Depressive Pseudodementia with Reversible AD-like Brain Hypometabolism: A Case Report and a Review of the Literature
Abstract
1. Introduction
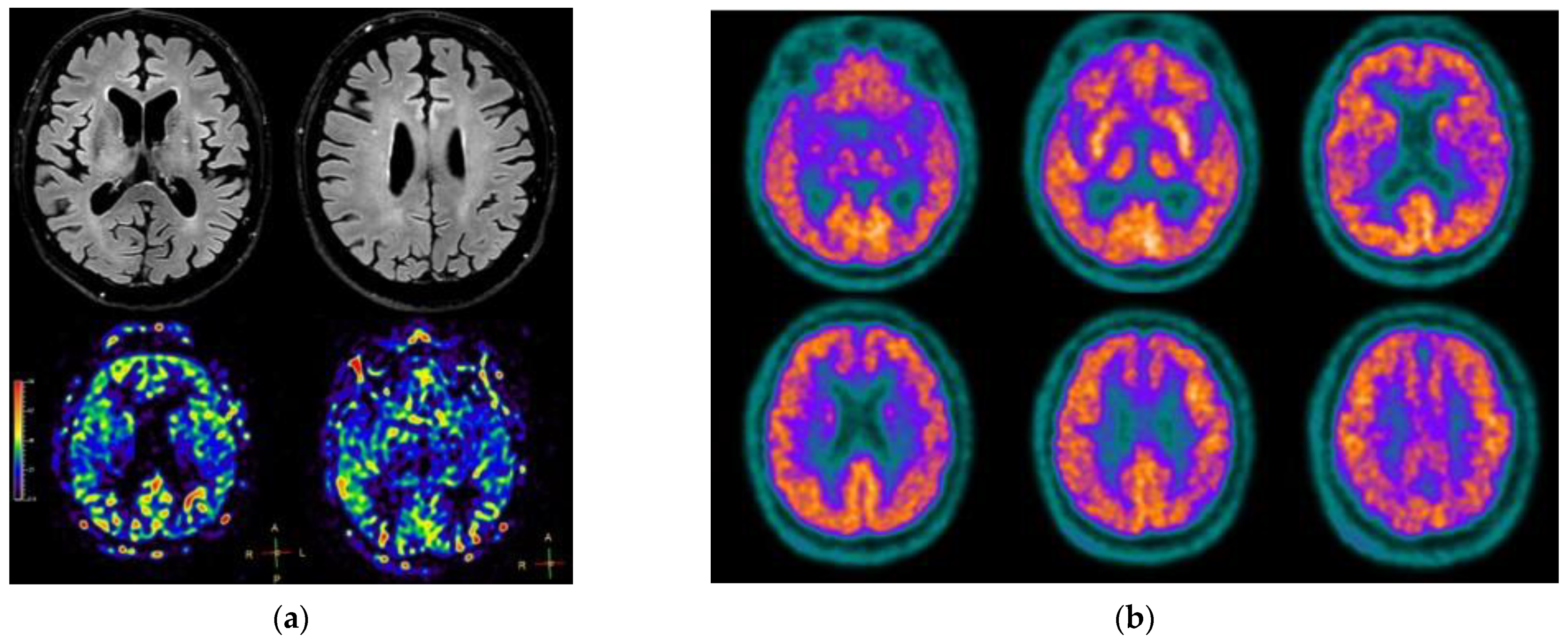
2. Case Presentation
3. Discussion
3.1. Meaning of FDG-PET Hypometabolism in Cognitive Impairment and Possible Implications
3.2. May Treatment Have Influenced the First PET?
3.3. A Review of Reversible Brain Hypometabolism in Pseudodementia
3.4. Interpretation of Amyloid PET in Our Case
3.5. Learning Points and Future Directions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Auning, E.; Selnes, P.; Grambaite, R.; Šaltyte Benth, J.; Haram, A.; Løvli Stav, A.; Bjørnerud, A.; Hessen, E.; Hol, P.K.; Muftuler løndalen, A.; et al. Neurobiological correlates of depressive symptoms in people with subjective and mild cognitive impairment. Acta Psychiatr. Scand. 2015, 131, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Brendel, M.; Pogarell, O.; Xiong, G.; Delker, A.; Bartenstein, P.; Rominger, A. Depressive symptoms accelerate cognitive decline in amyloid-positive MCI patients. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Marano, C.M.; Workman, C.I.; Lyman, C.H.; Kramer, E.; Hermann, C.R.; Ma, Y.; Dhawan, V.; Chaly, T.; Eidelberg, D.; Smith, G.S. The relationship between fasting serum glucose and cerebral glucose metabolism in late-life depression and normal aging. Psychiatry Res. Neuroimaging 2014, 222, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Emsell, L.; Vanhaute, H.; Vansteelandt, K.; De Winter, F.L.; Christiaens, D.; Van den Stock, J.; Vandenberghe, R.; Van Laere, K.; Sunaert, S.; Bouckaert, F.; et al. An optimized MRI and PET based clinical protocol for improving the differential diagnosis of geriatric depression and Alzheimer’s disease. Psychiatry Res. Neuroimaging 2022, 320, 111443. [Google Scholar] [CrossRef] [PubMed]
- De Crescenzo, F.; Ciliberto, M.; Menghini, D.; Treglia, G.; Ebmeier, K.P.; Janiri, L. Is 18F-FDG-PET suitable to predict clinical response to the treatment of geriatric depression? A systematic review of PET studies. Aging Ment. Health 2017, 21, 889–894. [Google Scholar] [CrossRef]
- Brodaty, H.; Connors, M.H. Pseudodementia, pseudo-pseudodementia, and pseudodepression. Assess. Dis. Monit. 2020, 12, e12027. [Google Scholar] [CrossRef]
- Wells, E. Pseudodementia. Am. J. Psychiatry 1979, 136, 895–900. [Google Scholar] [CrossRef]
- Caine, E.D. Pseudodementia. Arch. Gen. Psychiatry 1981, 38, 1359. [Google Scholar] [CrossRef]
- Bak, J.; Lee, S.M.; Kwon, Y.J.; Shim, S.H.; Kim, J.I. The normalization of brain 18F-fluorodeoxy-D-glucose positron emission tomography hypometabolism following electroconvulsive therapy in a 55-year-old woman with treatment-resistant late onset depression: A case report. Clin. Psychopharmacol. Neurosci. 2017, 15, 82–86. [Google Scholar] [CrossRef]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Ortner, M.M. The Use of 18F-FDG PET in the Diagnostic Workup of Alzheimer’s Dementia. In Methods in Molecular Biology; Perneczky, R., Ed.; Springer New York: New York, NY, USA, 2018; Volume 1750, pp. 213–219. [Google Scholar] [CrossRef]
- Nestor, P.J.; Altomare, D.; Festari, C.; Drzezga, A.; Rivolta, J.; Walker, Z.; Bouwman, F.; Orini, S.; Law, I.; Agosta, F.; et al. Clinical utility of FDG-PET for the differential diagnosis among the main forms of dementia. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1509–1525. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Shi, D.; Gao, Y.; Xu, J. Functional assessment of prefrontal lobes in patients with major depression disorder using a dual-mode technique of 3D-arterial spin labeling and 18F-fluorodeoxyglucose positron emission tomography/computed tomography. Exp. Ther. Med. 2017, 14, 1058–1064. [Google Scholar] [CrossRef]
- Fu, C.; Zhang, H.; Xuan, A.; Gao, Y.; Xu, J.; Shi, D. A combined study of 18F-FDG PET-CT and fMRI for assessing resting cerebral function in patients with major depressive disorder. Exp. Ther. Med. 2018, 16, 1873–1881. [Google Scholar] [CrossRef]
- Nobili, F.; Arbizu, J.; Bouwman, F.; Drzezga, A.; Agosta, F.; Nestor, P.; Walker, Z.; Boccardi, M.; Festari, C.; Altomare, D.; et al. European Association of Nuclear Medicine and European Academy of Neurology recommendations for the use of brain 18 F-fluorodeoxyglucose positron emission tomography in neurodegenerative cognitive impairment and dementia: Delphi consensus. Eur. J. Neurol. 2018, 25, 1201–1217. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Choo HIl Lee, D.Y.; Kim, J.W.; Seo, E.H.; Kim, S.G.; Park, S.Y.; Shin, J.H.; Kim, K.W.; Woo, J.I. Frontal dysfunction underlies depression in mild cognitive impairment: A FDG-PET study. Psychiatry Investig. 2010, 7, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Choo, I.H.; Jhoo, J.H.; Kim, K.W.; Youn, J.C.; Lee, D.S.; Kang, E.J.; Lee, J.S.; Kang, W.J.; Woo, J.I. Frontal dysfunction underlies depressive syndrome in Alzheimer disease: A FDG-PET study. Am. J. Geriatr. Psychiatry 2006, 14, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Hassamal, S.; Jolles, P.; Pandurangi, A. Reversal of cerebral glucose hypometabolism on positron emission tomography with electroconvulsive therapy in an elderly patient with a psychotic episode. Psychogeriatrics 2016, 16, 376–381. [Google Scholar] [CrossRef]
- Caroli, A.; Lorenzi, M.; Geroldi, C.; Nobili, F.; Paghera, B.; Bonetti, M.; Cotelli, M.; Frisoni, G. Metabolic compensation and depression in Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2010, 29, 37–45. [Google Scholar] [CrossRef]
- Lesman-Segev, O.H.; La Joie, R.; Iaccarino, L.; Lobach, I.; Rosen, H.J.; Seo, S.W.; Janabi, M.; Baker, S.L.; Bs, L.E.; Pham, J.; et al. Diagnostic Accuracy of Amyloid versus 18F-Fluorodeoxyglucose Positron Emission Tomography in Autopsy-Confirmed Dementia. Ann. Neurol. 2021, 89, 389–401. [Google Scholar] [CrossRef]
- Fessel, J. Does synaptic hypometabolism or synaptic dysfunction, originate cognitive loss? Analysis of the evidence. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2021, 7, e12177. [Google Scholar] [CrossRef]
- Krell-Roesch, J.; Syrjanen, J.A.; Vassilaki, M.; Lowe, V.J.; Vemuri, P.; Mielke, M.M.; Machulda, M.M.; Stokin, G.B.; Christianson, T.J.; Kremers, W.K.; et al. Brain Regional Glucose Metabolism, Neuropsychiatric Symptoms, and the Risk of Incident Mild Cognitive Impairment: The Mayo Clinic Study of Aging. Am. J. Geriatr. Psychiatry 2021, 29, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Holmes, S.E.; Scheinost, D.; Finnema, S.J.; Naganawa, M.; Davis, M.T.; DellaGioia, N.; Nabulsi, N.; Matuskey, D.; Angarita, G.A.; Pietrzak, R.H.; et al. Lower synaptic density is associated with depression severity and network alterations. Nat. Commun. 2019, 10, 1529. [Google Scholar] [CrossRef] [PubMed]
- Svensson, M.; Olsson, G.; Yang, Y.; Bachiller, S.; Ekemohn, M.; Ekstrand, J.; Deierborg, T. The effect of electroconvulsive therapy on neuroinflammation, behavior and amyloid plaques in the 5xFAD mouse model of Alzheimer’s disease. Sci. Rep. 2021, 11, 4910. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.; Campbell, K. Electroconvulsive Therapy for the Treatment of the Behavioural and Psychological Symptoms of Dementia: A Review of Clinical Effectiveness and Guidelines; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2019; pp. 1–23.
- Just, N.; Chevillard, P.M.; Migaud, M. Imaging and spectroscopic methods to investigate adult neurogenesis in vivo: New models and new avenues. Front. Neurosci. 2022, 16, 933947. [Google Scholar] [CrossRef]
- Kranaster, L.; Aksay, S.S.; Bumb, J.M.; Janke, C.; Alonso, A.; Hoyer, C.; Zerr, I.; Schmitz, M.; Hausner, L.; Frölich, L.; et al. Electroconvulsive therapy selectively enhances amyloid β 1–42 in the cerebrospinal fluid of patients with major depression: A prospective pilot study. Eur. Neuropsychopharmacol. 2016, 26, 1877–1884. [Google Scholar] [CrossRef]
- Drevets, W.C.; Bogers, W.; Raichle, M.E. Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. Eur. Neuropsychopharmacol. 2002, 12, 527–544. [Google Scholar] [CrossRef]
- Brody, A.L.; Saxena, S.; Silverman, D.H.S.; Alborzian, S.; Fairbanks, L.A.; Phelps, M.E.; Huang, S.-C.; Wu, H.-M.; Maidment, K.; Baxter, L.R.; et al. Brain metabolic changes in major depressive disorder from pre- to post-treatment with paroxetine. Psychiatry Res. Neuroimaging 1999, 91, 127–139. [Google Scholar] [CrossRef]
- Silva-Rodríguez, J.; García-Varela, L.; López-Arias, E.; Domínguez-Prado, I.; Cortés, J.; Pardo-Montero, J.; Fernández-Ferreiro, A.; Ruibal, Á.; Sobrino, T.; Aguiar, P. Impact of benzodiazepines on brain FDG-PET quantification after single-dose and chronic administration in rats. Nucl. Med. Biol. 2016, 43, 827–834. [Google Scholar] [CrossRef]
- Guenther, T.; Schönknecht, P.; Becker, G.; Olbrich, S.; Sander, C.; Hesse, S.; Meyer, P.M.; Luthardt, J.; Hegerl, U.; Sabri, O. Impact of EEG-vigilance on brain glucose uptake measured with [18F]FDG and PET in patients with depressive episode or mild cognitive impairment. Neuroimage 2011, 56, 93–101. [Google Scholar] [CrossRef]
- Awata, S.; Konno, M.; Kawashima, R.; Suzuki, K.; Sato, T.; Matsuoka, H.; Fukuda, H. Changes in regional cerebral blood flow abnormalities in late-life depression following response to electroconvulsive therapy. Psychiatry Clin. Neurosci. 2002, 56, 31–40. [Google Scholar] [CrossRef]
- Lajoie, C.; Levasseur, M.A.; Paquet, N. Complete normalization of severe brain 18F-FDG hypometabolism following electroconvulsive therapy in a major depressive episode. Clin. Nucl. Med. 2013, 38, 735–736. [Google Scholar] [CrossRef] [PubMed]
- Van Poeck, I.; Ahmad, R.; Van Laere, K.; Vandenbulcke, M. Reversible parietal hypometabolism in late-onset psychosis. J. Neuropsychiatry Clin. Neurosci. 2013, 25, 32–33. [Google Scholar] [CrossRef] [PubMed]
- Asada, T.; Takaya, S.; Takayama, Y.; Yamauchi, H.; Hashikawa, K.; Fukuyama, H. Reversible alcohol-related dementia: A five-year follow-up study using FDG-PET and neuropsychological tests. Int. Med. 2010, 49, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Kemppainen, J.; Kajander, S.; Yli-Kerttula, T.; Airas, L.; Seppänen, M. Reversible brain hypometabolism associated with central nervous system systemic lupus erythematosus. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 411. [Google Scholar] [CrossRef][Green Version]
- Mairal, E.; Barberon, B.; Laine, N.; Coulange, M.; Guedj, E. Reversible widespread brain 18F-FDG PET hypometabolism in chronic fatigue syndrome treated by hyperbaric oxygen therapy. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1680–1681. [Google Scholar] [CrossRef]
- Yokoyama, T.; Okamura, T.; Takahashi, M.; Momose, T.; Kondo, S. A case of recurrent depressive disorder presenting with Alice in Wonderland syndrome: Psychopathology and pre- and post-treatment FDG-PET findings. BMC Psychiatry 2017, 17, 150. [Google Scholar] [CrossRef]
- Miao, Q.; Zhang, S.; Guan, Y.H.; Ye, H.Y.; Zhang, Z.Y.; Zhang, Q.Y.; Xue, R.D.; Zeng, M.F.; Zuo, C.T.; Li, Y.M. Reversible changes in brain glucose metabolism following thyroid function normalization in hyperthyroidism. Am. J. Neuroradiol. 2011, 32, 1034–1042. [Google Scholar] [CrossRef]
- Bergeron, D.; Ossenkoppele, R.; Laforce, R. Evidence-based Interpretation of Amyloid-β PET Results: A Clinician’s Tool. Alzheimer Dis. Assoc. Disord. 2018, 32, 28–34. [Google Scholar] [CrossRef]
- Drzezga, A. The network degeneration hypothesis: Spread of neurodegenerative patterns along neuronal brain networks. J. Nucl. Med. 2018, 59, 1645–1648. [Google Scholar] [CrossRef]


| Neuropsychological Evaluation | July 2019 | July 2020 | January 2022 | Cut-Off Values |
|---|---|---|---|---|
| Global cognitive screening | ||||
| MMSE 1 | 30 | 27.86 | 28.86 | ≥23.8 |
| Attention and Executive Function | ||||
| Attentional Matrices | 46.25 | 43.25 | 48.25 | ≥41 |
| Digit Span—backwards | 4.21 | 4.21 | 5.21 | ≥2.65 |
| Stroop test: | ||||
| -time interference | 54 | 18.5 | 27 | ≤36.92 |
| -errors interference | 0.75 | 0.75 | 0.75 | ≤4.24 |
| FAB 1 | 18 | 17.15 | 18 | ≥13.5 |
| Raven Colored Progressive Matrices | 24 | 34 | 35 | ≥18 |
| Short-term and long-term memory | ||||
| Digit Span—forward | 8.27 | 8.27 | 5.27 | ≥4.26 |
| Rey Auditory Verbal Learning Test: | ||||
| immediate recall | 39.4 | 50.4 | 44.4 | ≥27.98 |
| delayed recall | 3.3 | 11.3 | 9.3 | ≥4.76 |
| Recognition | 31.2 | 27.2 | 27.2 | ≥22.59 |
| Recall of ROCF1 | 8.5 | 8.5 | 13.5 | ≥9.5 |
| Constructional and ideomotor apraxia | ||||
| Copy of ROCF 1 | 29.5 | 35.5 | 34.5 | ≥28.9 |
| Ideomotor apraxia (De Renzi’s test): | ||||
| right arm | 70 | / | 68 | ≥53 |
| left arm | 67 | / | 71 | ≥53 |
| Language | ||||
| Comprehension (subtest of ENPA 1): | ||||
| -Single word | 20 | 20 | 19.6 | ≥18.4 |
| -Sentences | 14 | 14 | 14 | ≥11.4 |
| Letter Fluency | 35.5 | 29.5 | 44.5 | ≥17.78 |
| Category Fluency | 49.31 | 40.31 | 39.31 | ≥28.34 |
| Picture Naming | 77.89 | 77.89 | 76.89 | ≥67.59 |
| Mood and behavioral evaluation | ||||
| Geriatric Depression Scale | 12 | 7 | 7 | <6 |
| Neuropsychiatric Inventory: | ||||
| -depression | 8 | 0 | 1 | =0 |
| -apathy | 6 | 0 | 1 | =0 |
| -eating change | 6 | 6 | 0 | =0 |
| -anxiety | 2 | 0 | 0 | =0 |
| Functional evaluation | ||||
| ADL 1—completely lost functions | 0 | 0 | 0 | =0 |
| ADL 1—partially lost functions | 1 | 1 | 1 | =0 |
| FAQ 1—completely lost functions | 0 | 1 | 1 | =0 |
| FAQ 1—partially lost functions | 3 | 0 | 0 | =0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozzi, F.E.; Licciardo, D.; Musarra, M.; Jonghi-Lavarini, L.; Crivellaro, C.; Basso, G.; Appollonio, I.; Ferrarese, C. Depressive Pseudodementia with Reversible AD-like Brain Hypometabolism: A Case Report and a Review of the Literature. J. Pers. Med. 2022, 12, 1665. https://doi.org/10.3390/jpm12101665
Pozzi FE, Licciardo D, Musarra M, Jonghi-Lavarini L, Crivellaro C, Basso G, Appollonio I, Ferrarese C. Depressive Pseudodementia with Reversible AD-like Brain Hypometabolism: A Case Report and a Review of the Literature. Journal of Personalized Medicine. 2022; 12(10):1665. https://doi.org/10.3390/jpm12101665
Chicago/Turabian StylePozzi, Federico Emanuele, Daniele Licciardo, Monica Musarra, Lorenzo Jonghi-Lavarini, Cinzia Crivellaro, Gianpaolo Basso, Ildebrando Appollonio, and Carlo Ferrarese. 2022. "Depressive Pseudodementia with Reversible AD-like Brain Hypometabolism: A Case Report and a Review of the Literature" Journal of Personalized Medicine 12, no. 10: 1665. https://doi.org/10.3390/jpm12101665
APA StylePozzi, F. E., Licciardo, D., Musarra, M., Jonghi-Lavarini, L., Crivellaro, C., Basso, G., Appollonio, I., & Ferrarese, C. (2022). Depressive Pseudodementia with Reversible AD-like Brain Hypometabolism: A Case Report and a Review of the Literature. Journal of Personalized Medicine, 12(10), 1665. https://doi.org/10.3390/jpm12101665








