Classification of Central Venous Catheter Tip Position on Chest X-ray Using Artificial Intelligence
Abstract
1. Introduction
2. Methods
2.1. Study Design
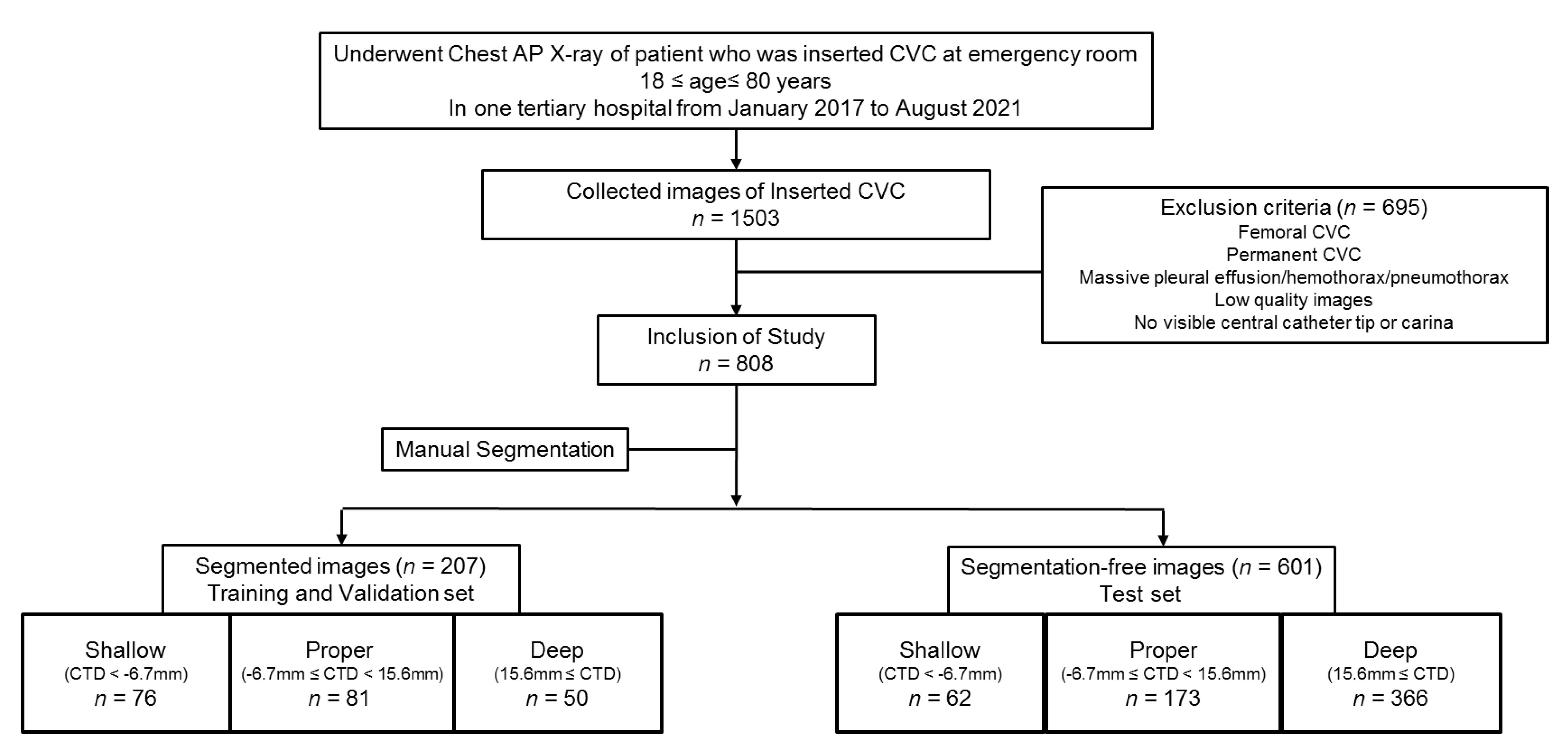
2.2. Dataset of Participants
2.2.1. Extraction and Categorization of Images on Chest Radiograph with CVC
2.2.2. Segmentation of the Trachea and the CVC on Images
2.3. Proposed Models for Classification of Three Classes of CVC Position with Application of Automatic Segmentation Using Deep CNN
2.4. Experiments
2.5. Primary Outcomes
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- McGee, D.C.; Gould, M.K. Preventing Complications of Central Venous Catheterization. N. Engl. J. Med. 2003, 348, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Leib, A.D.; England, B.S.; Kiel, J. Central Line; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Funaki, B. Central venous access: A primer for the diagnostic radiologist. AJR Am. J. Roentgenol. 2002, 179, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Nayeemuddin, M.; Pherwani, A.D.; Asquith, J.R. Imaging and management of complications of central venous catheters. Clin. Radiol. 2013, 68, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Muhm, M.; Sunder-Plassmann, G.; Apsner, R.; Pernerstorfer, T.; Rajek, A.; Lassnigg, A.; Prokesch, R.; Derfler, K.; Druml, W. Malposition of central venous catheters. Incidence, management and preventive practices. Wien Klin. Wochenschr. 1997, 109, 400–404. [Google Scholar] [PubMed]
- Hodzic, S.; Golic, D.; Smajic, J.; Sijercic, S.; Umihanic, S.; Umihanic, S. Complications Related to Insertion and Use of Central Venous Catheters (CVC). Med. Arch. 2014, 68, 300–303. [Google Scholar] [CrossRef]
- Kornbau, C.; Lee, K.C.; Hughes, G.D.; Firstenberg, M.S. Central line complications. Int. J. Crit. Illn. Inj. Sci. 2015, 5, 170–178. [Google Scholar] [CrossRef]
- Gibson, F.; Bodenham, A. Misplaced central venous catheters: Applied anatomy and practical management. BJA Br. J. Anaesth. 2013, 110, 333–346. [Google Scholar] [CrossRef]
- Petersen, J.; Delaney, J.H.; Brakstad, M.T.; Rowbotham, R.K.; Bagley, C.M. Silicone venous access devices positioned with their tips high in the superior vena cava are more likely to malfunction. Am. J. Surg. 1999, 178, 38–41. [Google Scholar] [CrossRef]
- Lamperti, M.; Biasucci, D.G.; Disma, N.; Pittiruti, M.; Breschan, C.; Vailati, D.; Subert, M.; Traškaitė, V.; Macas, A.; Estebe, J.P.; et al. European Society of Anaesthesiology guidelines on peri-operative use of ultrasound-guided for vascular access (PERSEUS vascular access). Eur. J. Anaesthesiol. 2020, 37, 344–376. [Google Scholar] [CrossRef]
- Pittiruti, M.; Pelagatti, F.; Pinelli, F. Intracavitary electrocardiography for tip location during central venous catheterization: A narrative review of 70 years of clinical studies. J. Vasc. Access 2021, 22, 778–785. [Google Scholar] [CrossRef]
- Wirsing, M.; Schummer, C.; Neumann, R.; Steenbeck, J.; Schmidt, P.; Schummer, W. Is traditional reading of the bedside chest radiograph appropriate to detect intraatrial central venous catheter position? Chest 2008, 134, 527–559. [Google Scholar] [CrossRef] [PubMed]
- Corradi, F.; Guarracino, F.; Santori, G.; Brusasco, C.; Tavazzi, G.; Via, G.; Mongodi, S.; Mojoli, F.; Biagini, R.U.D.; Isirdi, A.; et al. Ultrasound localization of central vein catheter tip by contrast-enhanced transthoracic ultrasonography: A comparison study with trans-esophageal echocardiography. Crit. Care 2022, 26, 113. [Google Scholar] [CrossRef] [PubMed]
- Gorski, L.A.; Hadaway, L.; Hagle, M.E.; Broadhurst, D.; Clare, S.; Kleidon, T.; Meyer, B.M.; Nickel, B.; Rowley, S.; Sharpe, E.; et al. Infusion Therapy Standards of Practice, 8th Edition. J. Infus. Nurs. 2021, 44, S1–S224. [Google Scholar] [CrossRef] [PubMed]
- Parmar, M.S. (F)utility of "routine" postprocedural chest radiograph after hemodialysis catheter (central venous catheter) insertion. J. Vasc. Access. 2021, 22, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Bae, J.; Moon, S.; Chung, T.N. Chest radiography for simplified evaluation of central venous catheter tip positioning for safe and accurate haemodynamic monitoring: A retrospective observational study. BMJ Open 2021, 11, e041101. [Google Scholar] [CrossRef]
- Tomaszewski, K.J.; Ferko, N.; Hollmann, S.S.; Eng, S.C.; Richard, H.M.; Rowe, L.; Sproule, S. Time and resources of peripherally inserted central catheter insertion procedures: A comparison between blind insertion/chest X-ray and a real time tip navigation and confirmation system. Clinicoecon. Outcomes Res. 2017, 7, 115–125. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, K.; Huang, L.; Zhao, B.; Zhang, X.; Guo, X.; Li, M.; Gu, Z.; Fu, G.; Hu, M.; et al. Detection of peripherally inserted central catheter (PICC) in chest X-ray images: A multi-task deep learning model. Comput. Methods Programs Biomed. 2020, 197, 105674. [Google Scholar] [CrossRef]
- Lee, H.; Mansouri, M.; Tajmir, S.; Lev, M.H.; Do, S. A Deep-Learning System for Fully-Automated Peripherally Inserted Central Catheter (PICC) Tip Detection. J. Digit. Imaging 2018, 31, 393–402. [Google Scholar] [CrossRef]
- Niehues, S.M.; Adams, L.C.; Gaudin, R.A.; Erxleben, C.; Keller, S.; Makowski, M.R.; Vahldiek, J.L.; Bressem, K.K. Deep-Learning-Based Diagnosis of Bedside Chest X-ray in Intensive Care and Emergency Medicine. Investig. Radiol. 2021, 56, 525–534. [Google Scholar] [CrossRef]
- Khan, A.B.M.; Ali, S.M.A. Early Detection of Malpositioned Catheters and Lines on Chest X-Rays using Deep Learning. In Proceedings of the 2021 International Conference on Artificial Intelligence and Computer Science Technology (ICAICST), Tangerang, Indonesia, 29–30 June 2021; pp. 51–55. [Google Scholar] [CrossRef]
- Subramanian, V.; Wang, H.; Wu, J.T.; Wong, K.C.L.; Sharma, A.; Syeda-Mahmood, T. Automated Detection and Type Classification of Central Venous Catheters in Chest X-rays. In Proceedings of the Medical Image Computing and Computer Assisted Intervention—MICCAI 2019, Shenzhen, China, 13–17 October 2019; Springer: Cham, Switzerland, 2019; Volume 11769. [Google Scholar] [CrossRef]
- Yi, X.; Adams, S.J.; Henderson, R.D.E.; Babyn, P. Computer-aided Assessment of Catheters and Tubes on Radiographs: How Good Is Artificial Intelligence for Assessment? Radiol. Artif. Intell. 2020, 2, e190082. [Google Scholar] [CrossRef]
- Lee, J.B.; Lee, Y.M. Pre-measured length using landmarks on posteroanterior chest radiographs for placement of the tip of a central venous catheter in the superior vena cava. J. Int. Med. Res. 2010, 38, 134–174. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.Y.; Lee, C.W.; Sohn, C.H.; Seo, D.W.; Yoon, J.C.; Koh, J.W.; Kim, W.; Lim, K.S.; Hong, S.B.; Lim, C.M.; et al. Optimal insertion depth of central venous catheters–is a formula required? A prospective cohort study. Injury 2012, 43, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.C.; Kim, C.; Oh, J.; Kim, T.H.; Kim, B.; Lee, J.; Chung, J.H.; Byun, H.; Yoon, M.S.; Lee, D.K. Position Classification of the Endotracheal Tube with Automatic Segmentation of the Trachea and the Tube on Plain Chest Radiography Using Deep Convolutional Neural Network. J. Pers. Med. 2022, 12, 1363. [Google Scholar] [CrossRef]
- Lakhani, P.; Flanders, A.; Gorniak, R. Endotracheal Tube Position Assessment on Chest Radiographs Using Deep Learning. Radiol. Artif. Intell. 2020, 3, e200026. [Google Scholar] [CrossRef]
- Kowalski, C.M.; Kaufman, J.A.; Rivitz, S.M.; Geller, S.C.; Waltman, A.C. Migration of central venous catheters: Implications for initial catheter tip positioning. J. Vasc. Interv. Radiol. 1997, 8, 443–449. [Google Scholar] [CrossRef]
- Aslamy, Z.; Dewald, C.L.; Heffner, J.E. MRI of central venous anatomy: Implications for central venous catheter insertion. Chest 1998, 114, 820–825. [Google Scholar] [CrossRef] [PubMed]

| The Distance from the Carina to Tip of the CVC (CTD), Median (IQR) | ||||
|---|---|---|---|---|
| Segmented Images for the Training and 5-Fold Validation | Segmentation-Free Images For the Test | p-Value | ||
| Labels | Shallow, mm | −16.39 (−26.04, −11.23) | −19.11 (−26.31, −12.46) | 0.30 |
| Proper, mm | 0 (−2.84, 9.865) | 4.57 (0, 10.45) | 0.02 | |
| Deep, mm | 34.19 (21.9, 46.18) | 32.61 (23.02, 45.17) | 0.99 | |
| 1-Fold | 2-Fold | 3-Fold | 4-Fold | 5-Fold | Total | |
|---|---|---|---|---|---|---|
| Overall Accuracy (F1 score) | 0.76 | 0.74 | 0.71 | 0.80 | 0.76 | 0.75 |
| (A) Confusion Matrix | Labels | ||||
| Shallow | Proper | Deep | Sum | ||
| Prediction | Shallow | 33 | 22 | 7 | 62 |
| Proper | 16 | 127 | 30 | 173 | |
| Deep | 10 | 77 | 279 | 366 | |
| Sum | 59 | 226 | 316 | 601 | |
| (B) Outcomes | Labels | ||||
| Shallow | Proper | Deep | Average | ||
| Overall Accuracy | 0.73 | ||||
| Accuracy | 0.91 | 0.76 | 0.79 | 0.82 | |
| Precision | 0.53 | 0.73 | 0.76 | 0.73 | |
| Recall | 0.56 | 0.56 | 0.88 | 0.73 | |
| F1 score | 0.55 | 0.64 | 0.82 | 0.73 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.; Oh, J.; Ryu, J.; Kim, J.; Lee, J.; Cho, Y.; Yoon, M.S.; Jeong, J.Y. Classification of Central Venous Catheter Tip Position on Chest X-ray Using Artificial Intelligence. J. Pers. Med. 2022, 12, 1637. https://doi.org/10.3390/jpm12101637
Jung S, Oh J, Ryu J, Kim J, Lee J, Cho Y, Yoon MS, Jeong JY. Classification of Central Venous Catheter Tip Position on Chest X-ray Using Artificial Intelligence. Journal of Personalized Medicine. 2022; 12(10):1637. https://doi.org/10.3390/jpm12101637
Chicago/Turabian StyleJung, Seungkyo, Jaehoon Oh, Jongbin Ryu, Jihoon Kim, Juncheol Lee, Yongil Cho, Myeong Seong Yoon, and Ji Young Jeong. 2022. "Classification of Central Venous Catheter Tip Position on Chest X-ray Using Artificial Intelligence" Journal of Personalized Medicine 12, no. 10: 1637. https://doi.org/10.3390/jpm12101637
APA StyleJung, S., Oh, J., Ryu, J., Kim, J., Lee, J., Cho, Y., Yoon, M. S., & Jeong, J. Y. (2022). Classification of Central Venous Catheter Tip Position on Chest X-ray Using Artificial Intelligence. Journal of Personalized Medicine, 12(10), 1637. https://doi.org/10.3390/jpm12101637







