Molecular Profiling in Non-Squamous Non-Small Cell Lung Carcinoma: Towards a Switch to Next-Generation Sequencing Reflex Testing
Abstract
1. Introduction
2. Approved Companion Tests (Figure 1)
2.1. EGFR
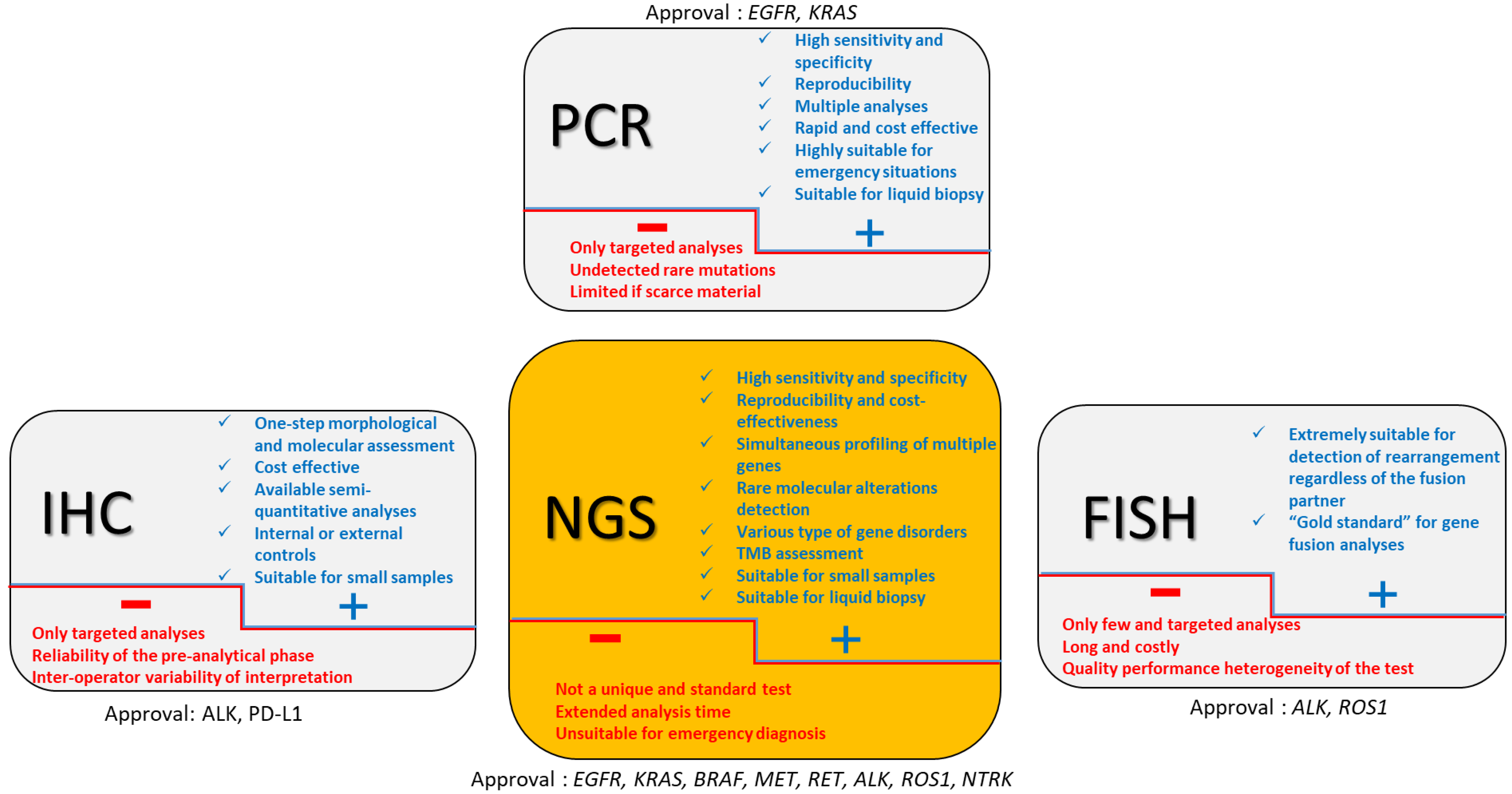
2.2. ALK
2.3. ROS1
2.4. BRAF
2.5. KRAS
2.6. MET
2.7. RET
2.8. NTRK
3. From Multiple Companion Biomarkers to NGS?
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- O’Keeffe, L.M.; Taylor, G.; Huxley, R.R.; Mitchell, P.; Woodward, M.; Peters, S.A.E. Smoking as a risk factor for lung cancer in women and men: A systematic review and meta-analysis. BMJ Open 2018, 8, e021611. [Google Scholar] [CrossRef]
- Pelosof, L.; Ahn, C.; Gao, A.; Horn, L.; Madrigales, A.; Cox, J.; McGavic, D.; Minna, J.D.; Gazdar, A.F.; Schiller, J. Proportion of Never-Smoker Non-Small Cell Lung Cancer Patients at Three Diverse Institutions. J. Natl. Cancer Inst. 2017, 109, djw295. [Google Scholar] [CrossRef] [PubMed]
- Sholl, L.M.; Aisner, D.L.; Varella-Garcia, M.; Berry, L.D.; Dias-Santagata, D.; Wistuba, I.I.; Chen, H.; Fujimoto, J.; Kugler, K.; Franklin, W.A.; et al. Multi-institutional oncogenic driver mutation analysis in lung adenocarcinoma: The lung cancer mutation consortium experience. J. Thorac. Oncol. 2015, 10, 768–777. [Google Scholar] [CrossRef]
- Smolle, E.; Pichler, M. Non-smoking-associated lung cancer: A distinct entity in terms of tumor biology, patient characteristics and impact of hereditary cancer predisposition. Cancers 2019, 11, 204. [Google Scholar] [CrossRef]
- Travis, W.D.; Rekhtman, N. Pathological diagnosis and classification of lung cancer in small biopsies and cytology: Strategic management of tissue for molecular testing. Semin. Respir. Crit. Care Med. 2011, 32, 22–31. [Google Scholar] [CrossRef]
- Walsh, S.L.F.; Lederer, D.J.; Ryerson, C.J.; Kolb, M.; Maher, T.M.; Nusser, R.; Poletti, V.; Richeldi, L.; Vancheri, C.; Wilsher, M.L.; et al. Diagnostic likelihood thresholds that define a working diagnosis of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2019, 200, 1146–1153. [Google Scholar] [CrossRef]
- Mok, T.S.; Wu, Y.-L.; Thongprasert, S.; Yang, C.-H.; Chu, D.-T.; Saijo, N.; Sunpaweravong, P.; Han, B.; Margono, B.; Ichinose, Y.; et al. Gefitinib or Carboplatin–Paclitaxel in Pulmonary Adenocarcinoma. N. Engl. J. Med. 2009, 361, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wu, Y.L.; Chen, G.; Feng, J.; Liu, X.Q.; Wang, C.; Zhang, S.; Wang, J.; Zhou, S.; Ren, S.; et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011, 12, 735–742. [Google Scholar] [CrossRef]
- Pao, W.; Miller, V.A.; Politi, K.A.; Riely, G.J.; Somwar, R.; Zakowski, M.F.; Kris, M.G.; Varmus, H. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005, 2, 0225–0235. [Google Scholar] [CrossRef]
- Papadimitrakopoulou, V.A.; Mok, T.S.; Han, J.Y.; Ahn, M.J.; Delmonte, A.; Ramalingam, S.S.; Kim, S.W.; Shepherd, F.A.; Laskin, J.; He, Y.; et al. Osimertinib versus platinum–pemetrexed for patients with EGFR T790M advanced NSCLC and progression on a prior EGFR-tyrosine kinase inhibitor: AURA3 overall survival analysis. Ann. Oncol. 2020, 31, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Leonetti, A.; Sharma, S.; Minari, R.; Perego, P.; Giovannetti, E.; Tiseo, M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br. J. Cancer 2019, 121, 725–737. [Google Scholar] [CrossRef]
- Schalm, S.S.; Dineen, T.; Lim, S.M.; Park, C.-W.; Hsieh, J.; Woessner, R.; Zhang, Z.; Wilson, K.; Eno, M.; Wilson, D.; et al. 1296P BLU-945, a highly potent and selective 4th generation EGFR TKI for the treatment of EGFR T790M/C797S resistant NSCLC. Ann. Oncol. 2020, 31, S839. [Google Scholar] [CrossRef]
- 14. Finlay, M.R.V.; Barton, P.; Bickerton, S.; Bista, M.; Colclough, N.; Cross, D.A.E.; Evans, L.; Floc’h, N.; Gregson, C.; Guérot, C.M.; et al. Potent and Selective Inhibitors of the Epidermal Growth Factor Receptor to Overcome C797S-Mediated Resistance. J. Med. Chem. 2021, 64, 13704–13718. [Google Scholar] [CrossRef] [PubMed]
- Hofman, P.; Rouleau, E.; Sabourin, J.C.; Denis, M.; Deleuze, J.F.; Barlesi, F.; Laurent-Puig, P. Predictive molecular pathology in non–small cell lung cancer in France: The past, the present and the perspectives. Cancer Cytopathol. 2020, 128, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.A.; Aisner, D.L.; Oxnard, G.R. Precision Medicine in Non–Small Cell Lung Cancer: Current Standards in Pathology and Biomarker Interpretation. Am. Soc. Clin. Oncol. Educ. B 2018, 38, 708–715. [Google Scholar] [CrossRef]
- Aisner, D.L.; Riely, G.J. Non-small cell lung cancer: Recommendations for biomarker testing and treatment. J. Natl. Compr. Cancer Netw. 2021, 19, 610–613. [Google Scholar] [CrossRef]
- Li, B.T.; Smit, E.F.; Goto, Y.; Nakagawa, K.; Udagawa, H.; Mazières, J.; Nagasaka, M.; Bazhenova, L.; Saltos, A.N.; Felip, E.; et al. Trastuzumab Deruxtecan in HER2 -Mutant Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2022, 386, 241–251. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Tsuboi, M.; He, J.; John, T.; Grohe, C.; Majem, M.; Goldman, J.W.; Laktionov, K.; Kim, S.-W.; Kato, T.; et al. Osimertinib in Resected EGFR -Mutated Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, C.; Bubendorf, L.; Cooper, W.A.; Illei, P.; Borralho Nunes, P.; Ong, B.H.; Tsao, M.S.; Yatabe, Y.; Kerr, K.M. Molecular testing in stage I–III non-small cell lung cancer: Approaches and challenges. Lung Cancer 2021, 162, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Schubart, C.; Stöhr, R.; Tögel, L.; Fuchs, F.; Sirbu, H.; Seitz, G.; Seggewiss-bernhardt, R.; Leistner, R.; Sterlacci, W.; Vieth, M.; et al. Met amplification in non-small cell lung cancer (Nsclc)—A consecutive evaluation using next-generation sequencing (ngs) in a real-world setting. Cancers 2021, 13, 5023. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Seto, T.; Han, J.-Y.; Reguart, N.; Garon, E.B.; Groen, H.J.M.; Tan, D.S.W.; Hida, T.; de Jonge, M.; Orlov, S.V.; et al. Capmatinib in MET Exon 14–Mutated or MET -Amplified Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 944–957. [Google Scholar] [CrossRef] [PubMed]
- Le, X.; Paz-Ares, L.G.; Van Meerbeeck, J.; Viteri, S.; Cabrera Galvez, C.; Vicente Baz, D.; Kim, Y.-C.; Kang, J.-H.; Schumacher, K.-M.; Karachaliou, N.; et al. Tepotinib in patients (pts) with advanced non-small cell lung cancer (NSCLC) with MET amplification (MET amp). J. Clin. Oncol. 2021, 39, 9021. [Google Scholar] [CrossRef]
- Jørgensen, J.T.; Mollerup, J. Companion Diagnostics and Predictive Biomarkers for MET-Targeted Therapy in NSCLC. Cancers 2022, 14, 2150. [Google Scholar] [CrossRef]
- Mendelsohn, J.; Baselga, J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J. Clin. Oncol. 2003, 21, 2787–2799. [Google Scholar] [CrossRef]
- Schlessinger, J. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell 2002, 110, 669–672. [Google Scholar] [CrossRef]
- Lynch, T.J.; Bell, D.W.; Sordella, R.; Gurubhagavatula, S.; Okimoto, R.A.; Brannigan, B.W.; Harris, P.L.; Haserlat, S.M.; Supko, J.G.; Haluska, F.G.; et al. Activating Mutations in the Epidermal Growth Factor Receptor Underlying Responsiveness of Non–Small-Cell Lung Cancer to Gefitinib. N. Engl. J. Med. 2004, 350, 2129–2139. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.S.; Vansteenkiste, J.; Planchard, D.; Cho, B.C.; Gray, J.E.; Ohe, Y.; Zhou, C.; Reungwetwattana, T.; Cheng, Y.; Chewaskulyong, B.; et al. Overall Survival with Osimertinib in Untreated, EGFR -Mutated Advanced NSCLC. N. Engl. J. Med. 2020, 382, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Dagogo-Jack, I.; Azzolli, C.G.; Fintelmann, F.; Mino-Kenudson, M.; Farago, A.F.; Gainor, J.F.; Jiang, G.; Piotrowska, Z.; Heist, R.S.; Lennes, I.T.; et al. Clinical Utility of Rapid EGFR Genotyping in Advanced Lung Cancer. JCO Precis. Oncol. 2018, 2, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gristina, V.; Malapelle, U.; Galvano, A.; Pisapia, P.; Pepe, F.; Rolfo, C.; Tortorici, S.; Bazan, V.; Troncone, G.; Russo, A. The significance of epidermal growth factor receptor uncommon mutations in non-small cell lung cancer: A systematic review and critical appraisal. Cancer Treat. Rev. 2020, 85, 101994. [Google Scholar] [CrossRef] [PubMed]
- Robichaux, J.P.; Le, X.; Vijayan, R.S.K.; Hicks, J.K.; Heeke, S.; Elamin, Y.Y.; Lin, H.Y.; Udagawa, H.; Skoulidis, F.; Tran, H.; et al. Structure-based classification predicts drug response in EGFR-mutant NSCLC. Nature 2021, 597, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.T.; Vyse, S.; Huang, P.H. Rare epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer. Semin. Cancer Biol. 2020, 61, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, C.; Rolfo, C.D.; Oxnard, G.R.; Gray, J.E.; Sholl, L.M.; Gandara, D.R. Strategies for the successful implementation of plasma-based NSCLC genotyping in clinical practice. Nat. Rev. Clin. Oncol. 2021, 18, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Olivier, T.; Prasad, V. Amivantamab and Mobocertinib in Exon 20 insertions EGFR Mutant Lung Cancer, Challenge to the Current Guidelines. Transl. Oncol. 2022, 23, 101475. [Google Scholar] [CrossRef]
- Park, K.; Haura, E.B.; Leighl, N.B.; Mitchell, P.; Shu, C.A.; Girard, N.; Viteri, S.; Han, J.Y.; Kim, S.W.; Lee, C.K.; et al. Amivantamab in egfr exon 20 insertion- mutated non-small-cell lung cancer progressing on platinum chemotherapy: Initial results from the chrysalis phase i study. J. Clin. Oncol. 2021, 39, 3391–3402. [Google Scholar] [CrossRef]
- Shu, C.A.; Goto, K.; Cho, B.C.; Griesinger, F.; Yang, J.C.-H.; Felip, E.; Xie, J.; Chen, J.; Mahoney, J.; Thayu, M.; et al. CHRYSALIS-2: A phase 1/1b study of lazertinib as monotherapy and in combination with amivantamab in patients with EGFR-mutant NSCLC. J. Clin. Oncol. 2021, 39, TPS9132. [Google Scholar] [CrossRef]
- Agrawal, T.; Artis, E.; Xie, J.; Bhattacharya, A.; Haddish-Berhane, N.; Gopen, T.; Curtin, J.C.; Karkera, J.; Roshak, A.; Knoblauch, R.E.; et al. P76.74 PAPILLON: Randomized Phase 3 Study of Amivantamab Plus Chemotherapy vs Chemotherapy Alone in EGFR Exon20ins NSCLC. J. Thorac. Oncol. 2021, 16, S621. [Google Scholar] [CrossRef]
- Zhou, C.; Ramalingam, S.S.; Kim, T.M.; Kim, S.W.; Yang, J.C.H.; Riely, G.J.; Mekhail, T.; Nguyen, D.; Garcia Campelo, M.R.; Felip, E.; et al. Treatment Outcomes and Safety of Mobocertinib in Platinum-Pretreated Patients with EGFR Exon 20 Insertion-Positive Metastatic Non-Small Cell Lung Cancer: A Phase 1/2 Open-label Nonrandomized Clinical Trial. JAMA Oncol. 2021, 7, 1772–1781. [Google Scholar] [CrossRef]
- Morris, S.W.; Kirstein, M.N.; Valentine, M.B.; Dittmer, K.G.; Shapiro, D.N.; Saltman, D.L.; Look, A.T. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science 1994, 263, 1281–1284. [Google Scholar] [CrossRef]
- Soda, M.; Choi, Y.L.; Enomoto, M.; Takada, S.; Yamashita, Y.; Ishikawa, S.; Fujiwara, S.I.; Watanabe, H.; Kurashina, K.; Hatanaka, H.; et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007, 448, 561–566. [Google Scholar] [CrossRef]
- Chia, P.L.; Dobrovic, A.; Dobrovic, A.; John, T. Prevalence and natural history of ALK positive non-small-cell lung cancer and the clinical impact of targeted therapy with ALK inhibitors. Clin. Epidemiol. 2014, 6, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Deng, C.; Qiu, Z.; Cao, C.; Wu, F. The Resistance Mechanisms and Treatment Strategies for ALK-Rearranged Non-Small Cell Lung Cancer. Front. Oncol. 2021, 11, 3945. [Google Scholar] [CrossRef]
- Kozuma, Y.; Toyokawa, G.; Seto, T. ALK testing methods: Is there a winner or loser? Expert Rev. Anticancer Ther. 2019, 19, 237–244. [Google Scholar] [CrossRef]
- Mino-Kenudson, M.; Chirieac, L.R.; Law, K.; Hornick, J.L.; Lindeman, N.; Mark, E.J.; Cohen, D.W.; Johnson, B.E.; Jänne, P.A.; Iafrate, A.J.; et al. A novel, highly sensitive antibody allows for the routine detection of ALK-rearranged lung adenocarcinomas by standard immunohistochemistry. Clin. Cancer Res. 2010, 16, 1561–1571. [Google Scholar] [CrossRef]
- Wynes, M.W.; Sholl, L.M.; Dietel, M.; Schuuring, E.; Tsao, M.S.; Yatabe, Y.; Tubbs, R.R.; Hirsch, F.R. An international interpretation study using the ALK IHC antibody D5F3 and a sensitive detection kit demonstrates high concordance between ALK IHC and ALK FISH and between evaluators. J. Thorac. Oncol. 2014, 9, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Kazdal, D.; Hofman, V.; Christopoulos, P.; Ilié, M.; Stenzinger, A.; Hofman, P. Fusion-positive non-small cell lung carcinoma: Biological principles, clinical practice, and diagnostic implications. Genes Chromosom. Cancer 2022, 61, 244–260. [Google Scholar] [CrossRef]
- Xia, P.; Zhang, L.; Li, P.; Liu, E.; Li, W.; Zhang, J.; Li, H.; Su, X.; Jiang, G. Molecular characteristics and clinical outcomes of complex ALK rearrangements identified by next-generation sequencing in non-small cell lung cancers. J. Transl. Med. 2021, 19, 308. [Google Scholar] [CrossRef] [PubMed]
- Chin, L.P.; Soo, R.A.; Soong, R.; Ou, S.H.I. Targeting ROS1 with anaplastic lymphoma kinase inhibitors: A promising therapeutic strategy for a newly defined molecular subset of non-small-cell lung cancer. J. Thorac. Oncol. 2012, 7, 1625–1630. [Google Scholar] [CrossRef]
- Moliner, L.; Arriola, E. ROS1 non-small cell lung cancer patients treatment approach. Precis. Cancer Med. 2021, 4, 25. [Google Scholar]
- Garrido, P.; Conde, E.; de Castro, J.; Gómez-Román, J.J.; Felip, E.; Pijuan, L.; Isla, D.; Sanz, J.; Paz-Ares, L.; López-Ríos, F. Updated guidelines for predictive biomarker testing in advanced non-small-cell lung cancer: A National Consensus of the Spanish Society of Pathology and the Spanish Society of Medical Oncology. Clin. Transl. Oncol. 2020, 22, 989–1003. [Google Scholar] [CrossRef]
- Conde, E.; Hernandez, S.; Benito, A.; Caminoa, A.; Garrido, P.; Lopez-Rios, F. Screening for ROS1 fusions in patients with advanced non-small cell lung carcinomas using the VENTANA ROS1 (SP384) Rabbit Monoclonal Primary Antibody. Expert Rev. Mol. Diagn. 2021, 21, 437–444. [Google Scholar] [CrossRef]
- Lindeman, N.I.; Cagle, P.T.; Aisner, D.L.; Arcila, M.E.; Beasley, M.B.; Bernicker, E.H.; Colasacco, C.; Dacic, S.; Hirsch, F.R.; Kerr, K.; et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors guideline from the college of American pathologists, the international association for the study of lung cancer, and the a. Arch. Pathol. Lab. Med. 2018, 142, 321–346. [Google Scholar] [CrossRef]
- Hofman, V.; Rouquette, I.; Long-Mira, E.; Piton, N.; Chamorey, E.; Heeke, S.; Vignaud, J.M.; Yguel, C.; Mazières, J.; Lepage, A.-L.; et al. Multicenter Evaluation of a Novel ROS1 Immunohistochemistry Assay (SP384) for Detection of ROS1 Rearrangements in a Large Cohort of Lung Adenocarcinoma Patients. J. Thorac. Oncol. 2019, 14, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Bubendorf, L.; Büttner, R.; Al-Dayel, F.; Dietel, M.; Elmberger, G.; Kerr, K.; López-Ríos, F.; Marchetti, A.; Öz, B.; Pauwels, P.; et al. Testing for ROS1 in non-small cell lung cancer: A review with recommendations. Virchows Arch. 2016, 469, 489–503. [Google Scholar] [CrossRef]
- Ito, K.; Sakata, S.; Otsubo, K.; Nakamura, A.; Teraoka, S.; Matsumoto, N.; Shiraishi, Y.; Haratani, K.; Tamiya, M.; Ikeda, S.; et al. MO4-5 A multicenter retrospective study of Oncomine Dx Target Test multi-CDx system for genetic profiling of NSCLC: WJOG13019L. Ann. Oncol. 2021, 32, S297. [Google Scholar] [CrossRef]
- Schwartzberg, L.S.; Li, G.; Tolba, K.; Bourla, A.B.; Schulze, K.; Gadgil, R.; Fine, A.; Lofgren, K.T.; Graf, R.P.; Oxnard, G.R.; et al. Complementary roles for tissue and blood based comprehensive genomic profiling for detection of actionable driver alterations in advanced NSCLC. JTO Clin. Res. Rep. 2022, 3, 100386. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Planchard, D.; Besse, B.; Groen, H.J.M.; Hashemi, S.M.S.; Mazieres, J.; Kim, T.M.; Quoix, E.; Souquet, P.J.; Barlesi, F.; Baik, C.; et al. Phase 2 Study of Dabrafenib Plus Trametinib in Patients With BRAF V600E-Mutant Metastatic NSCLC: Updated 5-Year Survival Rates and Genomic Analysis. J. Thorac. Oncol. 2022, 17, 103–115. [Google Scholar] [CrossRef]
- Planchard, D.; Smit, E.F.; Groen, H.J.M.; Mazieres, J.; Besse, B.; Helland, Å.; Giannone, V.; D’Amelio, A.M., Jr.; Zhang, P.; Mookerjee, B.; et al. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: An open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1307–1316. [Google Scholar] [CrossRef]
- Gow, C.H.; Hsieh, M.S.; Lin, Y.T.; Liu, Y.N.; Shih, J.Y. Validation of immunohistochemistry for the detection of BRAF V600E-mutated lung adenocarcinomas. Cancers 2019, 11, 866. [Google Scholar] [CrossRef]
- Sasaki, H.; Shimizu, S.; Tani, Y.; Shitara, M.; Okuda, K.; Hikosaka, Y.; Moriyama, S.; Yano, M.; Fujii, Y. Usefulness of immunohistochemistry for the detection of the BRAF V600E mutation in Japanese lung adenocarcinoma. Lung Cancer 2013, 82, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Hofman, V.; Benzaquen, J.; Heeke, S.; Lassalle, S.; Poudenx, M.; Long, E.; Lantéri, E.; Bordone, O.; Lespinet, V.; Tanga, V.; et al. Real-world assessment of the BRAF status in non-squamous cell lung carcinoma using VE1 immunohistochemistry: A single laboratory experience (LPCE, Nice, France). Lung Cancer 2020, 145, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Ilie, M.; Long, E.; Hofman, V.; Dadone, B.; Marquette, C.H.; Mouroux, J.; Vignaud, J.M.; Begueret, H.; Merlio, J.P.; Capper, D.; et al. Diagnostic value of immunohistochemistry for the detection of the BRAFV600E mutation in primary lung adenocarcinoma caucasian patients. Ann. Oncol. 2013, 24, 742–748. [Google Scholar] [CrossRef]
- Keohavong, P.; DeMichele, M.A.A.; Melacrinos, A.C.; Landreneau, R.J.; Weyant, R.J.; Siegfried, J.M. Detection of K-ras mutations in lung carcinomas: Relationship to prognosis. Clin. Cancer Res. 1996, 2, 411–418. [Google Scholar]
- Judd, J.; Karim, N.A.; Khan, H.; Naqash, A.R.; Baca, Y.; Xiu, J.; VanderWalde, A.M.; Mamdani, H.; Raez, L.E.; Nagasaka, M.; et al. Characterization of KRAS mutation subtypes in non-small cell lung cancer. Mol. Cancer Ther. 2021, 20, 2577–2584. [Google Scholar] [CrossRef]
- Bontoux, C.; Hofman, V.; Brest, P.; Ilié, M.; Mograbi, B.; Hofman, P. Daily Practice Assessment of KRAS Status in NSCLC Patients: A New Challenge for the Thoracic Pathologist Is Right around the Corner. Cancers 2022, 14, 1628. [Google Scholar] [CrossRef]
- Jänne, P.A.; Riely, G.J.; Gadgeel, S.M.; Heist, R.S.; Ou, S.-H.I.; Pacheco, J.M.; Johnson, M.L.; Sabari, J.K.; Leventakos, K.; Yau, E.; et al. Adagrasib in Non–Small-Cell Lung Cancer Harboring a KRAS G12C Mutation. N. Engl. J. Med. 2022, 387, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Xu, X.; Fan, Y. KRAS-Mutant Non-Small Cell Lung Cancer: An Emerging Promisingly Treatable Subgroup. Front. Oncol. 2021, 11, 672612. [Google Scholar] [CrossRef]
- Skoulidis, F.; Li, B.T.; Dy, G.K.; Price, T.J.; Falchook, G.S.; Wolf, J.; Italiano, A.; Schuler, M.; Borghaei, H.; Barlesi, F.; et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N. Engl. J. Med. 2021, 384, 2371–2381. [Google Scholar] [CrossRef]
- Sattler, M.; Reddy, M.M.; Hasina, R.; Gangadhar, T.; Salgia, R. The role of the c-Met pathway in lung cancer and the potential for targeted therapy. Ther. Adv. Med. Oncol. 2011, 3, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Paik, P.K.; Felip, E.; Veillon, R.; Sakai, H.; Cortot, A.B.; Garassino, M.C.; Mazieres, J.; Viteri, S.; Senellart, H.; Van Meerbeeck, J.; et al. Tepotinib in Non–Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N. Engl. J. Med. 2020, 383, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.S.; Lee, W.C.; Shin, J.Y.; Lee, S.; Bleazard, T.; Won, J.K.; Kim, Y.T.; Kim, J.-I.; Kang, J.H.; Seo, J.S. A transforming KIF5B and RET gene fusion in lung adenocarcinoma revealed from whole-genome and transcriptome sequencing. Genome Res. 2012, 22, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Tsuta, K.; Kohno, T.; Yoshida, A.; Shimada, Y.; Asamura, H.; Furuta, K.; Kushima, R. RET-rearranged non-small-cell lung carcinoma: A clinicopathological and molecular analysis. Br. J. Cancer 2014, 110, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Gainor, J.F.; Curigliano, G.; Kim, D.W.; Lee, D.H.; Besse, B.; Baik, C.S.; Doebele, R.C.; Cassier, P.A.; Lopes, G.; Tan, D.S.W.; et al. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): A multi-cohort, open-label, phase 1/2 study. Lancet Oncol. 2021, 22, 959–969. [Google Scholar] [CrossRef]
- Drilon, A.; Oxnard, G.R.; Tan, D.S.W.; Loong, H.H.F.; Johnson, M.; Gainor, J.; McCoach, C.E.; Gautschi, O.; Besse, B.; Cho, B.C.; et al. Efficacy of Selpercatinib in RET Fusion–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Laetsch, T.W.; Kummar, S.; DuBois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of Larotrectinib in TRK Fusion–Positive Cancers in Adults and Children. N. Engl. J. Med. 2018, 378, 731–739. [Google Scholar] [CrossRef]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- Marchiò, C.; Scaltriti, M.; Ladanyi, M.; Iafrate, A.J.; Bibeau, F.; Dietel, M.; Hechtman, J.F.; Troiani, T.; López-Rios, F.; Douillard, J.Y.; et al. ESMO recommendations on the standard methods to detect NTRK fusions in daily practice and clinical research. Ann. Oncol. 2019, 30, 1417–1427. [Google Scholar] [CrossRef]
- Uchibori, K.; Takano, N.; Manabe, R.; Tsugitomi, R.; Ogusu, S.; Tozuka, T.; Sakamoto, H.; Yoshida, H.; Amino, Y.; Ariyasu, R.; et al. Clinical influence of switching companion diagnostic tests for EGFR-TKs from Therascreen to Cobas v2. Thorac. Cancer 2021, 12, 906–913. [Google Scholar] [CrossRef]
- Gregg, J.P.; Li, T.; Yoneda, K.Y. Molecular testing strategies in non-small cell lung cancer: Optimizing the diagnostic journey. Transl. Lung Cancer Res. 2019, 8, 286–301. [Google Scholar] [CrossRef]
- Griesinger, F.; Eberhardt, W.; Nusch, A.; Reiser, M.; Zahn, M.O.; Maintz, C.; Bernhardt, C.; Losem, C.; Stenzinger, A.; Heukamp, L.C.; et al. Biomarker testing in non-small cell lung cancer in routine care: Analysis of the first 3,717 patients in the German prospective, observational, nation-wide CRISP Registry (AIO-TRK-0315). Lung Cancer 2021, 152, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Adizie, J.B.; Tweedie, J.; Khakwani, A.; Peach, E.; Hubbard, R.; Wood, N.; Gosney, J.R.; Harden, S.V.; Beckett, P.; Popat, S.; et al. Biomarker Testing for People With Advanced Lung Cancer in England. JTO Clin. Res. Rep. 2021, 2, 100176. [Google Scholar] [CrossRef] [PubMed]
- Friedlaender, A.; Subbiah, V.; Russo, A.; Banna, G.L.; Malapelle, U.; Rolfo, C.; Addeo, A. EGFR and HER2 exon 20 insertions in solid tumours: From biology to treatment. Nat. Rev. Clin. Oncol. 2022, 19, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.M.; Yin, Y.; Crossland, V.; Wu, Y.; Ou, S.H.I. EGFR Testing Patterns and Detection of EGFR Exon 20 Insertions in the United States. JTO Clin. Res. Rep. 2022, 3, 100285. [Google Scholar] [CrossRef]
- Bauml, J.M.; Viteri, S.; Minchom, A.; Bazhenova, L.; Ou, S.; Schaffer, M.; Le Croy, N.; Riley, R.; Mahadevia, P.; Girard, N. FP07.12 Underdiagnosis of EGFR Exon 20 Insertion Mutation Variants: Estimates from NGS-based Real-World Datasets. J. Thorac. Oncol. 2021, 16, S208–S209. [Google Scholar] [CrossRef]
- Hess, L.M.; Krein, P.M.; Haldane, D.; Han, Y.; Sireci, A.N. Biomarker Testing for Patients With Advanced/Metastatic Nonsquamous NSCLC in the United States of America, 2015 to 2021. JTO Clin. Res. Rep. 2022, 3, 100336. [Google Scholar] [CrossRef]
- Xie, F.; Zheng, X.; Mao, X.; Zhao, R.; Ye, J.; Zhang, Y.; Sun, J. Next-Generation Sequencing for Genotyping of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration Samples in Lung Cancer. Ann. Thorac. Surg. 2019, 108, 219–226. [Google Scholar] [CrossRef]
- Heitzer, E.; van den Broek, D.; Denis, M.G.; Hofman, P.; Hubank, M.; Mouliere, F.; Paz-Ares, L.; Schuuring, E.; Sültmann, H.; Vainer, G.; et al. Recommendations for a practical implementation of circulating tumor DNA mutation testing in metastatic non-small-cell lung cancer. ESMO Open 2022, 7, 100399. [Google Scholar] [CrossRef]
- Patel, A.; Hissong, E.; Rosado, L.; Burkhardt, R.; Cong, L.; Alperstein, S.A.; Siddiqui, M.T.; Park, H.J.; Song, W.; Velu, P.D.; et al. Next-Generation Sequencing of Cell-Free DNA Extracted From Pleural Effusion Supernatant: Applications and Challenges. Front. Med. 2021, 8, 662312. [Google Scholar] [CrossRef]
- Otake, S.; Goto, T.; Higuchi, R.; Nakagomi, T.; Hirotsu, Y.; Amemiya, K.; Oyama, T.; Mochizuki, H.; Omata, M. The Diagnostic Utility of Cell-Free DNA from Ex Vivo Bronchoalveolar Lavage Fluid in Lung Cancer. Cancers 2022, 14, 1764. [Google Scholar] [CrossRef]
- Shen, F.; Liang, N.; Fan, Z.; Zhao, M.; Kang, J.; Wang, X.; Hu, Q.; Mu, Y.; Wang, K.; Yuan, M.; et al. Genomic Alterations Identification and Resistance Mechanisms Exploration of NSCLC With Central Nervous System Metastases Using Liquid Biopsy of Cerebrospinal Fluid: A Real-World Study. Front. Oncol. 2022, 12, 889591. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Xi, L.; Cultraro, C.M.; Wei, F.; Jones, G.; Cheng, J.; Shafiei, A.; Pham, T.H.T.; Roper, N.; Akoth, E.; et al. Longitudinal circulating tumor dna analysis in blood and saliva for prediction of response to osimertinib and disease progression in egfr-mutant lung adenocarcinoma. Cancers 2021, 13, 3342. [Google Scholar] [CrossRef] [PubMed]
- Reckamp, K.L.; Melnikova, V.O.; Karlovich, C.; Sequist, L.V.; Camidge, D.R.; Wakelee, H.; Perol, M.; Oxnard, G.R.; Kosco, K.; Croucher, P.; et al. A highly sensitive and quantitative test platform for detection of NSCLC EGFR mutations in urine and plasma. J. Thorac. Oncol. 2016, 11, 1690–1700. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.; Chakravarty, D.; Dienstmann, R.; Jezdic, S.; Gonzalez-Perez, A.; Lopez-Bigas, N.; Ng, C.K.Y.; Bedard, P.L.; Tortora, G.; Douillard, J.Y.; et al. A framework to rank genomic alterations as targets for cancer precision medicine: The ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Ann. Oncol. 2018, 29, 1895–1902. [Google Scholar] [CrossRef]
- Pascual, J.; Attard, G.; Bidard, F.-C.; Curigliano, G.; De Mattos-Arruda, L.; Diehn, M.; Italiano, A.; Lindberg, J.; Merker, J.D.; Montagut, C.; et al. ESMO recommendations on the use of circulating tumour DNA assays for patients with cancer: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2022, 33, 750–768. [Google Scholar] [CrossRef]
- Martin Romano, P.; Mezquita, L.; Lacroix, L.; Rouleau, E.; Varga, A.; Baldini, C.; Postel-Vinay, S.; Friboulet, L.; Geraud, A.; Aldea, M.; et al. 1930O Genomic alterations in solid tumours according to ESMO scale for clinical actionability of molecular targets (ESCAT). Ann. Oncol. 2020, 31, S1092–S1093. [Google Scholar] [CrossRef]
- Verdaguer, H.; Saurí, T.; Acosta, D.A.; Guardiola, M.; Sierra, A.; Hernando, J.; Nuciforo, P.; Miquel, J.M.; Molero, C.; Peiró, S.P.; et al. ESMO Scale for Clinical Actionability of Molecular Targets Driving Targeted Treatment in Patients with Cholangiocarcinoma. Clin. Cancer Res. 2022, 28, 1662–1671. [Google Scholar] [CrossRef]
- Kalokairinou, L.; Howard, H.C.; Slokenberga, S.; Fisher, E.; Flatscher-Thöni, M.; Hartlev, M.; van Hellemondt, R.; Juškevičius, J.; Kapelenska-Pregowska, J.; Kováč, P.; et al. Legislation of direct-to-consumer genetic testing in Europe: A fragmented regulatory landscape. J. Community Genet. 2018, 9, 117–132. [Google Scholar] [CrossRef]
- Horgan, D.; Curigliano, G.; Rieß, O.; Hofman, P.; Büttner, R.; Conte, P.; Cufer, T.; Gallagher, W.M.; Georges, N.; Kerr, K.; et al. Identifying the Steps Required to Effectively Implement Next-Generation Sequencing in Oncology at a National Level in Europe. J. Pers. Med. 2022, 12, 72. [Google Scholar] [CrossRef]
- Steuten, L.; Goulart, B.; Meropol, N.J.; Pritchard, D.; Ramsey, S.D. Cost Effectiveness of Multigene Panel Sequencing for Patients With Advanced Non–Small-Cell Lung Cancer. JCO Clin. Cancer Inform. 2019, 3, 1–10. [Google Scholar] [CrossRef]
- Schluckebier, L.; Caetano, R.; Garay, O.U.; Montenegro, G.T.; Custodio, M.; Aran, V.; Gil Ferreira, C. Cost-effectiveness analysis comparing companion diagnostic tests for EGFR, ALK, and ROS1 versus next-generation sequencing (NGS) in advanced adenocarcinoma lung cancer patients. BMC Cancer 2020, 20, 875. [Google Scholar] [CrossRef]
- Pennell, N.A.; Mutebi, A.; Zhou, Z.-Y.; Ricculli, M.L.; Tang, W.; Wang, H.; Guerin, A.; Arnhart, T.; Dalal, A.; Sasane, M.; et al. Economic Impact of Next-Generation Sequencing Versus Single-Gene Testing to Detect Genomic Alterations in Metastatic Non–Small-Cell Lung Cancer Using a Decision Analytic Model. JCO Precis. Oncol. 2019, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Thunnissen, E.; Weynand, B.; Udovicic-Gagula, D.; Brcic, L.; Szolkowska, M.; Hofman, P.; Smojver-Ježek, S.; Anttila, S.; Calabrese, F.; Kern, I.; et al. Lung cancer biomarker testing: Perspective from Europe. Transl. Lung Cancer Res. 2020, 9, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Rolfo, C.; Mack, P.; Scagliotti, G.V.; Aggarwal, C.; Arcila, M.E.; Barlesi, F.; Bivona, T.; Diehn, M.; Dive, C.; Dziadziuszko, R.; et al. Liquid Biopsy for Advanced NSCLC: A Consensus Statement From the International Association for the Study of Lung Cancer. J. Thorac. Oncol. 2021, 16, 1647–1662. [Google Scholar] [CrossRef] [PubMed]
- Rijavec, E.; Coco, S.; Genova, C.; Rossi, G.; Longo, L.; Grossi, F. Liquid biopsy in non-small cell lung cancer: Highlights and challenges. Cancers 2020, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, J.-B.; Hou, L.K.; Yu, F.; Zhang, J.; Wu, W.; Tang, X.M.; Sun, F.; Lu, H.M.; Deng, J.; et al. Liquid biopsy in lung cancer: Significance in diagnostics, prediction, and treatment monitoring. Mol. Cancer 2022, 21, 25. [Google Scholar] [CrossRef]
- Bonanno, L.; Dal Maso, A.; Pavan, A.; Zulato, E.; Calvetti, L.; Pasello, G.; Guarneri, V.; Conte, P.F.; Indraccolo, S. Liquid biopsy and non-small cell lung cancer: Are we looking at the tip of the iceberg? Br. J. Cancer 2022, 127, 383–393. [Google Scholar] [CrossRef]
- Guibert, N.; Pradines, A.; Mazieres, J.; Favre, G. Current and future applications of liquid biopsy in nonsmall cell lung cancer from early to advanced stages. Eur. Respir. Rev. 2020, 29, 190052. [Google Scholar] [CrossRef]
- Choudhury, Y.; Tan, M.H.; Shi, J.L.; Tee, A.; Ngeow, K.C.; Poh, J.; Goh, R.R.; Mong, J. Complementing Tissue Testing With Plasma Mutation Profiling Improves Therapeutic Decision-Making for Patients With Lung Cancer. Front. Med. 2022, 9, 758464. [Google Scholar] [CrossRef]
- Mileham, K.F.; Schenkel, C.; Bruinooge, S.S.; Freeman-Daily, J.; Basu Roy, U.; Moore, A.; Smith, R.A.; Garrett-Mayer, E.; Rosenthal, L.; Garon, E.B.; et al. Defining comprehensive biomarker-related testing and treatment practices for advanced non-small-cell lung cancer: Results of a survey of U.S. oncologists. Cancer Med. 2022, 11, 530–538. [Google Scholar] [CrossRef]
- Ilié, M.; Hofman, V.; Bontoux, C.; Heeke, S.; Lespinet-Fabre, V.; Bordone, O.; Lassalle, S.; Lalvée, S.; Tanga, V.; Allegra, M.; et al. Setting Up an Ultra-Fast Next-Generation Sequencing Approach as Reflex Testing at Diagnosis of Non-Squamous Non-Small Cell Lung Cancer; Experience of a Single Center (LPCE, Nice, France). Cancers 2022, 14, 2258. [Google Scholar] [CrossRef] [PubMed]
- Momeni-Boroujeni, A.; Salazar, P.; Zheng, T.; Mensah, N.; Rijo, I.; Dogan, S.; Yao, J.Y.; Moung, C.; Vanderbilt, C.; Benhamida, J.; et al. Rapid EGFR Mutation Detection Using the Idylla Platform: Single-Institution Experience of 1200 Cases Analyzed by an In-House Developed Pipeline and Comparison with Concurrent Next-Generation Sequencing Results. J. Mol. Diagn. 2021, 23, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.C.; Yee, S.S.; Troxel, A.B.; Savitch, S.L.; Fan, R.; Balli, D.; Lieberman, D.B.; Morrissette, J.D.; Evans, T.L.; Bauml, J.; et al. Detection of therapeutically targetable driver and resistance mutations in lung cancer patients by next-generation sequencing of cell-free circulating tumor DNA. Clin. Cancer Res. 2016, 22, 5772–5782. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Hondelink, L.M.; Solleveld-Westerink, N.; Uljee, S.M.; Ruano, D.; Cleton-Jansen, A.M.; von der Thüsen, J.H.; Ramai, S.R.S.; Postmus, P.E.; Graadt van Roggen, J.F.; et al. Optimizing Mutation and Fusion Detection in NSCLC by Sequential DNA and RNA Sequencing. J. Thorac. Oncol. 2020, 15, 1000–1014. [Google Scholar] [CrossRef] [PubMed]
- Nong, L.; Zhang, Z.; Xiong, Y.; Zheng, Y.; Li, X.; Li, D.; He, Q.; Li, T. Comparison of next-generation sequencing and immunohistochemistry analysis for targeted therapy-related genomic status in lung cancer patients. J. Thorac. Dis. 2019, 11, 4992–5003. [Google Scholar] [CrossRef]
- Durães, C.; Gomes, C.P.; Costa, J.L. Demystifying the Discussion of Sequencing Panel Size in Oncology Genetic Testing. Eur. Med. J. 2022, 7, 68–77. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pujol, N.; Heeke, S.; Bontoux, C.; Boutros, J.; Ilié, M.; Hofman, V.; Marquette, C.-H.; Hofman, P.; Benzaquen, J. Molecular Profiling in Non-Squamous Non-Small Cell Lung Carcinoma: Towards a Switch to Next-Generation Sequencing Reflex Testing. J. Pers. Med. 2022, 12, 1684. https://doi.org/10.3390/jpm12101684
Pujol N, Heeke S, Bontoux C, Boutros J, Ilié M, Hofman V, Marquette C-H, Hofman P, Benzaquen J. Molecular Profiling in Non-Squamous Non-Small Cell Lung Carcinoma: Towards a Switch to Next-Generation Sequencing Reflex Testing. Journal of Personalized Medicine. 2022; 12(10):1684. https://doi.org/10.3390/jpm12101684
Chicago/Turabian StylePujol, Nina, Simon Heeke, Christophe Bontoux, Jacques Boutros, Marius Ilié, Véronique Hofman, Charles-Hugo Marquette, Paul Hofman, and Jonathan Benzaquen. 2022. "Molecular Profiling in Non-Squamous Non-Small Cell Lung Carcinoma: Towards a Switch to Next-Generation Sequencing Reflex Testing" Journal of Personalized Medicine 12, no. 10: 1684. https://doi.org/10.3390/jpm12101684
APA StylePujol, N., Heeke, S., Bontoux, C., Boutros, J., Ilié, M., Hofman, V., Marquette, C.-H., Hofman, P., & Benzaquen, J. (2022). Molecular Profiling in Non-Squamous Non-Small Cell Lung Carcinoma: Towards a Switch to Next-Generation Sequencing Reflex Testing. Journal of Personalized Medicine, 12(10), 1684. https://doi.org/10.3390/jpm12101684







