Deciphering the Impact of HER2 Alterations on Non-Small-Cell Lung Cancer: From Biological Mechanisms to Therapeutic Approaches
Abstract
1. Introduction
2. Biology of the HER2 Receptor
3. HER2 Alterations and Carcinogenesis
4. Epidemiology of HER2 Alterations in NSCLC
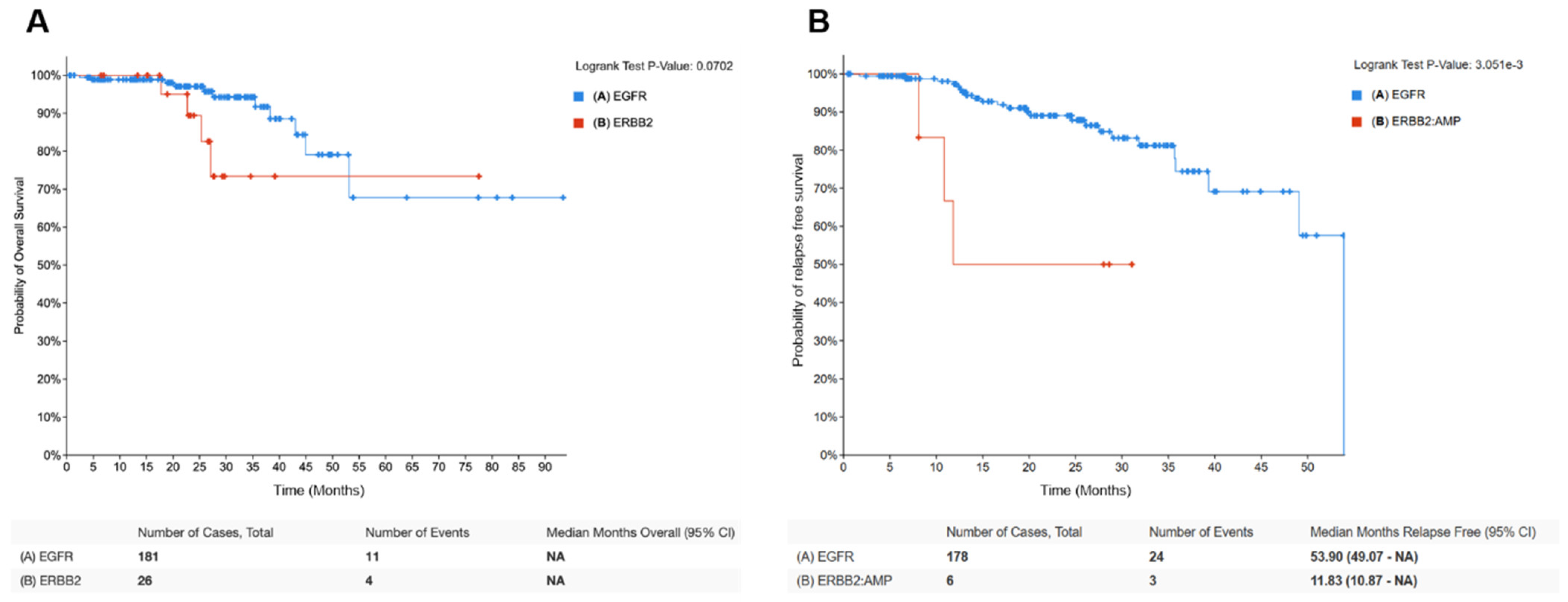
4.1. HER2 Amplification/Overexpression in Lung Cancer (HER2-Positive NSCLC)
4.2. HER2 Mutations in Lung Cancer (HER2-Mutant NSCLC)
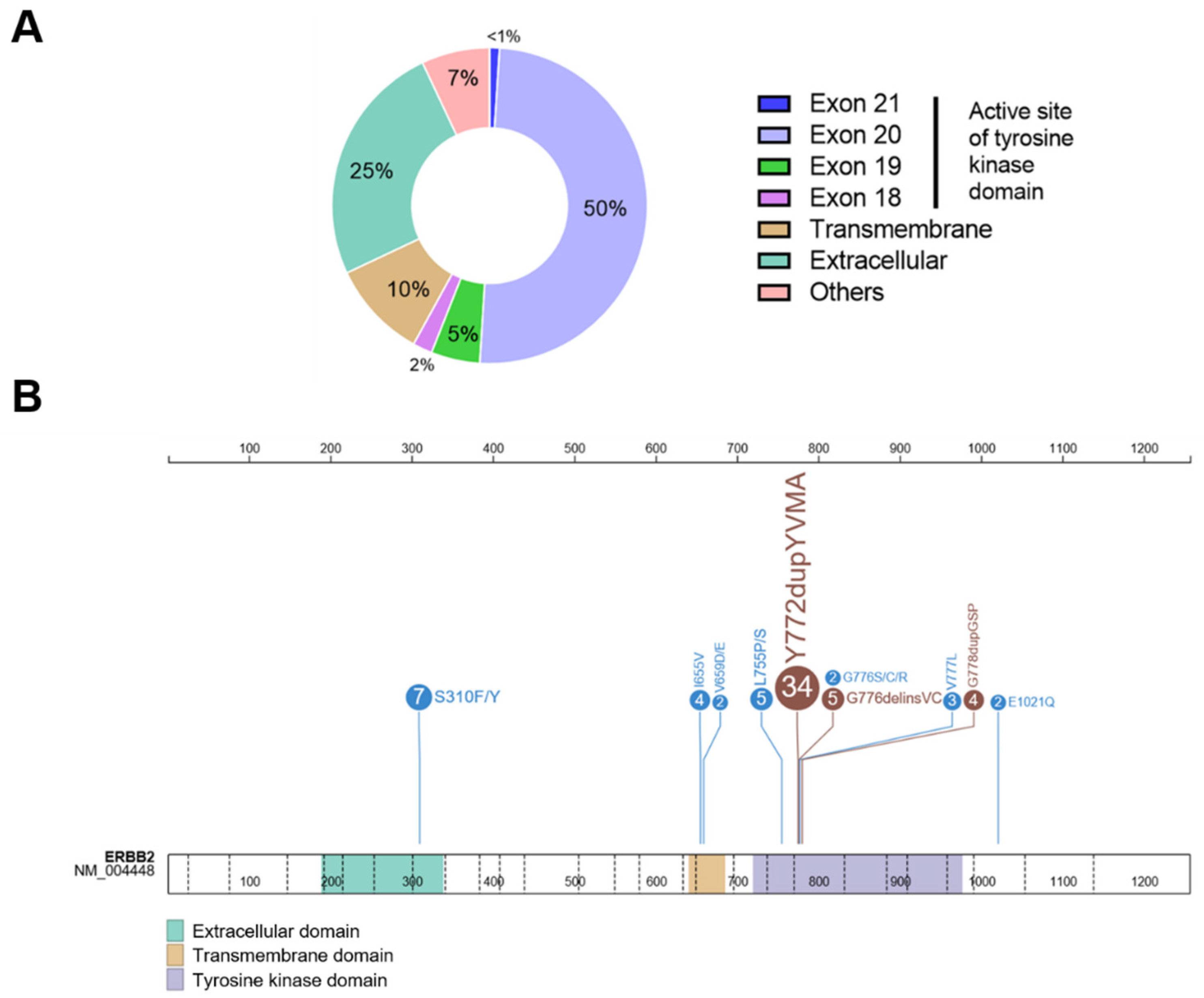
5. Detection Methods for HER2 Alterations in NSCLC
5.1. HER2 Amplification/Overexpression Detection
5.2. HER2 Mutation Detection
| Main Method (Interpretation) | Alternative Methods (Interpretation) | |
|---|---|---|
| HER2 mutation | Sequencing techniques - Next-generation sequencing - Sanger - Pyrosequencing | RT-PCR qPCR |
| HER2 amplification | FISH (HER2/CEP17 ratio >2 and/or HER2 copy number > 6) | NGS (gene copy number > 6) |
| HER2 overexpression | IHC based on membranous staining according to breast guidelines [74]: >0: HER2 negative; >1+: needs to be confirmed by further studies whether 1+ should be considered negative or as having a HER2 low expression; >2+, 3+: HER2 positive. Due to the poor concordance between FISH and IHC in NSCLC, FISH confirmation is not required for NSCLC patients with IHC 2+/3+ to define positive HER2 expression. |
6. HER2-Targeted Therapy in NSCLC
6.1. Non-Selective HER2 Tyrosine Kinase Inhibitors
6.2. Selective HER2 Tyrosine Kinase Inhibitors
6.2.1. Poziotinib
6.2.2. Pyrotinib
6.2.3. Tarloxotinib
6.2.4. Mobocertinib
6.3. Antibody–Drug Conjugates (ADC) against HER2
6.3.1. Trastuzumab Emtansine
6.3.2. Trastuzumab Deruxtecan
6.4. Immune Checkpoint Inhibitors (ICI)
7. Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kandoth, C.; McLellan, M.D.; Vandin, F.; Ye, K.; Niu, B.; Lu, C.; Xie, M.; Zhang, Q.; McMichael, J.F.; Wyczalkowski, M.A.; et al. Mutational Landscape and Significance across 12 Major Cancer Types. Nature 2013, 502, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.H.; Tokheim, C.; Porta-Pardo, E.; Sengupta, S.; Bertrand, D.; Weerasinghe, A.; Colaprico, A.; Wendl, M.C.; Kim, J.; Reardon, B.; et al. Comprehensive Characterization of Cancer Driver Genes and Mutations. Cell 2018, 173, 371–385.e18. [Google Scholar] [CrossRef] [PubMed]
- Majeed, U.; Manochakian, R.; Zhao, Y.; Lou, Y. Targeted Therapy in Advanced Non-Small Cell Lung Cancer: Current Advances and Future Trends. J. Hematol. Oncol. 2021, 14, 108. [Google Scholar] [CrossRef] [PubMed]
- Lynch, T.J.; Bell, D.W.; Sordella, R.; Gurubhagavatula, S.; Okimoto, R.A.; Brannigan, B.W.; Harris, P.L.; Haserlat, S.M.; Supko, J.G.; Haluska, F.G.; et al. Activating Mutations in the Epidermal Growth Factor Receptor Underlying Responsiveness of Non-Small-Cell Lung Cancer to Gefitinib. N. Engl. J. Med. 2004, 350, 2129–2139. [Google Scholar] [CrossRef] [PubMed]
- Paez, J.G.; Jänne, P.A.; Lee, J.C.; Tracy, S.; Greulich, H.; Gabriel, S.; Herman, P.; Kaye, F.J.; Lindeman, N.; Boggon, T.J.; et al. EGFR Mutations in Lung Cancer: Correlation with Clinical Response to Gefitinib Therapy. Science 2004, 304, 1497–1500. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, F.; Rodrigues Pereira, J.; Ciuleanu, T.; Tan, E.; Hirsh, V.; Thongprasert, S.; Campos, D.; Maoleekoonpiroj, S.; Smylie, M.; Martins, R.; et al. Erlotinib in Previously Treated Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2005, 353, 123–132. [Google Scholar] [CrossRef]
- Planchard, D.; Besse, B.; Groen, H.J.M.; Souquet, P.J.; Quoix, E.; Baik, C.S.; Barlesi, F.; Kim, T.M.; Mazieres, J.; Novello, S.; et al. Dabrafenib plus Trametinib in Patients with Previously Treated BRAF(V600E)-Mutant Metastatic Non-Small Cell Lung Cancer: An Open-Label, Multicentre Phase 2 Trial. Lancet Oncol. 2016, 17, 984–993. [Google Scholar] [CrossRef]
- Shaw, A.T.; Kim, D.-W.; Nakagawa, K.; Seto, T.; Crinó, L.; Ahn, M.-J.; de Pas, T.; Besse, B.; Solomon, B.J.; Blackhall, F.; et al. Crizotinib versus Chemotherapy in Advanced ALK-Positive Lung Cancer. N. Engl. J. Med. 2013, 368, 2385–2394. [Google Scholar] [CrossRef]
- Kazdal, D.; Hofman, V.; Christopoulos, P.; Ilié, M.; Stenzinger, A.; Hofman, P. Fusion-Positive Non-Small Cell Lung Carcinoma: Biological Principles, Clinical Practice, and Diagnostic Implications. Genes Chromosomes Cancer 2022, 61, 244–260. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 2.2021. J. Natl. Compr. Cancer Netw. 2021, 19, 254–266. [Google Scholar] [CrossRef]
- Bontoux, C.; Hofman, V.; Brest, P.; Ilié, M.; Mograbi, B.; Hofman, P. Daily Practice Assessment of KRAS Status in NSCLC Patients: A New Challenge for the Thoracic Pathologist Is Right around the Corner. Cancers 2022, 14, 1628. [Google Scholar] [CrossRef]
- Moasser, M.M. The Oncogene HER2: Its Signaling and Transforming Functions and Its Role in Human Cancer Pathogenesis. Oncogene 2007, 26, 6469–6487. [Google Scholar] [CrossRef]
- Buttitta, F.; Barassi, F.; Fresu, G.; Felicioni, L.; Chella, A.; Paolizzi, D.; Lattanzio, G.; Salvatore, S.; Camplese, P.P.; Rosini, S.; et al. Mutational Analysis of the HER2 Gene in Lung Tumors from Caucasian Patients: Mutations Are Mainly Present in Adenocarcinomas with Bronchioloalveolar Features. Int. J. Cancer 2006, 119, 2586–2591. [Google Scholar] [CrossRef]
- Mishra, R.; Hanker, A.B.; Garrett, J.T. Genomic Alterations of ERBB Receptors in Cancer: Clinical Implications. Oncotarget 2017, 8, 114371–114392. [Google Scholar] [CrossRef]
- Collisson, E.A.; Campbell, J.D.; Brooks, A.N.; Berger, A.H.; Lee, W.; Chmielecki, J.; Beer, D.G.; Cope, L.; Creighton, C.J.; Danilova, L.; et al. Comprehensive Molecular Profiling of Lung Adenocarcinoma. Nature 2014, 511, 543–550. [Google Scholar] [CrossRef]
- Romond, E.H.; Perez, E.A.; Bryant, J.; Suman, V.J.; Geyer, C.E.; Davidson, N.E.; Tan-Chiu, E.; Martino, S.; Paik, S.; Kaufman, P.A.; et al. Trastuzumab plus Adjuvant Chemotherapy for Operable HER2-Positive Breast Cancer. N. Engl. J. Med. 2005, 353, 1673–1684. [Google Scholar] [CrossRef]
- Piccart-Gebhart, M.J.; Procter, M.; Leyland-Jones, B.; Goldhirsch, A.; Untch, M.; Smith, I.; Gianni, L.; Baselga, J.; Bell, R.; Jackisch, C.; et al. Trastuzumab after Adjuvant Chemotherapy in HER2-Positive Breast Cancer. N. Engl. J. Med. 2005, 353, 1659–1672. [Google Scholar] [CrossRef]
- Heinmöller, P.; Gross, C.; Beyser, K.; Schmidtgen, C.; Maass, G.; Pedrocchi, M.; Rüschoff, J. HER2 Status in Non-Small Cell Lung Cancer: Results from Patient Screening for Enrollment to a Phase II Study of Herceptin. Clin. Cancer Res. 2003, 9, 5238–5243. [Google Scholar] [CrossRef]
- Cappuzzo, F.; Bemis, L.; Varella-Garcia, M. HER2 Mutation and Response to Trastuzumab Therapy in Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2006, 354, 2619–2621. [Google Scholar] [CrossRef]
- Ricciardi, G.R.R.; Russo, A.; Franchina, T.; Ferraro, G.; Zanghì, M.; Picone, A.; Scimone, A.; Adamo, V. NSCLC and HER2: Between Lights and Shadows. J. Thorac. Oncol. 2014, 9, 1750–1762. [Google Scholar] [CrossRef]
- Hotta, K.; Aoe, K.; Kozuki, T.; Ohashi, K.; Ninomiya, K.; Ichihara, E.; Kubo, T.; Ninomiya, T.; Chikamori, K.; Harada, D.; et al. A Phase II Study of Trastuzumab Emtansine in HER2-Positive Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, 273–279. [Google Scholar] [CrossRef]
- Li, B.T.; Smit, E.F.; Goto, Y.; Nakagawa, K.; Udagawa, H.; Mazières, J.; Nagasaka, M.; Bazhenova, L.; Saltos, A.N.; Felip, E.; et al. Trastuzumab Deruxtecan in HER2-Mutant Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2022, 386, 241–251. [Google Scholar] [CrossRef]
- Passaro, A.; Peters, S. Targeting HER2 -Mutant NSCLC—The Light Is On. N. Engl. J. Med. 2022, 386, 286–289. [Google Scholar] [CrossRef]
- Ilié, M.; Hofman, V.; Bontoux, C.; Heeke, S.; Lespinet-Fabre, V.; Bordone, O.; Lassalle, S.; Lalvée, S.; Tanga, V.; Allegra, M.; et al. Setting Up an Ultra-Fast Next-Generation Sequencing Approach as Reflex Testing at Diagnosis of Non-Squamous Non-Small Cell Lung Cancer; Experience of a Single Center (LPCE, Nice, France). Cancers 2022, 14, 2258. [Google Scholar] [CrossRef]
- Cho, H.S.; Mason, K.; Ramyar, K.X.; Stanley, A.M.; Gabelli, S.B.; Denney, D.W.; Leahy, D.J. Structure of the Extracellular Region of HER2 Alone and in Complex with the Herceptin Fab. Nature 2003, 421, 756–760. [Google Scholar] [CrossRef]
- Ushiro, H.; Cohen, S. Identification of Phosphotyrosine as a Product of Epidermal Growth Factor-Activated Protein Kinase in A-431 Cell Membranes. Yale J. Biol. Med. 1980, 255, 8363–8365. [Google Scholar] [CrossRef]
- Ferguson, K.M.; Berger, M.B.; Mendrola, J.M.; Cho, H.S.; Leahy, D.J.; Lemmon, M.A. EGF Activates Its Receptor by Removing Interactions That Autoinhibit Ectodomain Dimerization. Mol. Cell 2003, 11, 507–517. [Google Scholar] [CrossRef]
- Graus-Porta, D.; Beerli, R.R.; Daly, J.M.; Hynes, N.E. ErbB-2, the Preferred Heterodimerization Partner of All ErbB Receptors, Is a Mediator of Lateral Signaling. EMBO J. 1997, 16, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, T.; Mashiro, Y. Recent Advances in the HER2 Targeted Therapy of Gastric Cancer. World J. Clin. Cases 2015, 3, 42. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Batty, K.M.; Crowe, P.J.; Goldstein, D.; Yang, J.L. The Potential of PanHER Inhibition in Cancer. Front. Oncol. 2015, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of Chemotherapy plus a Monoclonal Antibody against HER2 for Metastatic Breast Cancer That Overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef]
- Buza, N.; English, D.P.; Santin, A.D.; Hui, P. Toward Standard HER2 Testing of Endometrial Serous Carcinoma: 4-Year Experience at a Large Academic Center and Recommendations for Clinical Practice. Mod. Pathol. 2013, 26, 1605–1612. [Google Scholar] [CrossRef]
- Grabsch, H.; Sivakumar, S.; Gray, S.; Gabbert, H.E.; Müller, W. HER2 Expression in Gastric Cancer: Rare, Heterogeneous and of No Prognostic Value-Conclusions from 924 Cases of Two Independent Series. Cell Oncol. 2010, 32, 57–65. [Google Scholar] [CrossRef]
- Bang, Y.J.; van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in Combination with Chemotherapy versus Chemotherapy Alone for Treatment of HER2-Positive Advanced Gastric or Gastro-Oesophageal Junction Cancer (ToGA): A Phase 3, Open-Label, Randomised Controlled Trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Fader, A.N.; Roque, D.M.; Siegel, E.; Buza, N.; Hui, P.; Abdelghany, O.; Chambers, S.K.; Secord, A.A.; Havrilesky, L.; O’Malley, D.M.; et al. Randomized Phase II Trial of Carboplatin-Paclitaxel Versus Carboplatin-Paclitaxel-Trastuzumab in Uterine Serous Carcinomas That Overexpress Human Epidermal Growth Factor Receptor 2/Neu. J. Clin. Oncol. 2018, 36, 2044–2051. [Google Scholar] [CrossRef]
- Diao, W.; Ding, C.; Yuan, B.; Li, Z.; Sun, N.; Huang, J. Expression and Clinical Significance of HER2 Gene and DNMT1 in Non-Small-Cell Lung Cancer. Dis. Markers 2022, 2022, 8426384. [Google Scholar] [CrossRef]
- Chi, F.; Wu, R.; Jin, X.; Jiang, M.; Zhu, X. HER2 Induces Cell Proliferation and Invasion of Non-Small-Cell Lung Cancer by Upregulating COX-2 Expression via MEK/ERK Signaling Pathway. Onco Targets Ther. 2016, 9, 2709. [Google Scholar] [CrossRef]
- Herter-Sprie, G.S.; Greulich, H.; Wong, K.K. Activating Mutations in ERBB2 and Their Impact on Diagnostics and Treatment. Front. Oncol. 2013, 3, 86. [Google Scholar] [CrossRef]
- Pahuja, K.B.; Nguyen, T.T.; Jaiswal, B.S.; Prabhash, K.; Thaker, T.M.; Senger, K.; Chaudhuri, S.; Kljavin, N.M.; Antony, A.; Phalke, S.; et al. Actionable Activating Oncogenic ERBB2/HER2 Transmembrane and Juxtamembrane Domain Mutations. Cancer Cell 2018, 34, 792–806.e5. [Google Scholar] [CrossRef]
- Cocco, E.; Lopez, S.; Santin, A.D.; Scaltriti, M. Prevalence and Role of HER2 Mutations in Cancer. Pharmacol. Ther. 2019, 199, 188–196. [Google Scholar] [CrossRef]
- Robichaux, J.P.; Elamin, Y.Y.; Vijayan, R.S.K.; Nilsson, M.B.; Hu, L.; He, J.; Zhang, F.; Pisegna, M.; Poteete, A.; Sun, H.; et al. Pan-Cancer Landscape and Analysis of ERBB2 Mutations Identifies Poziotinib as a Clinically Active Inhibitor and Enhancer of T-DM1 Activity. Cancer Cell 2019, 36, 444–457.e7. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Johnson, A.M.; Ileana Dumbrava, E.E.; Raghav, K.; Balaji, K.; Bhatt, M.; Murthy, R.K.; Rodon, J.; Piha-Paul, S.A. Advances in HER2-Targeted Therapy: Novel Agents and Opportunities Beyond Breast and Gastric Cancer. Clin. Cancer Res. 2019, 25, 2033–2041. [Google Scholar] [CrossRef]
- Pillai, R.N.; Behera, M.; Berry, L.D.; Rossi, M.R.; Kris, M.G.; Johnson, B.E.; Bunn, P.A.; Ramalingam, S.S.; Khuri, F.R. HER2 Mutations in Lung Adenocarcinomas: A Report from the Lung Cancer Mutation Consortium. Cancer 2017, 123, 4099–4105. [Google Scholar] [CrossRef]
- Ren, S.; Wang, J.; Ying, J.; Mitsudomi, T.; Lee, D.H.; Wang, Z.; Chu, Q.; Mack, P.C.; Cheng, Y.; Duan, J.; et al. Consensus for HER2 Alterations Testing in Non-Small-Cell Lung Cancer. ESMO Open 2022, 7, 100395. [Google Scholar] [CrossRef]
- Kim, E.K.; Kim, K.A.; Lee, C.Y.; Shim, H.S. The Frequency and Clinical Impact of HER2 Alterations in Lung Adenocarcinoma. PLoS ONE 2017, 12, e0171280. [Google Scholar] [CrossRef]
- Li, B.T.; Ross, D.S.; Aisner, D.L.; Chaft, J.E.; Hsu, M.; Kako, S.L.; Kris, M.G.; Varella-Garcia, M.; Arcila, M.E. HER2 Amplification and HER2 Mutation Are Distinct Molecular Targets in Lung Cancers. J. Thorac. Oncol. 2016, 11, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Kuyama, S.; Hotta, K.; Tabata, M.; Segawa, Y.; Fujiwara, Y.; Takigawa, N.; Kiura, K.; Ueoka, H.; Eguchi, K.; Tanimoto, M. Impact of HER2 Gene and Protein Status on the Treatment Outcome of Cisplatin-Based Chemoradiotherapy for Locally Advanced Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2008, 3, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, K.; Hata, T.; Yoshioka, H.; Ohashi, K.; Bessho, A.; Hosokawa, S.; Ishikawa, N.; Yamasaki, M.; Shibayama, T.; Aoe, K.; et al. A Prospective Cohort Study to Define the Clinical Features and Outcome of Lung Cancers Harboring HER2 Aberration in Japan (HER2-CS STUDY). Chest 2019, 156, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Hoyo, A.; Moliner, L.; Arriola, E. Acquired Mechanisms of Resistance to Osimertinib—The Next Challenge. Cancers 2022, 14, 1931. [Google Scholar] [CrossRef]
- Wen, W.; Chen, W.S.; Xiao, N.; Bender, R.; Ghazalpour, A.; Tan, Z.; Swensen, J.; Millis, S.Z.; Basu, G.; Gatalica, Z.; et al. Mutations in the Kinase Domain of the HER2/ERBB2 Gene Identified in a Wide Variety of Human Cancers. J. Mol. Diagn. 2015, 17, 487–495. [Google Scholar] [CrossRef]
- Kris, M.G.; Camidge, D.R.; Giaccone, G.; Hida, T.; Li, B.T.; O’Connell, J.; Taylor, I.; Zhang, H.; Arcila, M.E.; Goldberg, Z.; et al. Targeting HER2 Aberrations as Actionable Drivers in Lung Cancers: Phase II Trial of the Pan-HER Tyrosine Kinase Inhibitor Dacomitinib in Patients with HER2-Mutant or Amplified Tumors. Ann. Oncol. 2015, 26, 1421–1427. [Google Scholar] [CrossRef]
- Oh, I.J.; Hur, J.Y.; Park, C.K.; Kim, Y.C.; Kim, S.J.; Lee, M.K.; Kim, H.J.; Lee, K.Y.; Lee, J.C.; Choi, C.M. Clinical Activity of Pan-HER Inhibitors Against HER2-Mutant Lung Adenocarcinoma. Clin. Lung Cancer 2018, 19, e775–e781. [Google Scholar] [CrossRef]
- Liu, S.; Li, S.; Hai, J.; Wang, X.; Chen, T.; Quinn, M.M.; Gao, P.; Zhang, Y.; Ji, H.; Cross, D.A.E.; et al. Targeting HER2 Aberrations in Non-Small Cell Lung Cancer with Osimertinib. Clin. Cancer Res. 2018, 24, 2594–2604. [Google Scholar] [CrossRef]
- Lee, K.; Jung, H.A.; Sun, J.M.; Lee, S.H.; Ahn, J.S.; Park, K.; Ahn, M.J. Clinical Characteristics and Outcomes of Non-Small Cell Lung Cancer Patients with HER2 Alterations in Korea. Cancer Res. Treat. 2020, 52, 292–300. [Google Scholar] [CrossRef]
- Zhao, S.; Fang, W.; Pan, H.; Yang, Y.; Liang, Y.; Yang, L.; Dong, X.; Zhan, J.; Wang, K.; Zhang, L. Conformational Landscapes of HER2 Exon 20 Insertions Explain Their Sensitivity to Kinase Inhibitors in Lung Adenocarcinoma. J. Thorac. Oncol. 2020, 15, 962–972. [Google Scholar] [CrossRef]
- Fang, W.; Zhao, S.; Liang, Y.; Yang, Y.; Yang, L.; Dong, X.; Zhang, L.; Tang, Y.; Wang, S.; Yang, Y.; et al. Mutation Variants and Co-Mutations as Genomic Modifiers of Response to Afatinib in HER2 -Mutant Lung Adenocarcinoma. Oncologist 2020, 25, e545–e554. [Google Scholar] [CrossRef]
- Mazières, J.; Peters, S.; Lepage, B.; Cortot, A.B.; Barlesi, F.; Beau-Faller, M.; Besse, B.; Blons, H.; Mansuet-Lupo, A.; Urban, T.; et al. Lung Cancer That Harbors an HER2 Mutation: Epidemiologic Characteristics and Therapeutic Perspectives. J. Clin. Oncol. 2013, 31, 1997–2003. [Google Scholar] [CrossRef]
- Arcila, M.E.; Chaft, J.E.; Nafa, K.; Roy-Chowdhuri, S.; Lau, C.; Zaidinski, M.; Paik, P.K.; Zakowski, M.F.; Kris, M.G.; Ladanyi, M. Prevalence, Clinicopathologic Associations, and Molecular Spectrum of ERBB2 (HER2) Tyrosine Kinase Mutations in Lung Adenocarcinomas. Clin. Cancer Res. 2012, 18, 4910–4918. [Google Scholar] [CrossRef]
- Greulich, H.; Kaplan, B.; Mertins, P.; Chen, T.H.; Tanaka, K.E.; Yun, C.H.; Zhang, X.; Lee, S.H.; Cho, J.; Ambrogio, L.; et al. Functional Analysis of Receptor Tyrosine Kinase Mutations in Lung Cancer Identifies Oncogenic Extracellular Domain Mutations of ERBB2. Proc. Natl. Acad. Sci. USA 2012, 109, 14476–14481. [Google Scholar] [CrossRef]
- Wei, X.W.; Gao, X.; Zhang, X.C.; Yang, J.J.; Chen, Z.H.; Wu, Y.L.; Zhou, Q. Mutational Landscape and Characteristics of ERBB2 in Non-Small Cell Lung Cancer. Thorac. Cancer 2020, 11, 1512–1521. [Google Scholar] [CrossRef]
- Ou, S.H.I.; Schrock, A.B.; Bocharov, E.V.; Klempner, S.J.; Haddad, C.K.; Steinecker, G.; Johnson, M.; Gitlitz, B.J.; Chung, J.; Campregher, P.V.; et al. HER2 Transmembrane Domain (TMD) Mutations (V659/G660) That Stabilize Homo- and Heterodimerization Are Rare Oncogenic Drivers in Lung Adenocarcinoma That Respond to Afatinib. J. Thorac. Oncol. 2017, 12, 446–457. [Google Scholar] [CrossRef]
- Yuan, B.; Zhao, J.; Zhou, C.; Wang, X.; Zhu, B.; Zhuo, M.; Dong, X.; Feng, J.; Yi, C.; Yang, Y.; et al. Co-Occurring Alterations of ERBB2 Exon 20 Insertion in Non-Small Cell Lung Cancer (NSCLC) and the Potential Indicator of Response to Afatinib. Front. Oncol. 2020, 10, 729. [Google Scholar] [CrossRef]
- Offin, M.; Feldman, D.; Ni, A.; Myers, M.L.; Lai, W.V.; Pentsova, E.; Boire, A.; Daras, M.; Jordan, E.J.; Solit, D.B.; et al. Frequency and Outcomes of Brain Metastases in Patients with HER2-Mutant Lung Cancers. Cancer 2019, 125, 4380–4387. [Google Scholar] [CrossRef]
- Mazieres, J.; Lafitte, C.; Ricordel, C.; Greillier, L.; Negre, E.; Zalcman, G.; Domblides, C.; Madelaine, J.; Bennouna, J.; Mascaux, C.; et al. Combination of Trastuzumab, Pertuzumab, and Docetaxel in Patients With Advanced Non-Small-Cell Lung Cancer Harboring HER2 Mutations: Results From the IFCT-1703 R2D2 Trial. J. Clin. Oncol. 2022, 40, 719–728. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Y.; Zhao, C.; Li, X.; Liu, Q.; Mao, S.; Liu, Y.; Yu, X.; Wang, W.; Tian, Q.; et al. Exon 20 YVMA Insertion Is Associated with High Incidence of Brain Metastasis and Inferior Outcome of Chemotherapy in Advanced Non-Small Cell Lung Cancer Patients with HER2 Kinase Domain Mutations. Transl. Lung Cancer Res. 2021, 10, 753–765. [Google Scholar] [CrossRef]
- Zhou, C.; Lu, Y.; Kim, S.-W.; Reungwetwattana, T.; Zhou, J.; Zhang, Y.; He, J.; Yang, J.-J.; Cheng, Y.; Lee, S.H.; et al. Mechanisms of Acquired Resistance to First-Line Osimertinib: Preliminary Data from the Phase III FLAURA Study. Ann. Oncol. 2018, 29, 740. [Google Scholar] [CrossRef]
- Bunn, P.; Helfrich, B.; Soriano, A.; Franklin, W.; Varella-Garcia, M.; Hirsch, F.; Baron, A.; Zeng, C.; Chan, D. Expression of Her-2/Neu in Human Lung Cancer Cell Lines by Immunohistochemistry and Fluorescence in Situ Hybridization and Its Relationship to in Vitro Cytotoxicity by Trastuzumab and Chemotherapeutic Agents. Clin. Cancer Res. 2001, 7, 3239–3250. [Google Scholar]
- Giltnane, J.M.; Murren, J.R.; Rimm, D.L.; King, B.L. AQUA and FISH Analysis of HER-2/Neu Expression and Amplification in a Small Cell Lung Carcinoma Tissue Microarray. Histopathology 2006, 49, 161–169. [Google Scholar] [CrossRef]
- Peters, S.; Zimmermann, S. Targeted Therapy in NSCLC Driven by HER2 Insertions. Transl. Lung Cancer Res. 2014, 3, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Li, X.; Wang, Q.; Gao, G.; Zhang, Y.; Chen, J.; Shu, Y.; Hu, Y.; Fan, Y.; Fang, J.; et al. Pyrotinib in HER2-Mutant Advanced Lung Adenocarcinoma After Platinum-Based Chemotherapy: A Multicenter, Open-Label, Single-Arm, Phase II Study. J. Clin. Oncol. 2020, 38, 2753–2761. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, F.R.; Varella-Garcia, M.; Franklin, W.A.; Veve, R.; Chen, L.; Helfrich, B.; Zeng, C.; Baron, A.; Bunn, P.A. Evaluation of HER-2/Neu Gene Amplification and Protein Expression in Non-Small Cell Lung Carcinomas. Br. J. Cancer 2002, 86, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Wang, L.; Arcila, M.E.; Balasubramanian, S.; Greenbowe, J.R.; Ross, J.S.; Stephens, P.; Lipson, D.; Miller, V.A.; Kris, M.G.; et al. Broad, Hybrid Capture-Based Next-Generation Sequencing Identifies Actionable Genomic Alterations in Lung Adenocarcinomas Otherwise Negative for Such Alterations by Other Genomic Testing Approaches. Clin. Cancer Res. 2015, 21, 3631–3639. [Google Scholar] [CrossRef] [PubMed]
- Li, B.T.; Janku, F.; Jung, B.; Hou, C.; Madwani, K.; Alden, R.; Razavi, P.; Reis-Filho, J.S.; Shen, R.; Isbell, J.M.; et al. Ultra-Deep next-Generation Sequencing of Plasma Cell-Free DNA in Patients with Advanced Lung Cancers: Results from the Actionable Genome Consortium. Ann. Oncol. 2019, 30, 597–603. [Google Scholar] [CrossRef]
- Wolff, A.C.; Elizabeth, M.; Hammond, H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef]
- Graziano, S.L.; Tatum, A.; Herndon, J.E.; Box, J.; Memoli, V.; Green, M.R.; Kern, J.A. Use of Neuroendocrine Markers, P53, and HER2 to Predict Response to Chemotherapy in Patients with Stage III Non-Small Cell Lung Cancer: A Cancer and Leukemia Group B Study. Lung Cancer 2001, 33, 115–123. [Google Scholar] [CrossRef]
- Cappuzzo, F.; Ligorio, C.; Toschi, L.; Rossi, E.; Trisolini, R.; Paioli, D.; Magrini, E.; Finocchiaro, G.; Bartolini, S.; Cancellieri, A.; et al. EGFR and HER2 Gene Copy Number and Response to First-Line Chemotherapy in Patients with Advanced Non-Small Cell Lung Cancer (NSCLC). J. Thorac. Oncol. 2007, 2, 423–429. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Wu, F.; Zhao, J.; Li, X.; Zhao, C.; Ren, S.; Zhou, C. Outcomes of Pemetrexed-Based Chemotherapies in HER2-Mutant Lung Cancers. BMC Cancer 2018, 18, 326. [Google Scholar] [CrossRef]
- van Berge Henegouwen, J.M.; Jebbink, M.; Hoes, L.R.; van der Wijngaart, H.; Zeverijn, L.J.; van der Velden, D.L.; Roepman, P.; de Leng, W.W.J.; Jansen, A.M.L.; van Werkhoven, E.; et al. Trastuzumab and Pertuzumab Combination Therapy for Advanced Pre-Treated HER2 Exon 20-Mutated Non-Small Cell Lung Cancer. Eur. J. Cancer 2022, 171, 114–123. [Google Scholar] [CrossRef]
- Mazières, J.; Barlesi, F.; Filleron, T.; Besse, B.; Monnet, I.; Beau-Faller, M.; Peters, S.; Dansin, E.; Früh, M.; Pless, M.; et al. Lung Cancer Patients with HER2 Mutations Treated with Chemotherapy and HER2-Targeted Drugs: Results from the European EUHER2 Cohort. Ann. Oncol. 2016, 27, 281–286. [Google Scholar] [CrossRef]
- Lai, W.V.; Lebas, L.; Barnes, T.A.; Milia, J.; Ni, A.; Gautschi, O.; Peters, S.; Ferrara, R.; Plodkowski, A.J.; Kavanagh, J.; et al. Afatinib in Patients with Metastatic or Recurrent HER2-Mutant Lung Cancers: A Retrospective International Multicentre Study. Eur. J. Cancer 2019, 109, 28–35. [Google Scholar] [CrossRef]
- Dziadziuszko, R.; Smit, E.F.; Dafni, U.; Wolf, J.; Wasąg, B.; Biernat, W.; Finn, S.P.; Kammler, R.; Tsourti, Z.; Rabaglio, M.; et al. Afatinib in NSCLC With HER2 Mutations: Results of the Prospective, Open-Label Phase II NICHE Trial of European Thoracic Oncology Platform (ETOP). J. Thorac. Oncol. 2019, 14, 1086–1094. [Google Scholar] [CrossRef]
- Hyman, D.M.; Piha-Paul, S.A.; Won, H.; Rodon, J.; Saura, C.; Shapiro, G.I.; Juric, D.; Quinn, D.I.; Moreno, V.; Doger, B.; et al. HER Kinase Inhibition in Patients with HER2- and HER3-Mutant Cancers. Nature 2018, 554, 189–194. [Google Scholar] [CrossRef]
- Peters, S.; Curioni-Fontecedro, A.; Nechushtan, H.; Shih, J.Y.; Liao, W.Y.; Gautschi, O.; Spataro, V.; Unk, M.; Chih-Hsin Yang, J.; Lorence, R.M.; et al. Activity of Afatinib in Heavily Pretreated Patients With ERBB2 Mutation-Positive Advanced NSCLC: Findings From a Global Named Patient Use Program. J. Thorac. Oncol. 2018, 13, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- de Grève, J.; Moran, T.; Graas, M.P.; Galdermans, D.; Vuylsteke, P.; Canon, J.L.; Schallier, D.; Decoster, L.; Teugels, E.; Massey, D.; et al. Phase II Study of Afatinib, an Irreversible ErbB Family Blocker, in Demographically and Genotypically Defined Lung Adenocarcinoma. Lung Cancer 2015, 88, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Robichaux, J.P.; Elamin, Y.Y.; Tan, Z.; Carter, B.W.; Zhang, S.; Liu, S.; Li, S.; Chen, T.; Poteete, A.; Estrada-Bernal, A.; et al. Mechanisms and Clinical Activity of an EGFR and HER2 Exon 20-Selective Kinase Inhibitor in Non-Small Cell Lung Cancer. Nat. Med. 2018, 24, 638. [Google Scholar] [CrossRef] [PubMed]
- Koga, T.; Kobayashi, Y.; Tomizawa, K.; Suda, K.; Kosaka, T.; Sesumi, Y.; Fujino, T.; Nishino, M.; Ohara, S.; Chiba, M.; et al. Activity of a Novel HER2 Inhibitor, Poziotinib, for HER2 Exon 20 Mutations in Lung Cancer and Mechanism of Acquired Resistance: An in Vitro Study. Lung Cancer 2018, 126, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.M.; Lee, K.W.; Oh, D.Y.; Lee, J.S.; Im, S.A.; Kim, D.W.; Han, S.W.; Kim, Y.J.; Kim, T.Y.; Kim, J.H.; et al. Phase 1 Studies of Poziotinib, an Irreversible Pan-HER Tyrosine Kinase Inhibitor in Patients with Advanced Solid Tumors. Cancer Res. Treat. 2018, 50, 835–842. [Google Scholar] [CrossRef]
- Elamin, Y.Y.; Robichaux, J.P.; Carter, B.W.; Altan, M.; Gibbons, D.L.; Fossella, F.V.; Lam, V.K.; Patel, A.B.; Negrao, M.V.; Le, X.; et al. Poziotinib for Patients With HER2 Exon 20 Mutant Non-Small-Cell Lung Cancer: Results From a Phase II Trial. J. Clin. Oncol. 2022, 40, 702–709. [Google Scholar] [CrossRef]
- Prelaj, A.; Bottiglieri, A.; Proto, C.; lo Russo, G.; Signorelli, D.; Ferrara, R.; Galli, G.; de Toma, A.; Viscardi, G.; Brambilla, M.; et al. Poziotinib for EGFR and HER2 Exon 20 Insertion Mutation in Advanced NSCLC: Results from the Expanded Access Program. Eur. J. Cancer 2021, 149, 235–248. [Google Scholar] [CrossRef]
- Le, X.; Cornelissen, R.; Garassino, M.; Clarke, J.M.; Tchekmedyian, N.; Goldman, J.W.; Leu, S.Y.; Bhat, G.; Lebel, F.; Heymach, J.V.; et al. Poziotinib in Non-Small-Cell Lung Cancer Harboring HER2 Exon 20 Insertion Mutations After Prior Therapies: ZENITH20-2 Trial. J. Clin. Oncol. 2022, 40, 710–718. [Google Scholar] [CrossRef]
- Cornelissen, R.; Sun, S.; Wollner, M.; Garassino, M.C.C.; Prelaj, A.; Haura, E.B.; Piotrowska, Z.; Goldman, J.W.; Socinski, M.; Dreling, L.; et al. Efficacy and Safety of Poziotinib in Treatment-Naïve NSCLC Harboring HER2 Exon 20 Mutations: A Multinational Phase II Study (ZENITH20-4)|OncologyPRO. Ann. Oncol. 2021, 32, S1283–S1346. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, T.; Qin, Z.; Jiang, J.; Wang, Q.; Yang, S.; Rivard, C.; Gao, G.; Ng, T.L.; Tu, M.M.; et al. HER2 Exon 20 Insertions in Non-Small-Cell Lung Cancer Are Sensitive to the Irreversible Pan-HER Receptor Tyrosine Kinase Inhibitor Pyrotinib. Ann. Oncol. 2019, 30, 447–455. [Google Scholar] [CrossRef]
- Song, Z.; Lv, D.; Chen, S.Q.; Huang, J.; Li, Y.; Ying, S.; Wu, X.; Hua, F.; Wang, W.; Xu, C.; et al. Pyrotinib in Patients with HER2-Amplified Advanced Non-Small Cell Lung Cancer: A Prospective, Multicenter, Single-Arm Trial. Clin. Cancer Res. 2022, 28, 461–467. [Google Scholar] [CrossRef]
- Estrada-Bernal, A.; Le, A.T.; Doak, A.E.; Tirunagaru, V.G.; Silva, S.; Bull, M.R.; Smaill, J.B.; Patterson, A.V.; Kim, C.; Liu, S.V.; et al. Tarloxotinib Is a Hypoxia-Activated Pan-HER Kinase Inhibitor Active Against a Broad Range of HER-Family Oncogenes. Clin. Cancer Res. 2021, 27, 1463–1475. [Google Scholar] [CrossRef]
- Liu, S.V.; Villaruz, L.C.; Lee, V.H.F.; Zhu, V.W.; Baik, C.S.; Sacher, A.; McCoach, C.E.; Nguyen, D.; Li, J.Y.-C.; Pacheco, J.M.; et al. LBA61 First Analysis of RAIN-701: Study of Tarloxotinib in Patients with Non-Small Cell Lung Cancer (NSCLC) EGFR Exon 20 Insertion, HER2-Activating Mutations & Other Solid Tumours with NRG1/ERBB Gene Fusions. Ann. Oncol. 2020, 31, S1189. [Google Scholar] [CrossRef]
- Han, H.; Li, S.; Chen, T.; Fitzgerald, M.; Liu, S.; Peng, C.; Tang, K.H.; Cao, S.; Chouitar, J.; Wu, J.; et al. Targeting HER2 Exon 20 Insertion-Mutant Lung Adenocarcinoma with a Novel Tyrosine Kinase Inhibitor Mobocertinib. Cancer Res. 2021, 81, 5311–5324. [Google Scholar] [CrossRef]
- Riely, G.J.; Neal, J.W.; Camidge, D.R.; Spira, A.I.; Piotrowska, Z.; Costa, D.B.; Tsao, A.S.; Patel, J.D.; Gadgeel, S.M.; Bazhenova, L.; et al. Activity and Safety of Mobocertinib (TAK-788) in Previously Treated Non–Small Cell Lung Cancer With EGFR Exon 20 Insertion Mutations From a Phase 1/2 Trial. Cancer Discov. 2021, 11, 1688. [Google Scholar] [CrossRef]
- Lewis Phillips, G.D.; Li, G.; Dugger, D.L.; Crocker, L.M.; Parsons, K.L.; Mai, E.; Blättler, W.A.; Lambert, J.M.; Chari, R.V.J.; Lutz, R.J.; et al. Targeting HER2-Positive Breast Cancer with Trastuzumab-DM1, an Antibody-Cytotoxic Drug Conjugate. Cancer Res. 2008, 68, 9280–9290. [Google Scholar] [CrossRef]
- Li, B.T.; Shen, R.; Buonocore, D.; Olah, Z.T.; Ni, A.; Ginsberg, M.S.; Ulaner, G.A.; Offin, M.; Feldman, D.; Hembrough, T.; et al. Ado-Trastuzumab Emtansine for Patients With HER2-Mutant Lung Cancers: Results From a Phase II Basket Trial. J. Clin. Oncol. 2018, 36, 2532–2537. [Google Scholar] [CrossRef]
- Li, B.T.; Makker, V.; Buonocore, D.J.; Offin, M.D.; Olah, Z.T.; Panora, E.; Shen, R.; Ho, A.L.; Yaeger, R.; Iyer, G.; et al. A Multi-Histology Basket Trial of Ado-Trastuzumab Emtansine in Patients with HER2 Amplified Cancers. J. Clin. Oncol. 2018, 36, 2502. [Google Scholar] [CrossRef]
- Peters, S.; Stahel, R.; Bubendorf, L.; Bonomi, P.; Villegas, A.; Kowalski, D.M.; Baik, C.S.; Isla, D.; de Castro Carpeno, J.; Garrido, P.; et al. Trastuzumab Emtansine (T-DM1) in Patients with Previously Treated HER2-Overexpressing Metastatic Non-Small Cell Lung Cancer: Efficacy, Safety, and Biomarkers. Clin. Cancer Res. 2019, 25, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Iwama, E.; Zenke, Y.; Sugawara, S.; Daga, H.; Morise, M.; Yanagitani, N.; Sakamoto, T.; Murakami, H.; Kishimoto, J.; Matsumoto, S.; et al. Trastuzumab Emtansine for Patients with Non–Small Cell Lung Cancer Positive for Human Epidermal Growth Factor Receptor 2 Exon-20 Insertion Mutations. Eur. J. Cancer 2022, 162, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Ogitani, Y.; Hagihara, K.; Oitate, M.; Naito, H.; Agatsuma, T. Bystander Killing Effect of DS-8201a, a Novel Anti-human Epidermal Growth Factor Receptor 2 Antibody–Drug Conjugate, in Tumors with Human Epidermal Growth Factor Receptor 2 Heterogeneity. Cancer Sci. 2016, 107, 1039. [Google Scholar] [CrossRef] [PubMed]
- Ogitani, Y.; Aida, T.; Hagihara, K.; Yamaguchi, J.; Ishii, C.; Harada, N.; Soma, M.; Okamoto, H.; Oitate, M.; Arakawa, S.; et al. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin. Cancer Res. 2016, 22, 5097–5108. [Google Scholar] [CrossRef]
- Tsurutani, J.; Iwata, H.; Krop, I.; Jänne, P.A.; Doi, T.; Takahashi, S.; Park, H.; Redfern, C.; Tamura, K.; Wise-Draper, T.M.; et al. Targeting HER2 with Trastuzumab Deruxtecan: A Dose-Expansion, Phase I Study in Multiple Advanced Solid Tumors. Cancer Discov. 2020, 10, 688–701. [Google Scholar] [CrossRef]
- Nakagawa, K.; Nagasaka, M.; Felip, E.; Pacheco, J.; Baik, C.; Goto, Y.; Saltos, A.; Li, B.; Udagawa, H.; Gadgeel, S.; et al. OA04.05 Trastuzumab Deruxtecan in HER2-Overexpressing Metastatic Non-Small Cell Lung Cancer: Interim Results of DESTINY-Lung01. J. Thorac. Oncol. 2021, 16, S109–S110. [Google Scholar] [CrossRef]
- Li, B.T.; Michelini, F.; Misale, S.; Cocco, E.; Baldino, L.; Cai, Y.; Shifman, S.; Tu, H.Y.; Myers, M.L.; Xu, C.; et al. HER2-Mediated Internalization of Cytotoxic Agents in ERBB2 Amplified or Mutant Lung Cancers. Cancer Discov. 2020, 10, 674–687. [Google Scholar] [CrossRef]
- Negrao, M.V.; Reuben, A.; Robichaux, J.P.; Le, X.; Nilsson, M.B.; Wu, C.; Zhang, J.; Landry, L.C.A.; Roarty, E.; Rinsurongkawong, W.; et al. Association of EGFR and HER-2 Exon 20 Mutations with Distinct Patterns of Response to Immune Checkpoint Blockade in Non-Small Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 9052. [Google Scholar] [CrossRef]
- Lai, W.-C.V.; Feldman, D.L.; Buonocore, D.J.; Brzostowski, E.B.; Rizvi, H.; Plodkowski, A.J.; Ni, A.; Sabari, J.K.; Offin, M.D.; Kris, M.G.; et al. PD-L1 Expression, Tumor Mutation Burden and Response to Immune Checkpoint Blockade in Patients with HER2-Mutant Lung Cancers. J. Clin. Oncol. 2018, 36, 9060. [Google Scholar] [CrossRef]
- Calles, A.; Riess, J.W.; Brahmer, J.R. Checkpoint Blockade in Lung Cancer With Driver Mutation: Choose the Road Wisely. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, 372–384. [Google Scholar] [CrossRef]
- Guisier, F.; Dubos-Arvis, C.; Viñas, F.; Doubre, H.; Ricordel, C.; Ropert, S.; Janicot, H.; Bernardi, M.; Fournel, P.; Lamy, R.; et al. Efficacy and Safety of Anti-PD-1 Immunotherapy in Patients With Advanced NSCLC With BRAF, HER2, or MET Mutations or RET Translocation: GFPC 01-2018. J. Thorac. Oncol. 2020, 15, 628–636. [Google Scholar] [CrossRef]
- Mazieres, J.; Drilon, A.; Lusque, A.; Mhanna, L.; Cortot, A.B.; Mezquita, L.; Thai, A.A.; Mascaux, C.; Couraud, S.; Veillon, R.; et al. Immune Checkpoint Inhibitors for Patients with Advanced Lung Cancer and Oncogenic Driver Alterations: Results from the IMMUNOTARGET Registry. Ann. Oncol. 2019, 30, 1321–1328. [Google Scholar] [CrossRef]
- Chen, K.; Pan, G.; Cheng, G.; Zhang, F.; Xu, Y.; Huang, Z.; Fan, Y. Immune Microenvironment Features and Efficacy of PD-1/PD-L1 Blockade in Non-Small Cell Lung Cancer Patients with EGFR or HER2 Exon 20 Insertions. Thorac. Cancer 2021, 12, 218–226. [Google Scholar] [CrossRef]
- Chu, X.; Qiang, H.; Xie, M.; Li, X.; Zhao, J.; Wu, Y.; Zhou, J.; Ye, J.; Zhao, C.; Han, C.; et al. Treatment Efficacy of HER2-Mutant Lung Adenocarcinoma by Immune Checkpoint Inhibitors: A Multicenter Retrospective Study. Cancer Immunol. Immunother. 2022, 71, 1625–1631. [Google Scholar] [CrossRef]
- Saalfeld, F.C.; Wenzel, C.; Christopoulos, P.; Merkelbach-Bruse, S.; Reissig, T.M.; Laßmann, S.; Thiel, S.; Stratmann, J.A.; Marienfeld, R.; Berger, J.; et al. Efficacy of Immune Checkpoint Inhibitors Alone or in Combination With Chemotherapy in NSCLC Harboring ERBB2 Mutations. J. Thorac. Oncol. 2021, 16, 1952–1958. [Google Scholar] [CrossRef]
- Tian, P.; Zeng, H.; Ji, L.; Ding, Z.; Ren, L.; Gao, W.; Fan, Z.; Li, L.; Le, X.; Li, P.; et al. Lung Adenocarcinoma with ERBB2 Exon 20 Insertions: Comutations and Immunogenomic Features Related to Chemoimmunotherapy. Lung Cancer 2021, 160, 50–58. [Google Scholar] [CrossRef]
- Tchekmedyian, N.; Paxton, B.; Lebel, F.; Keossayan, L.; Heymach, J.V. Prolonged Central Nervous System Response in a Patient With HER2 Mutant NSCLC Treated With First-Line Poziotinib. JTO Clin. Res. Rep. 2020, 1, 100081. [Google Scholar] [CrossRef]
| Class | Drugs | Clinical Trial | Population | Cohort Size (n) | HER2 Alteration | ORR n (%) | DCR n (%) | Median PFS, Months (95% CI) | Median OS, Months (95% CI) | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Selective TKI | Poziotinib | Phase II study (NCT03066206) | Metastatic, recurrent NSCLC | 12 | HER2 mutation (Y772dupYVMA (9) Or G778dupGSP (3)) | 5 (42) | 10 (83) | 5.6 (NA) | NA | Robichaux et al [41]. |
| Selective TKI | Poziotinib | Phase II study (NCT03066206). | Stage IV or recurrent NSCLC, 90% of patients were pretreated | 30 | HER2 mutation (Y772_A775dupYVMA (23), G778_P780dupGSP (5) or G776delinsVC (2)) | 8 (27) | 22 (73) | 5.5 (4.0–7.0) | 15 (9.0–NE) | Elamin et al. [88]. |
| Selective TKI | Poziotinib | Phase II study, expanded access program | Advanced NSCLC | 8 a | HER2 exon 20 insertion | 4 (50) | 6 (75) | 5.6 (3.6–6.7) b | 9.5 (5.3–NE) b | Prelaj et al. [89] |
| Selective TKI | Poziotinib | Phase II Basket trial ZENITH20 study (NCT03318939) | Pretreated, advanced NSCLC | 90 (cohort 2) | HER2 mutation (Y772_A775dupYVMA (65), G776delinsVC (11), G778_P780dupGSP (7) or other mutant (7)) | 25 (27.8) | 63 (70) | 5.5 (3.9–5.8) | NA | Le et al. [90]. |
| Selective TKI | Poziotinib | Phase II Basket trial ZENITH20 study (NCT03318939) | Treatment naïve, advanced NSCLC | 48 (cohort 4) | HER2 exon 20 insertion | 21 (44) | 5.6 (NA) | NA | Cornelissen et al. [91]. | |
| Selective TKI | Pyrotinib | Phase I/II study (NCT02535507) | Pretreated, advanced NSCLC | 15 | HER2 exon 20 insertion (A775_ G776insYVMA (10)) | 8 (53.3) | 11 (73.3) | 6.4 (1.6–11.2) | 12.9 (2.1–23.8) | Wang et al. [92]. |
| Selective TKI | Pyrotinib | Phase II, single-arm study (NCT02834936) | Pretreated, advanced NSCLC | 60 | HER2 mutations (12-bp exon 20 insertion (44) G776 mutation (6) G778_P780dupGSP (5), L755P (4), or V777L (1)) | 18 (30) | 51 (85) | 6.9 (5.5–8.3) | 14.4 (12.3–21.3) | Zhou et al. [70]. |
| Selective TKI | Pyrotinib | Prospective, single-arm study (ChiCTR1800020262) | Stage IIIB/IV NSCLC | 27 | HER2 amplification | 6 (22.2) | 18 (81.5) | 6.3 (3.0–9.6) | 12.5 (8.2–16.8) | Song et al. [93]. |
| Selective TKI | Tarloxotinib | Phase II Basket trial RAIN-701 study (NCT03805841) | Progressive disease after platinum-based CT | 11 (cohort B), 9 were evaluable | HER2 mutations (not specified) | 2 (22) | 6 (67) | NA | NA | Liu et al. [95]. |
| ADC | T-DM1 | Phase II, single-arm study | Pretreated, advanced NSCLC | 7/15 | HER2 mutation (A775_ G776insYVMA (5)) | 1 (4.3) | 5 (71.4) | 2.0 (1.2–4) | 10.9 (4.4–12) | Hotta et al. [21]. |
| 8/15 | HER2 amplification/ overexpression (IHC3+ or IHC2+ confirmed by FISH) | 0 (0) | 3 (37.5) | NA | NA | |||||
| ADC | T-DM1 | Phase II Basket trial (NCT02675829) | Advanced NSCLC, 83% pretreated with CT | 28/49 | HER2 mutation (subtypes not specified) | 14 (50) | NA | 5 (3.5–5.9) | NA | Li et al. [99,100]. |
| 11/49 | HER2 amplification | 6 (55) | NA | |||||||
| ADC | T-DM1 | Phase II, single-arm study | Locally advanced or metastatic NSCLC, pretreated with ≥1 CT | 29 | HER2 overexpression IHC2+ | 0 (0) | 8 (28) | 2.6 (1.4–2.8) | 12.2 (3.8–23.3) | Peters et al. [101]. |
| ADC | T-DM1 | Phase II, single-arm study | Stage III/IV NSCLC pretreated with CT or NSCLC with postoperative recurrence | 22 | HER2 exon 20 mutation (A775_ G776insYVMA (19)) | 8 (38) | 11 (52) | 2.8 | 8.1 | Iwama et al. [102] |
| 20 | HER2 overexpression IHC3+ | 4 (20) | 8 (40) | 2.7 (1.4–8.3) | 15.3 (4.1–NE) | |||||
| ADC | T-Dxd | Phase I study (NCT02564900) | 11 | HER2 mutation (44.4% exon 20 insertions) | 8 (72.7) | 10 (90.9) | 11.3 (8.1–14.3) | 17.3 (17.3–NE) | Tsurutani et al. [105]. | |
| ADC | T-Dxd | Phase II study Two-cohort and two-arm DESTINYLung01 (NCT03505710) | Pretreated, metastatic NSCLC | 49 | HER2 overexpression (IHC2+/3+) | 12 (24.5) | 34 (69.4) | 5.4 (2.8–7.0) | 11.3 (7.8–NR) | Nakagawa et al. [106]. |
| Pretreated, unresectable, or metastatic NSCLC | 91 | HER2 mutation (exon 20 insertion, (78), mutation in HER2 TKD exon 19 or 20 (7), or mutation in HER2 ECD exon 8 (6)) | 50 (55) | 84 (92) | 8.2 (6.0–11.9) | 17.8 (13.8–22.1) | Li et al. [22]. | |||
| Single ICI | Anti-PD1 (Nivolumab 89.6%) | Retrospective study—IMMUNOTARGET Registry c | Pretreated, advanced NSCLC | 29 | HER2 mutation (not specified) | 2 (7.4) | 9 (31) | 2.5 (1.8–3.5) | 20.3 (7.8–NR) | Mazieres et al. [112]. |
| Single ICI | Anti-PD1 (Nivolumab 83%) | Retrospective study—French Lung Cancer Group (GFPC) d | Pretreated, advanced NSCLC | 23 | HER2 mutation (not specified) | 6 (27.3) | 11 (50) | 2.2 (1.7–15.2) | 20.4 (9.3–NR) | Guisier et al. [111]. |
| ICI + CT | Combined therapy (pembrolizumab 80%) | Retrospective study | Treatment-naïve, advanced NSCLC | 27 (21 patients were assessable) | HER2 mutation (Exon 20 Insertion (16), TKD Mutation (1), ECD mutation (4)) | 11 (52) | NA | 6 (6–14) | NA | Saalfeld et al. [115]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bontoux, C.; Benzaquen, J.; Hofman, V.; Heeke, S.; Hannetel, P.; Capela-Brosseau-Laborde, P.; Marquette, C.-H.; Ilié, M.; Hofman, P. Deciphering the Impact of HER2 Alterations on Non-Small-Cell Lung Cancer: From Biological Mechanisms to Therapeutic Approaches. J. Pers. Med. 2022, 12, 1651. https://doi.org/10.3390/jpm12101651
Bontoux C, Benzaquen J, Hofman V, Heeke S, Hannetel P, Capela-Brosseau-Laborde P, Marquette C-H, Ilié M, Hofman P. Deciphering the Impact of HER2 Alterations on Non-Small-Cell Lung Cancer: From Biological Mechanisms to Therapeutic Approaches. Journal of Personalized Medicine. 2022; 12(10):1651. https://doi.org/10.3390/jpm12101651
Chicago/Turabian StyleBontoux, Christophe, Jonathan Benzaquen, Véronique Hofman, Simon Heeke, Paul Hannetel, Pierre Capela-Brosseau-Laborde, Charles-Hugo Marquette, Marius Ilié, and Paul Hofman. 2022. "Deciphering the Impact of HER2 Alterations on Non-Small-Cell Lung Cancer: From Biological Mechanisms to Therapeutic Approaches" Journal of Personalized Medicine 12, no. 10: 1651. https://doi.org/10.3390/jpm12101651
APA StyleBontoux, C., Benzaquen, J., Hofman, V., Heeke, S., Hannetel, P., Capela-Brosseau-Laborde, P., Marquette, C.-H., Ilié, M., & Hofman, P. (2022). Deciphering the Impact of HER2 Alterations on Non-Small-Cell Lung Cancer: From Biological Mechanisms to Therapeutic Approaches. Journal of Personalized Medicine, 12(10), 1651. https://doi.org/10.3390/jpm12101651







