Evaluation of Plaque Vulnerability via Combination of Hemodynamic Analysis and Simultaneous Non-Contrast Angiography and Intraplaque Hemorrhage (SNAP) Sequence for Carotid Intraplaque Hemorrhage
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. MR Imaging Acquisition
- (1)
- MRI with SNAP sequence: repetition time/echo time (TR/TE) 10/4.7 ms, flip angle 11°, field-of-view (FOV) 149 × 149 mm, matrix = 187 × 216, acquisition time 3 min 30 s;
- (2)
- TOF-MRA: TR/TE 21/3.6 ms, flip angle 18°, slice thickness 0.6 mm, FOV 172 × 200 mm, matrix = 384 × 198, acquisition time 4 min 25 s, echo train length 1.
2.3. MR Imaging Review
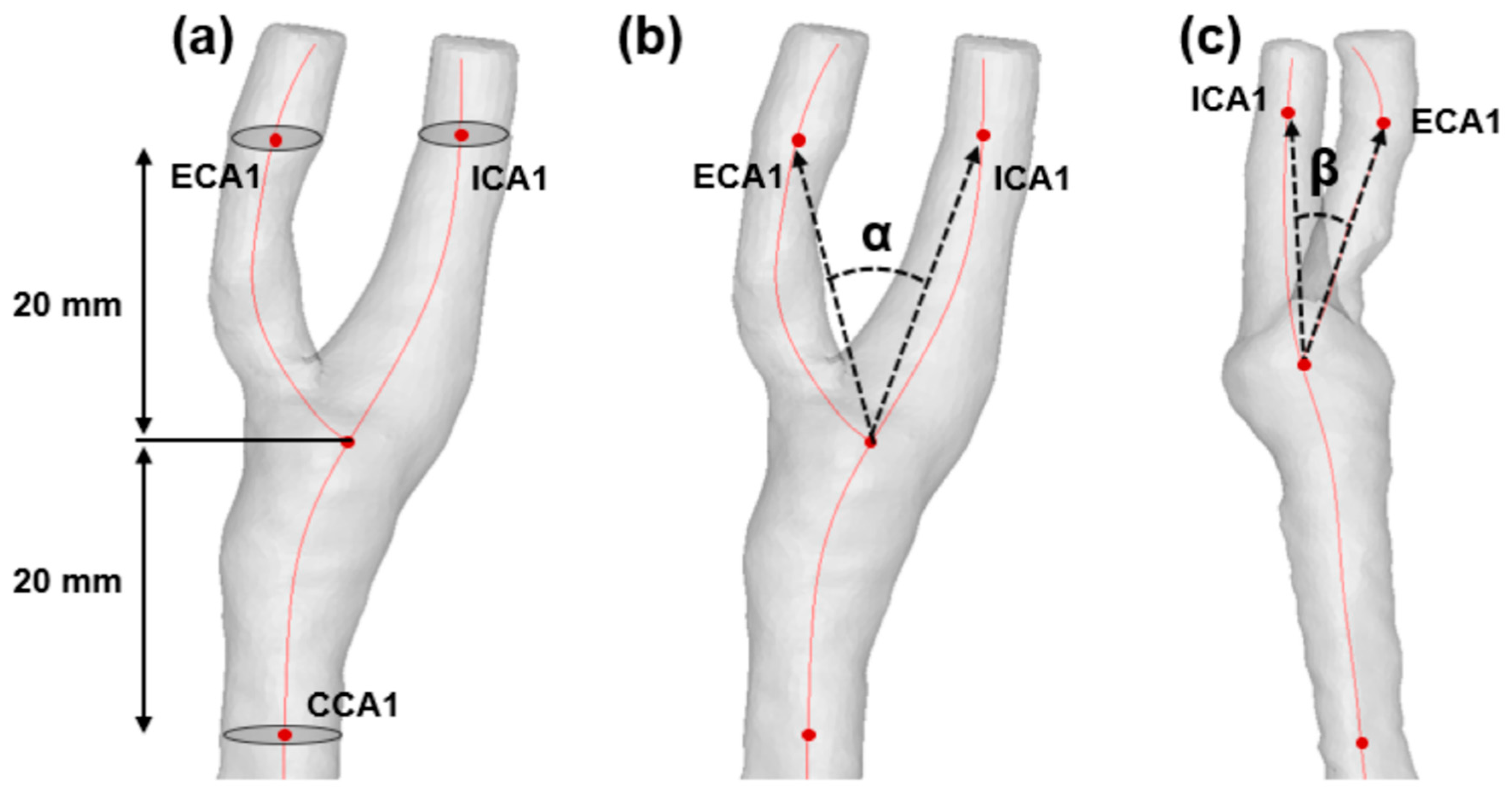
2.4. Quantification of Morphological Factors
2.5. Computational Fluid Dynamics
2.6. Analysis of Hemodynamic Factors
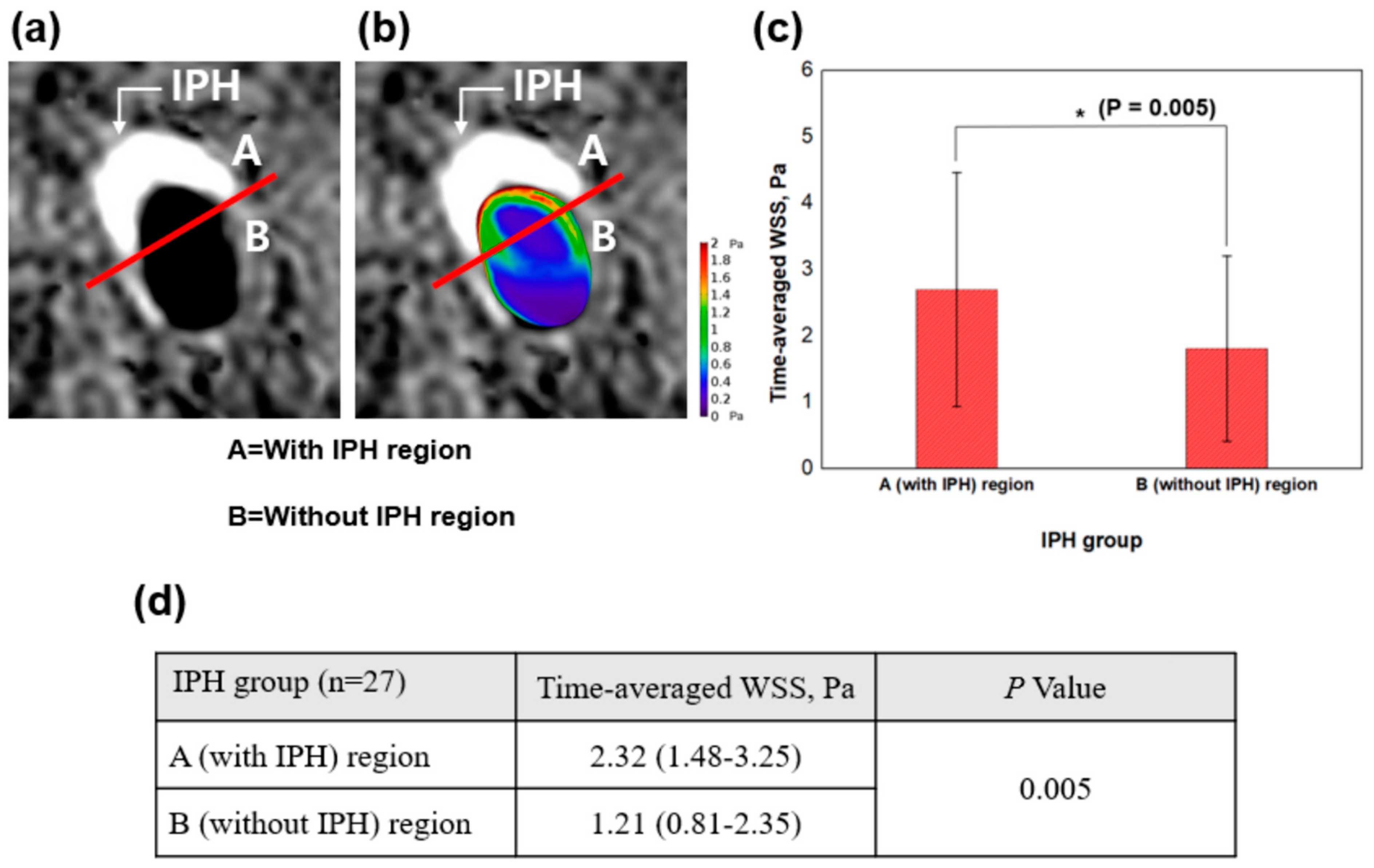
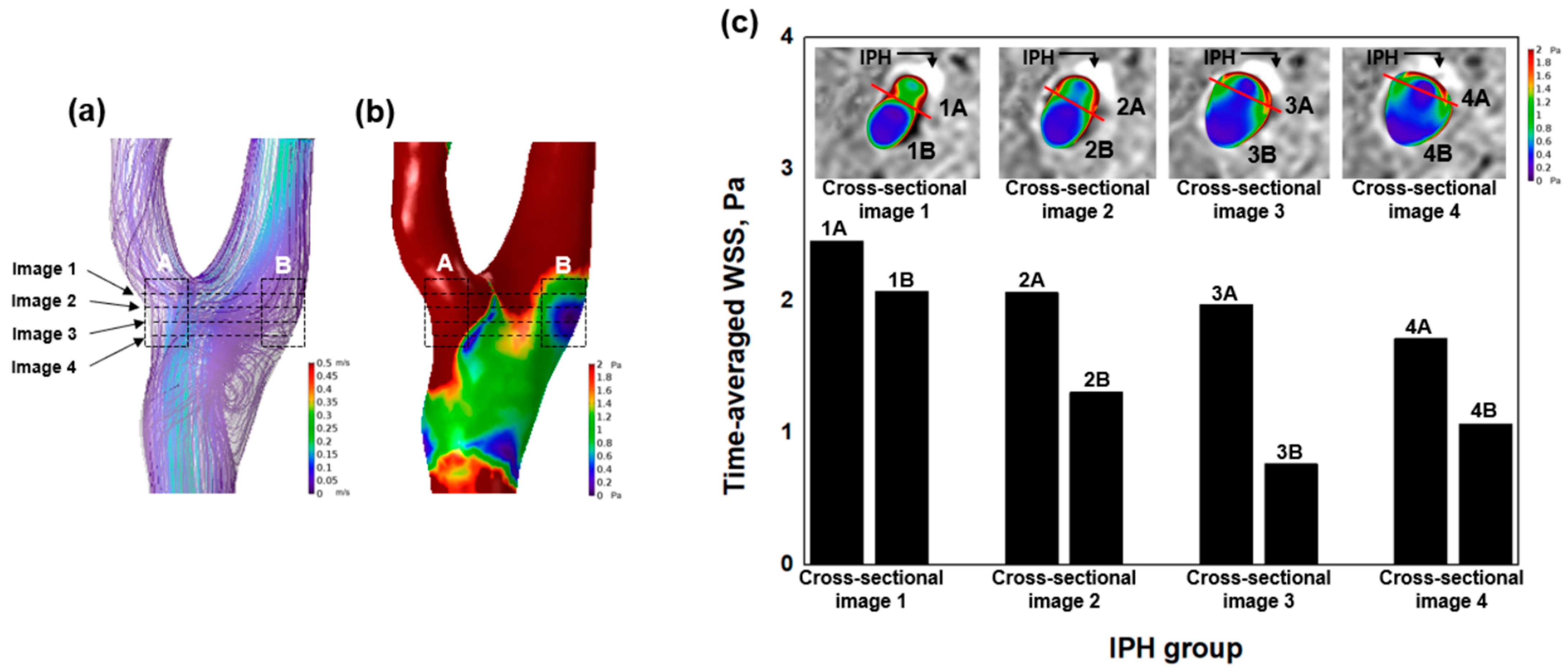
2.7. Combined Analysis Using SNAP Sequence and WSS
2.8. Statistical Analysis
3. Results
3.1. Comparison of Morphological Factors between IPH and Non-IPH Groups
3.2. Hemodynamic Characteristics of IPH
4. Discussion
4.1. High WSS and IPH
4.2. Combination of Hemodynamic Analysis and SNAP Sequence
4.3. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Noh, S.-M.; Kang, H.G. Clinical significance of the internal carotid artery angle in ischemic stroke. Sci. Rep. 2019, 9, 1–5. [Google Scholar] [CrossRef]
- Jiang, P.; Chen, Z.; Hippe, D.S.; Watase, H.; Sun, B.; Lin, R.; Yang, Z.; Xue, Y.; Zhao, X.; Yuan, C. Associations between Carotid Bifurcation Geometry and Atherosclerotic Plaque Vulnerability: A Chinese Atherosclerosis Risk Evaluation II Study. Mol. Cell. Biomech. 2020, 40, 1383–1391. [Google Scholar] [CrossRef]
- Selwaness, M.; Bouwhuijsen, Q.V.D.; van Onkelen, R.S.; Hofman, A.; Franco, O.H.; van der Lugt, A.; Wentzel, J.J.; Vernooij, M. Atherosclerotic Plaque in the Left Carotid Artery Is More Vulnerable than in the Right. Stroke 2014, 45, 3226–3230. [Google Scholar] [CrossRef]
- Tuenter, A.; Selwaness, M.; Lorza, A.A.; Schuurbiers, J.; Speelman, L.; Cibis, M.; van der Lugt, A.; de Bruijne, M.; van der Steen, A.; Franco, O.; et al. High shear stress relates to intraplaque haemorrhage in asymptomatic carotid plaques. Atherosclerosis 2016, 251, 348–354. [Google Scholar] [CrossRef]
- Groen, H.C.; Gijsen, F.J.; van der Lugt, A.; Ferguson, M.S.; Hatsukami, T.S.; van der Steen, A.F.; Yuan, C.; Wentzel, J.J. Plaque rupture in the carotid artery is localized at the high shear stress region: A case report. Stroke 2007, 38, 2379–2381. [Google Scholar] [CrossRef] [PubMed]
- Saam, T.; Hetterich, H.; Hoffmann, V.S.; Yuan, C.; Dichgans, M.; Poppert, H.; Koeppel, T.; Hoffmann, U.; Reiser, M.F.; Bamberg, F. Meta-Analysis and Systematic Review of the Predictive Value of Carotid Plaque Hemorrhage on Cerebrovascular Events by Magnetic Resonance Imaging. J. Am. Coll. Cardiol. 2013, 62, 1081–1091. [Google Scholar] [CrossRef]
- Kwak, H.S.; Yang, H.J.; Hwang, S.B.; Chung, G.H. Carotid Wall Imaging with Routine Brain MRI to Facilitate Early Detection of Carotid Plaque and Intraplaque Hemorrhage. J. Stroke 2017, 19, 107–108. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Qian, Y.; Zhang, M.; Li, Y.; Lv, P.; Tang, X.; Javadzadegan, A.; Lin, J. Associations between local haemodynamics and carotid intraplaque haemorrhage with different stenosis severities: A preliminary study based on MRI and CFD. J. Clin. Neurosci. 2019, 66, 220–225. [Google Scholar] [CrossRef]
- Kim, M.J.; Kwak, H.S.; Hwang, S.B.; Chung, G.H. One-step evaluation of intraplaque hemorrhage in the carotid artery and vertebrobasilar artery using simultaneous non-contrast angiography and intraplaque hemorrhage. Eur. J. Radiol. 2021, 141, 109824. [Google Scholar] [CrossRef]
- Kamenskiy, A.V.; MacTaggart, J.N.; Pipinos, I.I.; Bikhchandani, J.; Dzenis, Y.A. Three-Dimensional Geometry of the Human Carotid Artery. J. Biomech. Eng. 2012, 134, 064502. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Teng, Z.; Canton, G.; Ferguson, M.; Yuan, C.; Tang, D. Intraplaque hemorrhage is associated with higher structural stresses in human atherosclerotic plaques: An In Vivo MRI-based 3d fluid-structure interaction study. Biomed. Eng. Online 2010, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Eshtehardi, P.; Teng, Z. Protective or destructive: High wall shear stress and atherosclerosis. Atherosclerosis 2016, 251, 501–503. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thomas, J.B.; Antiga, L.; Che, S.L.; Milner, J.S.; Hangan Steinman, D.A.; Spence, J.D.; Rutt, B.K.; Steinman, D.A. Variation in the carotid bifurcation geometry of young versus older adults: Implications for geometric risk of atherosclerosis. Stroke 2005, 36, 2450–2456. [Google Scholar] [CrossRef]
- Jeon, S.J.; Kwak, H.S.; Chung, G.H. Widening and Rotation of Carotid Artery with Age: Geometric Approach. J. Stroke Cerebrovasc. Dis. 2018, 27, 865–870. [Google Scholar] [CrossRef]
- Ngo, M.T.; Kim, C.I.; Jung, J.; Chung, G.H.; Lee, D.H.; Kwak, H.S. Four-Dimensional Flow Magnetic Resonance Imaging for Assessment of Velocity Magnitudes and Flow Patterns in The Human Carotid Artery Bifurcation: Comparison with Computational Fluid Dynamics. Diagnostics 2019, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Lv, P.; Javadzadegan, A.; Tang, X.; Qian, Y.; Lin, J. Hemodynamic analysis of carotid artery after endarterectomy: A preliminary and quantitative imaging study based on computational fluid dynamics and magnetic resonance angiography. Quant. Imaging Med. Surg. 2018, 8, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Hoi, Y.; Wasserman, B.A.; Xie, Y.J.; Najjar, S.S.; Ferruci, L.; Lakatta, E.; Gerstenblith, G.; A Steinman, D. Characterization of volumetric flow rate waveforms at the carotid bifurcations of older adults. Physiol. Meas. 2010, 31, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-W.; Antiga, L.; Spence, J.D.; Steinman, D.A. Geometry of the Carotid Bifurcation Predicts Its Exposure to Disturbed Flow. Stroke 2008, 39, 2341–2347. [Google Scholar] [CrossRef]
- Compagne, K.; Dilba, K.; Postema, E.; Van Es, A.; Emmer, B.; Majoie, C.; Van Zwam, W.; Dippel, D.; Wentzel, J.; Van Der Lugt, A.; et al. Flow Patterns in Carotid Webs: A Patient-Based Computational Fluid Dynamics Study. Am. J. Neuroradiol. 2019, 40, 703–708. [Google Scholar] [CrossRef]
- Eshtehardi, P.; Brown, A.J.; Bhargava, A.; Costopoulos, C.; Hung, O.Y.; Corban, M.T.; Hosseini, H.; Gogas, B.D.; Giddens, D.P.; Samady, H. High wall shear stress and high-risk plaque: An emerging concept. Int. J. Cardiovasc. Imaging 2017, 33, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Duan, W.; Qiao, A. Finite element analysis of mechanics of neovessels with intraplaque hemorrhage in carotid atherosclerosis. Biomed. Eng. Online 2015, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.; Wang, P.; Kim, S.-G.; Jin, T. Sensitivity and Source of Amine-Proton Exchange and Amide-Proton Transfer Magnetic Resonance Imaging in Cerebral Ischemia. Magn. Reson. Med. 2014, 71, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Moerman, A.M.; Dilba, K.; Korteland, S.; Poot, D.H.J.; Klein, S.; Van Der Lugt, A.; Rouwet, E.V.; Van Gaalen, K.; Wentzel, J.J.; Van Der Steen, A.F.W.; et al. An MRI-based method to register patient-specific wall shear stress data to histology. PLoS ONE 2019, 14, e0217271. [Google Scholar] [CrossRef] [PubMed]
| Variables | IPH Group (n = 27) | Non-IPH Group (n = 27) | p Value |
|---|---|---|---|
| Age, median (IQR) | 72.00 (70.00–78.00) | 72.00 (70.50–78.00) | 0.952 |
| Male, n (%) | 19 (70.37) | 19 (70.37) | 1.000 |
| Medical history, n (%) | |||
| Hypertension | 19 (70.37) | 22 (81.48) | 0.524 |
| Diabetes mellitus | 11 (40.74) | 14 (51.85) | 0.585 |
| Dyslipidemia | 9 (33.33) | 4 (14.81) | 0.203 |
| History of cardiovascular disease | 3 (11.11) | 6 (22.22) | 0.465 |
| History of cerebrovascular disease | 9 (33.33) | 5 (18.52) | 0.352 |
| Smoking | 6 (22.22) | 4 (14.81) | 0.726 |
| Atrial fibrillation | 2 (7.41) | 2 (7.41) | 1.000 |
| Morphological Variables | IPH Group (n = 27) | Non-IPH Group (n = 27) | p Value |
|---|---|---|---|
| ICA diameter, mm | 4.80 (4.38–5.05) | 5.04 (4.64–5.86) | 0.115 |
| ECA diameter, mm | 3.91 ± 0.72 | 4.18 ± 0.73 | 0.174 |
| CCA diameter, mm | 6.73 ± 0.97 | 7.12 ± 0.96 | 0.148 |
| ICA area, mm2 | 17.91 (14.96–19.93) | 19.75 (16.49–26.63) | 0.132 |
| ECA area, mm2 | 12.25 ± 4.52 | 13.96 ± 4.95 | 0.189 |
| CCA area, mm2 | 36.16 ± 10.61 | 40.51 ± 10.95 | 0.145 |
| Bifurcation angle (α), degree° | 39.47 (29.27–48.97) | 47.60 (38.94–53.77) | 0.041 |
| ICA angle (β), degree° | 44.64 (32.05–54.64) | 48.85 (40.19–56.91) | 0.156 |
| Hemodynamic variables | |||
| Time-averaged WSS, Pa | 1.84 (1.21–2.77) | 1.10 (0.80–1.58) | 0.007 |
| Minimum WSS, Pa | 0.60 (0.35–1.05) | 0.42 (0.27–0.50) | 0.015 |
| Maximum WSS, Pa | 4.27 (3.11–5.76) | 2.41 (2.07–4.04) | 0.005 |
| Oscillatory shear index | 0.39 (0.34–0.41) | 0.38 (0.35–0.40) | 0.494 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, U.Y.; Kwak, H.S. Evaluation of Plaque Vulnerability via Combination of Hemodynamic Analysis and Simultaneous Non-Contrast Angiography and Intraplaque Hemorrhage (SNAP) Sequence for Carotid Intraplaque Hemorrhage. J. Pers. Med. 2021, 11, 856. https://doi.org/10.3390/jpm11090856
Lee UY, Kwak HS. Evaluation of Plaque Vulnerability via Combination of Hemodynamic Analysis and Simultaneous Non-Contrast Angiography and Intraplaque Hemorrhage (SNAP) Sequence for Carotid Intraplaque Hemorrhage. Journal of Personalized Medicine. 2021; 11(9):856. https://doi.org/10.3390/jpm11090856
Chicago/Turabian StyleLee, Ui Yun, and Hyo Sung Kwak. 2021. "Evaluation of Plaque Vulnerability via Combination of Hemodynamic Analysis and Simultaneous Non-Contrast Angiography and Intraplaque Hemorrhage (SNAP) Sequence for Carotid Intraplaque Hemorrhage" Journal of Personalized Medicine 11, no. 9: 856. https://doi.org/10.3390/jpm11090856
APA StyleLee, U. Y., & Kwak, H. S. (2021). Evaluation of Plaque Vulnerability via Combination of Hemodynamic Analysis and Simultaneous Non-Contrast Angiography and Intraplaque Hemorrhage (SNAP) Sequence for Carotid Intraplaque Hemorrhage. Journal of Personalized Medicine, 11(9), 856. https://doi.org/10.3390/jpm11090856






