinically valid studies for patients presenting for evaluation of cardiac ischemia or arrhythmia with body mass index equal to or greater than 30? J. Nucl. Med. Technol. 2013, 41, 274–278. [Google Scholar] [PubMed]
- Kharabsheh, S.M.; Al-Sugair, A.; Al-Buraiki, J.; Farhan, J. Overview of Exercise Stress Testing. Ann. Saudi Med. 2006, 26, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Peretto, G.; Sala, S.; Rizzo, S.; Palmisano, A.; Esposito, A.; De Cobelli, F.; Campochiaro, C.; De Luca, G.; Foppoli, L.; Dagna, L.; et al. Ventricular Arrhythmias in Myocarditis: Characterization and Relationships with Myocardial Inflammation. J. Am. Coll. Cardiol. 2020, 75, 1046–1057. [Google Scholar] [CrossRef]
- Gentile, P.; Merlo, M.; Peretto, G.; Ammirati, E.; Sala, S.; Della Bella, P.; Aquaro, G.D.; Imazio, M.; Potena, L.; Campodonico, J.; et al. Post-discharge arrhythmic risk stratification of patients with acute myocarditis and life-threatening ventricular tachyarrhythmias. Eur. J. Heart Fail. 2021, 23, 2045–2054. [Google Scholar] [CrossRef]
- Camici, P.G.; d’Amati, G.; Rimoldi, O. Coronary microvascular dysfunction: Mechanisms and functional assessment. Nat. Rev. Cardiol. 2015, 12, 48–62. [Google Scholar] [CrossRef]
- Schmidt-Lucke, C.; Zobel, T.; Schrepfer, S.; Kuhl, U.; Wang, D.; Klingel, K.; Becher, P.M.; Fechner, H.; Pozzuto, T.; Van Linthout, S.; et al. Impaired Endothelial Regeneration Through Human Parvovirus B19-Infected Circulating Angiogenic Cells in Patients with Cardiomyopathy. J. Infect. Dis. 2015, 212, 1070–1081. [Google Scholar] [CrossRef]
- Peretto, G.; Sala, S.; Basso, C.; Della Bella, P. Programmed ventricular stimulation in patients with active vs previous arrhythmic myocarditis. J. Cardiovasc. Electrophysiol. 2020, 31, 692–701. [Google Scholar] [CrossRef]
- Schultheiss, H.-P.; Baumeier, C.; Aleshcheva, G.; Bock, C.-T.; Escher, F. Viral Myocarditis-From Pathophysiology to Treatment. J. Clin. Med. 2021, 10, 5240. [Google Scholar] [CrossRef]
- Ukena, C.; Mahfoud, F.; Kindermann, I.; Kandolf, R.; Kindermann, M.; Böhm, M. Prognostic electrocardiographic parameters in patients with suspected myocarditis. Eur. J. Heart Fail. 2011, 13, 398–405. [Google Scholar] [CrossRef]
- Peretto, G.; Basso, C.; Della Bella, P.; Sala, S. Thyroid dysfunction in adult patients with biopsy-proved myocarditis: Screening and characterization. Eur. J. Intern. Med. 2020, 71, 98–100. [Google Scholar] [CrossRef]
- Peretto, G.; Sala, S.; Camaschella, C. Iron deficiency in chronic myocarditis: Assessment and prognostic significance. Eur. J. Intern. Med. 2021, 89, 129–131. [Google Scholar] [CrossRef]
- Peretto, G.; Sala, S.; De Luca, G.; Campochiaro, C.; Sartorelli, S.; Cappelletti, A.M.; Rizzo, S.; Palmisano, A.; Esposito, A.; Margonato, A.; et al. Impact of systemic immune-mediated diseases on clinical features and prognosis of patients with biopsy-proved myocarditis. Int. J. Cardiol. 2019, 280, 110–116. [Google Scholar] [CrossRef]
- Peretto, G.; Sala, S.; Basso, C.; Rizzo, S.; Radinovic, A.; Frontera, A.; Limite, L.R.; Paglino, G.; Bisceglia, C.; De Luca, G.; et al. Inflammation as a Predictor of Recurrent Ventricular Tachycardia After Ablation in Patients with Myocarditis. J. Am. Coll. Cardiol. 2020, 76, 1644–1656. [Google Scholar] [CrossRef]
- Peretto, G.; Sala, S.; De Luca, G.; Marcolongo, R.; Campochiaro, C.; Sartorelli, S.; Tresoldi, M.; Foppoli, L.; Palmisano, A.; Esposito, A.; et al. Immunosuppressive therapy and risk stratification of patients with myocarditis presenting with ventricular arrhythmias. JACC Clin. Electrophysiol. 2020, 6, 1221–1234. [Google Scholar] [CrossRef]
- Cooper, L.T., Jr. Ventricular Arrhythmias and Sudden Cardiac Death in Lymphocytic Myocarditis. J. Am. Coll. Cardiol. 2020, 75, 1058–1060. [Google Scholar] [CrossRef]
- Peretto, G.; Mazzone, P.; Paglino, G.; Marzi, A.; Tsitsinakis, G.; Rizzo, S.; Basso, C.; Della Bella, P.; Sala, S. Continuous Electrical Monitoring in Patients with Arrhythmic Myocarditis: Insights from a Referral Center. J. Clin. Med. 2021, 10, 5142. [Google Scholar] [CrossRef]



| Arrhythmic n = 64 | Nonarrhythmic n = 64 | p | ||
|---|---|---|---|---|
| Clinical features | ||||
| Age (y) Male gender | Mean ± SD n (%) | 42 ± 10 45 (70) | 40 ± 9 44 (68) | 0.237 1.000 |
| History of myocarditis History of SCD or CMP Agonism | n (%) n (%) n (%) | 3 (5) 5 (8) 17 (27) | 4 (6) 4 (6) 20 (31) | 1.000 1.000 0.697 |
| Anemia Thyroid dysfunction SIDs | n (%) n (%) n (%) | 7 (11) 9 (14) 6 (9) | 6 (9) 7 (11) 4 (6) | 1.000 0.790 0.744 |
| Presentation | ||||
| ACS-like HF Sustained VT/VF NSVT VE Lown’s grade ≥ 2 * | n (%) n (%) n (%) n (%) n (%) | 0 (0) 0 (0) 32 (50) 18 (28) 14 (22) | 34 (53) 30 (47) 0 (0) 0 (0) 0 (0) | <0.001 <0.001 <0.001 <0.001 <0.001 |
| Blood exams | ||||
| T-Troponin (ng/L) NTproBNP (pg/mL) C-reactive protein (mg/L) | Median ± IQR Median ± IQR Median ± IQR | 46 (19–312) 507 (118–1965) 5 (3–14) | 78 (22–517) 396 (89–2170) 6 (3–25) | 0.326 0.512 0.618 |
| ECG | ||||
| PQ (ms) QRS (ms) QTc (ms) LBBB | Mean ± SD Mean ± SD Mean ± SD n (%) | 174 ± 39 103 ± 24 416 ± 31 3 (5) | 168 ± 42 99 ± 26 409 ± 33 5 (8) | 0.404 0.368 0.218 0.718 |
| Echocardiogram | ||||
| LVEDVi (mL/m2) LVEF (%) E/E’ RVEDD (mm) TAPSE (mm) Pericardial effusion | Mean ± SD Mean ± SD Mean ± SD Mean ± SD Mean ± SD n (%) | 72 ± 20 50 ± 10 7 ± 2 29 ± 3 21 ± 3 2 (3) | 68 ± 28 52 ± 16 7 ± 3 29 ± 4 22 ± 4 6 (9) | 0.404 0.398 1.000 1.000 0.312 0.273 |
| Myocarditis diagnosis | ||||
| CMR-proven (LLC) STIR, T2 LGE, T1, ECV EMB-proven (ESC criteria) CD3+ TCL > 7/mm2 Viral PCR | n (%) n (%) n (%) n (%) n (%) n (%) | 39 (61) 39 (61) 60 (94) 62 (97) 62 (97) 7 (11) | 58 (91) 58 (91) 60 (94) 54 (84) 54 (84) 13 (20) | <0.001 <0.001 1.000 0.030 0.030 0.223 |
| Treatment at discharge | ||||
| ACE-inhibitors Betablockers Diuretics Antiarrhythmics IST ICD | n (%) n (%) n (%) n (%) n (%) n (%) | 56 (88) 61 (95) 7 (11) 50 (78) 49 (77) 30 (47) | 50 (78) 47 (73) 14 (22) 3 (5) 37 (58) 9 (14) | 0.241 0.001 0.151 <0.001 0.038 <0.001 |
| Total n = 128 | Arrhythmic n = 64 | Nonarrhythmic n = 64 | p | ||
|---|---|---|---|---|---|
| Time from clinical presentation | Mean ± SD | 15 ± 4 | 19 ± 4 | 12 ± 3 | <0.001 |
| Treadmill Bicycle | n (%) n (%) | 117 (91) 11 (9) | 59 (92) 5 (8) | 58 (91) 6 (9) | 1.000 1.000 |
| On treatment on betablockers on antiarrhythmics Off treatment | n (%) n (%) n (%) n (%) | 79 (62) 75 (59) 14 (11) 49 (38) | 59 (92) 55 (86) 14 (22) 5 (8) | 20 (31) 20 (31) 0 (0) 44 (69) | <0.001 <0.001 <0.001 <0.001 |
| Maximal power (W) Maximal METs Peak SBP (mmHg) Peak HR (bpm) Peak RPP (*102) % MTHR (%) | Mean ± SD Mean ± SD Mean ± SD Mean ± SD Mean ± SD Mean ± SD | 142 ± 9 10 ± 3 157 ± 14 150 ± 12 24 ± 5 85 ± 6 | 133 ± 8 9 ± 2 153 ± 16 146 ± 13 23 ± 5 84 ± 6 | 151 ± 11 11 ± 4 159 ± 15 154 ± 14 25 ± 6 86 ± 6 | <0.001 0.001 0.031 0.001 0.043 0.062 |
| Maximal negative test on betablockers off betablockers Submaximal negative test on betablockers off betablockers Positive test on betablockers off betablockers | n (%) n (%) n (%) n (%) n (%) n (%) n (%) n (%) n (%) | 64 (50) 24 (19) 40 (31) 10 (8) 9 (7) 1 (1) 54 (42) 42 (33) 12 (9) | 14 (22) 8 (13) 6 (9) 6 (9) 6 (9) 0 (0) 44 (69) 41 (64) 3 (5) | 50 (78) 16 (25) 34 (53) 4 (6) 3 (5) 1 (2) 10 (16) 1 (2) 9 (14) | <0.001 0.112 <0.001 0.744 0.492 1.000 <0.001 <0.001 0.127 |
| VA Sustained VT/VF * NSVT VE | n (%) n (%) n (%) n (%) | 47 (37) 5 (4) 24 (19) 40 (31) | 43 (67) 5 (8) 24 (38) 36 (56) | 4 (6) 0 (0) 0 (0) 4 (6) | <0.001 0.058 <0.001 <0.001 |
| Ischemia * ST-T changes Angina-like chest pain Uninterpretable for LBBB | n (%) n (%) n (%) n (%) | 7 (5) 4 (3) 3 (2) 8 (6) | 1 (2) 0 (0) 1 (2) 3 (5) | 6 (9) 4 (6) 2 (3) 5 (8) | 0.115 0.119 1.000 0.718 |
| PID | Age (y) | Gender | Presentation | Baseline LVEF (%) | Baseline Myocarditis | Baseline Treatment | Malignant VA during EST | Management |
|---|---|---|---|---|---|---|---|---|
| P45 | 54 | Male | NSVT | 55 | EMB-proven, virus-negative | sotalol, ramipril | Presyncopal sustained VT | ICD implant. EMB: chronically active virus-negative lymphocytic myocarditis. IST for 12 months until FDG-PET normalization |
| P64 | 32 | Male | Sustained VT | 60 | CMR-proven | metoprolol | Tolerated sustained VT | EMB: chronically active virus-negative lymphocytic myocarditis. IST for 12 months until CMR normalization. Flecainide |
| P67 | 34 | Male | VF | 51 | EMB-proven, viral | metoprolol, amiodarone, ICD | Presyncopal sustained VT | VT ablation. Subsequent uneventful follow-up |
| P78 | 34 | Male | Sustained VT | 66 | EMB-proven, virus-negative | flecainide, metoprolol, prior IST (prednisone, azathioprine), ICD | Tolerated sustained VT | FDG-PET: normal. EMB: replacement fibrosis, no myocarditis. VT ablation. Subsequent uneventful follow-up |
| P124 | 44 | Male | Sustained VT | 60 | EMB-proven, virus-negative | flecainide, metoprolol, prior IST (prednisone, azathioprine), ICD | Syncopal sustained VT | EMB: replacement fibrosis, no myocarditis. VT ablation. Subsequent uneventful follow-up |
| PID | Age (y) | Gender | Presentation | Baseline LVEF (%) | Baseline Myocarditis | Baseline Treatment | Ischemia during EST | Management |
| P37 | 68 | Male | ACS-like | 58 | CMR-proven | ramipril, ivabradine | ST-T changes, asymptomatic | Coronary angiography: normal. CMR: persistently active myocarditis. EMB: chronically active viral lymphocytic myocarditis (parvovirus B19). No etiology-specific treatment |
| P41 | 59 | Male | ACS-like | 60 | CMR-proven and EMB-proven, virus-negative | ramipril, prior IST (prednisone, azathioprine) | Angina-like chest pain, no ST-T changes | Coronary CT scan: normal. CMR: persistently active myocarditis. EMB: chronically active virus-negative lymphocytic myocarditis. IST for additional 6 months until CMR normalization |
| P63 | 41 | Male | HF | 25 | CMR-proven after LVEF recovery up to 50% | enalapril, furosemide | ST-T changes, asymptomatic | Coronary CT scan: normal. CMR: normal. Bisoprolol. No additional diagnostic workup |
| P98 | 40 | Male | HF | 38 | CMR-proven | sacubitril/valsartan | ST-T changes, asymptomatic | Coronary CT scan: normal. CMR: persistently active myocarditis. EMB: chronically active virus-negative lymphocytic myocarditis. Bisoprolol and IST for 12 months until CMR normalization |
| P99 | 52 | Male | ACS-like | 55 | EMB-proven, virus-negative | ramipril, prior IST (prednisone, azathioprine) | Angina-like chest pain, no ST-T changes, abnormal T-troponin | Coronary CT scan: normal. CMR: persistently active myocarditis. EMB: chronically active virus-negative lymphocytic myocarditis. Bisoprolol and IST for additional 6 months until CMR normalization |
| P103 | 64 | Male | ACS-like | 62 | CMR-proven | none | Angina-like chest pain, no ST-T changes | CMR: normal. Coronary CT scan: normal. No additional diagnostic workup |
| P119 | 23 | Female | Sustained VT | 44 | CMR-proven and EMB-proven, virus-negative | metoprolol, ramipril, prior IST (prednisone, azathioprine), ICD | Angina-like chest pain, no ST-T changes, abnormal T-troponin | FDG-PET scan: persistently active myocarditis. EMB: chronically active virus-negative lymphocytic myocarditis. IST for additional 12 months until FDG-PET normalization |
| Total n = 128 | Arrhythmic n = 64 | Nonarrhythmic n = 64 | p | EST+ n = 54 | EST- n = 74 | p | ||
|---|---|---|---|---|---|---|---|---|
| Adverse events | n (%) | 52 (41) | 39 (61) | 13 (20) | <0.001 | 40 (74) | 12 (16) | <0.001 |
| Cardiac death | n (%) | 3 (2) | 3 (5) | 0 (0) | 0.244 | 3 (6) | 0 (0) | 0.073 |
| Disease-related hospitalizations | n (%) | 37 (29) | 24 (38) | 13 (20) | 0.050 | 27 (50) | 10 (14) | <0.001 |
| Malignant VA * | n (%) | 23 (18) | 21 (33) | 2 (3) | <0.001 | 19 (35) | 4 (5) | <0.001 |
| Proven active myocarditis | n (%) | 10 (8) | 7 (11) | 3 (5) | 0.324 | 8 (15) | 2 (3) | 0.017 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peretto, G.; Gulletta, S.; Slavich, M.; Campochiaro, C.; Vignale, D.; De Luca, G.; Palmisano, A.; Villatore, A.; Rizzo, S.; Cavalli, G.; et al. Exercise Stress Test Late after Arrhythmic versus Nonarrhythmic Presentation of Myocarditis. J. Pers. Med. 2022, 12, 1702. https://doi.org/10.3390/jpm12101702
Peretto G, Gulletta S, Slavich M, Campochiaro C, Vignale D, De Luca G, Palmisano A, Villatore A, Rizzo S, Cavalli G, et al. Exercise Stress Test Late after Arrhythmic versus Nonarrhythmic Presentation of Myocarditis. Journal of Personalized Medicine. 2022; 12(10):1702. https://doi.org/10.3390/jpm12101702
Chicago/Turabian StylePeretto, Giovanni, Simone Gulletta, Massimo Slavich, Corrado Campochiaro, Davide Vignale, Giacomo De Luca, Anna Palmisano, Andrea Villatore, Stefania Rizzo, Giulio Cavalli, and et al. 2022. "Exercise Stress Test Late after Arrhythmic versus Nonarrhythmic Presentation of Myocarditis" Journal of Personalized Medicine 12, no. 10: 1702. https://doi.org/10.3390/jpm12101702
APA StylePeretto, G., Gulletta, S., Slavich, M., Campochiaro, C., Vignale, D., De Luca, G., Palmisano, A., Villatore, A., Rizzo, S., Cavalli, G., De Gaspari, M., Busnardo, E., Gianolli, L., Dagna, L., Basso, C., Esposito, A., Sala, S., Della Bella, P., & Mazzone, P. (2022). Exercise Stress Test Late after Arrhythmic versus Nonarrhythmic Presentation of Myocarditis. Journal of Personalized Medicine, 12(10), 1702. https://doi.org/10.3390/jpm12101702









