Frenemies within: An Endocarditis Case in Behçet’s Disease
Abstract
:1. Introduction
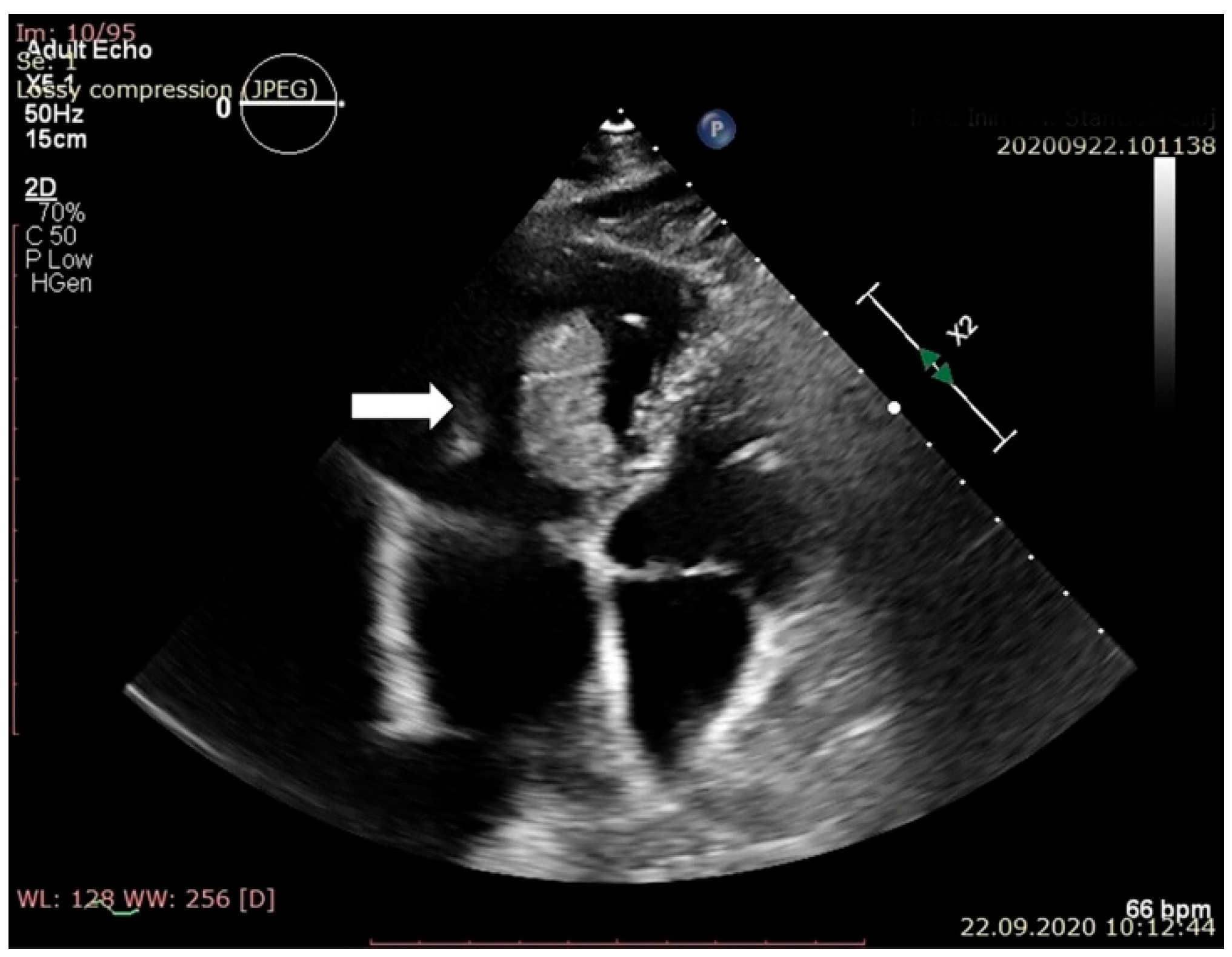
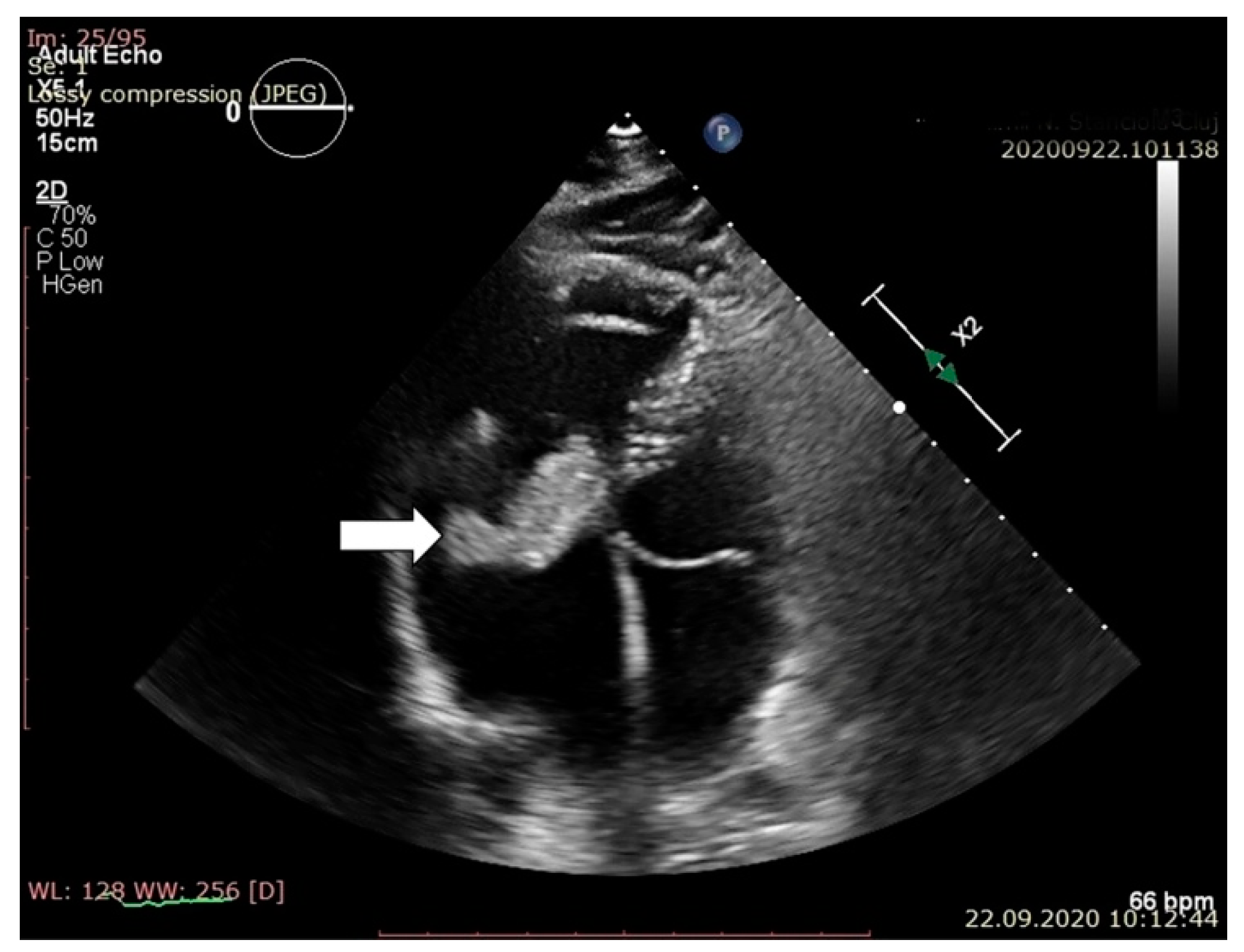
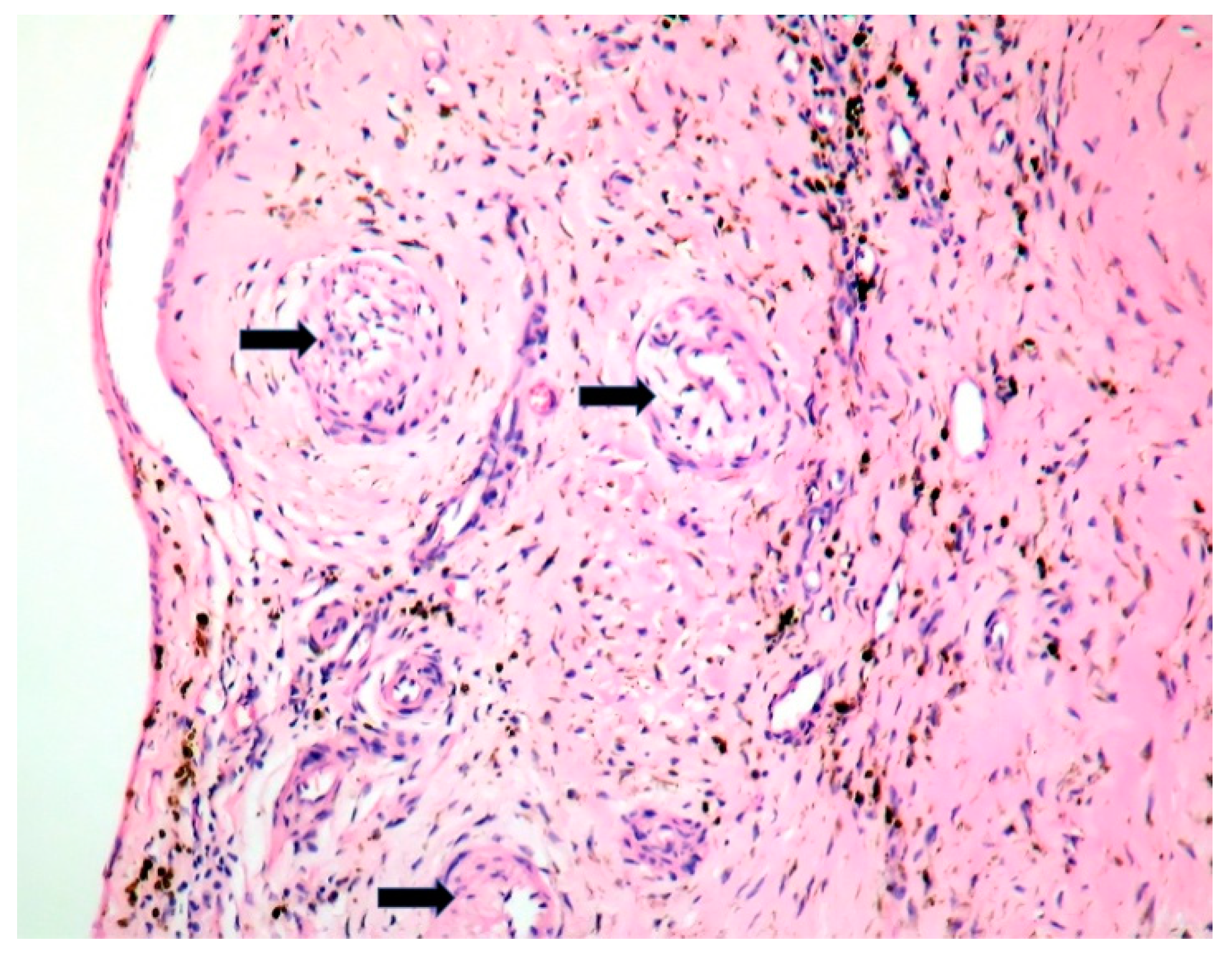
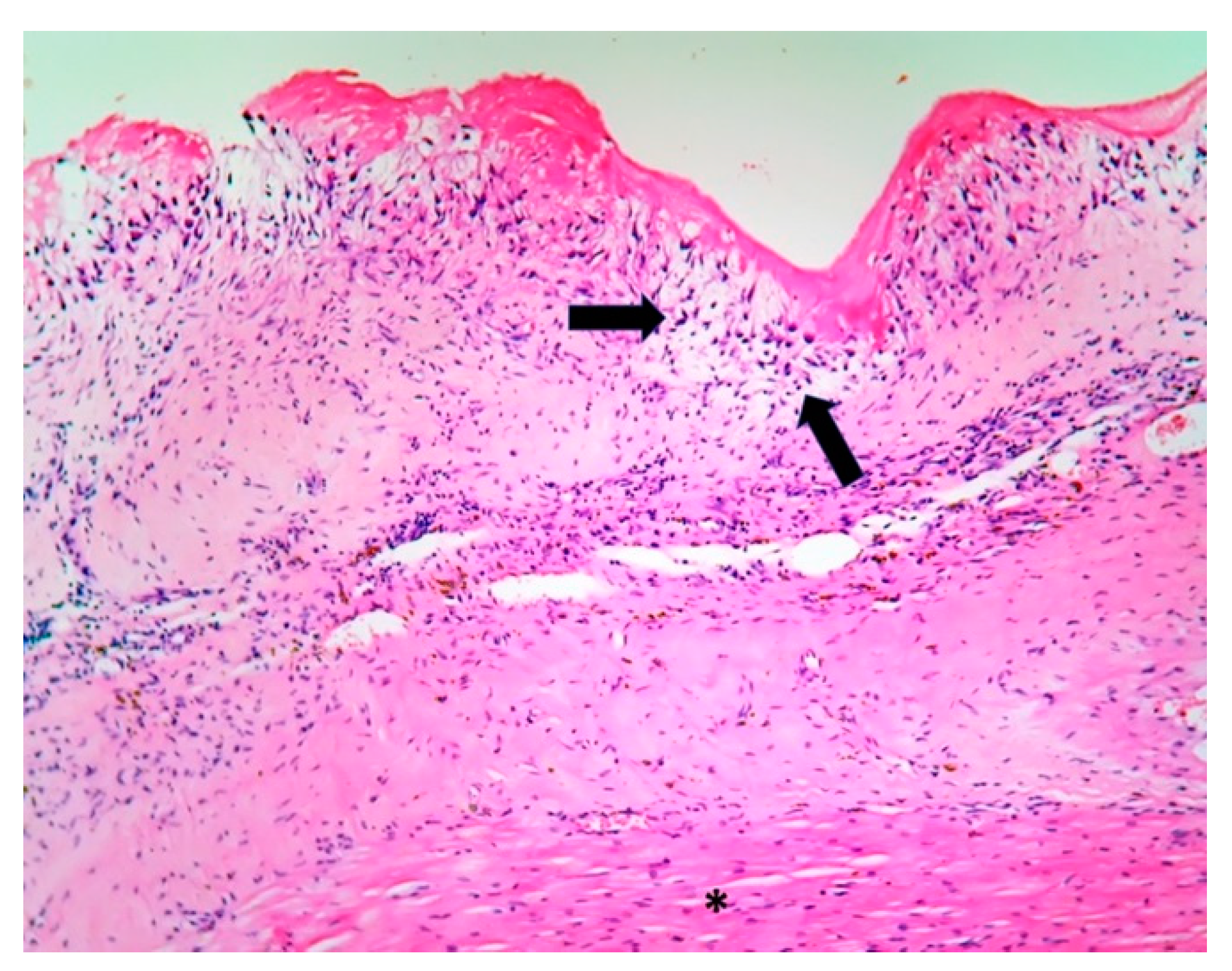
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burette, M.; Bonazzi, M. From neglected to dissected: How technological advances are leading the way to the study of Coxiella burnetii pathogenesis. Cell Microbiol. 2020, 22, e13180. [Google Scholar] [CrossRef] [Green Version]
- Million, M.; Thuny, F.; Bardin, N.; Angelakis, E.; Edouard, S.; Bessis, S.; Guimard, T.; Weitten, T.; Martin-Barbaz, F.; Texereau, M.; et al. Antiphospholipid Antibody Syndrome with Valvular Vegetations in Acute Q Fever. Clin. Infect. Dis. 2016, 62, 537–544. [Google Scholar] [CrossRef]
- Nygren, D.; Älverbrandt, M.; Sunnerhagen, T.; Fagman, E.; Ostenfeld, E.; Rasmussen, M. Aortitis caused by Abiotrophia defectiva: Description of two cases. Infect. Dis. Rep. 2018, 10, 7746. [Google Scholar] [CrossRef] [Green Version]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis. Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.J.; Pan, J.B.; Song, G.; Wen, X.T.; Wu, Z.Y.; Chen, S.; Mo, W.X.; Zhang, F.C.; Qian, J.; Zhu, H.; et al. Identification of Novel Biomarkers for Behçet Disease Diagnosis Using Human Proteome Microarray Approach. Mol. Cell Proteom. 2017, 16, 147–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Direskeneli, H. Autoimmunity vs autoinflammation in Behçet’s disease: Do we oversimplify a complex disorder? Rheumatology 2006, 45, 1461–1465. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.A.; Alam, S.S.; Kundu, S.; Prodhan, A.S.; Khandker, S.S.; Reshetnyak, T.; Kotyla, P.J.; Hassan, R.; Hossan, T. Prevalence of antiphospholipid antibodies in Behçet’s disease: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0227836. [Google Scholar] [CrossRef] [Green Version]
- Owlia, M.B.; Mehrpoor, G. Behçet’s Disease: New Concepts in Cardiovascular Involvements and Future Direction for Treatment. ISRN Pharmacol. 2012, 2012, 760484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demirelli, S.; Degirmenci, H.; Inci, S.; Arisoy, A. Cardiac manifestations in Behçet’s disease. Intractable Rare Dis. Res. 2015, 4, 70–75. [Google Scholar] [CrossRef] [Green Version]
- Mazzoni, C.; Scheggi, V.; Mariani, T. Cardiac involvement in Behçet disease presenting as non-bacterial thrombotic endocarditis: A case report. J. Cardiol. Cases 2021. [Google Scholar] [CrossRef]
- Serban, A.B.N.; Molnar, A.; Kovacs, E. Multimodality imaging of a papillary fibroelastoma of the tricuspid valve-case report. Rom. J. Cardiol. 2018, 28, 38–43. [Google Scholar]
- Moldovan, H.; Popescu, D.; Buliga, T.; Filip, A.; Antoniac, I.; Gheorghiţӑ, D.; Molnar, A. Gastric Adenocarcinoma Associated with Acute Endocarditis of the Aortic Valve and Coronary Artery Disease in a 61-Year-Old Male with Multiple Comorbidities-Combined Surgical Management-Case Report. Medicina 2019, 55, 242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Issartel, B.; Gauduchon, V.; Chalabreysse, L.; Celard, M.; Ninet, J.; Lepidi, H.; Etienne, J.; Vandenesch, F. Clinically and histologically silent Q fever endocarditis accidentally diagnosed by PCR. Clin. Microbiol. Infect. 2002, 8, 113–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Ruiz, M.; López-Medrano, F.; Alonso-Navas, F.; Aguado, J.M. Coxiella burnetii infection of left atrial thrombus mimicking an atrial myxoma. Int. J. Infect. Dis. 2010, 14 (Suppl. 3), e319–e321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukocheva, O.A.; Manavis, J.; Kok, T.W.; Turra, M.; Izzo, A.; Blumbergs, P.; Marmion, B.P. Coxiella burnetii dormancy in a fatal ten-year multisystem dysfunctional illness: Case report. BMC Infect. Dis. 2016, 16, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kampschreur, L.M.; Wegdam-Blans, M.C.; Wever, P.C.; Renders, N.H.; Delsing, C.E.; Sprong, T.; van Kasteren, M.E.; Bijlmer, H.; Notermans, D.; Oosterheert, J.J.; et al. Chronic Q fever diagnosis- consensus guideline versus expert opinion. Emerg. Infect. Dis 2015, 21, 1183–1188. [Google Scholar] [CrossRef] [Green Version]
- Wielders, C.C.; Morroy, G.; Wever, P.C.; Coutinho, R.A.; Schneeberger, P.M.; van der Hoek, W. Strategies for early detection of chronic Q-fever: A systematic review. Eur. J. Clin. Investig. 2013, 43, 616–639. [Google Scholar] [CrossRef] [PubMed]
- Melenotte, C.; Protopopescu, C.; Million, M.; Edouard, S.; Carrieri, M.P.; Eldin, C.; Angelakis, E.; Djossou, F.; Bardin, N.; Fournier, P.E.; et al. Clinical Features and Complications of Coxiella burnetii Infections from the French National Reference Center for Q Fever. JAMA Netw. Open 2018, 1, e181580. [Google Scholar] [CrossRef] [Green Version]
- Molnar, A.; Sacui, D.; Manole, S.; Radulescu, A.; Beyer, R. The value of transthoracic and transesophageal echocardiography for the diagnosis of the native aortic infective endocarditis valve complications: A case report and literature review. Med. Ultrason 2016, 18, 253–256. [Google Scholar] [CrossRef] [Green Version]
- Eldin, C.; Mélenotte, C.; Mediannikov, O.; Ghigo, E.; Million, M.; Edouard, S.; Mege, J.-L.; Maurin, M.; Raoult, D. From Q Fever to Coxiella burnetii Infection: A Paradigm Change. Clin. Microbiol. Rev. 2017, 30, 115–190. [Google Scholar] [CrossRef] [Green Version]
- Kiernan, T.J.; O’Flaherty, N.; Gilmore, R.; Ho, E.; Hickey, M.; Tolan, M.; Mulcahy, D.; Moore, D.P. Abiotrophia defectiva endocarditis and associated hemophagocytic syndrome—A first case report and review of the literature. Int. J. Infect. Dis. 2008, 12, 478–482. [Google Scholar] [CrossRef] [Green Version]
- Faria, C.; Guerreiro, R.; Cruz, S.; Fernandes, M. Mitral Valve Subacute Endocarditis Caused by Abiotrophia Defectiva: A Case Report. Clin. Pr. 2021, 11, 162–166. [Google Scholar] [CrossRef]
- Jang, Y.R.; Song, J.S.; Jin, C.E.; Ryu, B.H.; Park, S.Y.; Lee, S.O.; Choi., S.H.; Kim, Y.S.; Woo, J.H.; Song, J.K.; et al. Molecular detection of Coxiella burnetii in heart valve tissue from patients with culture-negative infective endocarditis. Medicina 2018, 97, e11881. [Google Scholar] [CrossRef]
- Lee, H.S.; Choi, W.S.; Kim, K.H.; Kang, J.K.; Kim, N.Y.; Park, S.H.; Park, Y.; Nam, E.J.; Yang, D.H.; Park, H.S.; et al. Aseptic Endocarditis in Behçet’s Disease Presenting as Tricuspid Valve Stenosis. Korean Circ. J. 2011, 41, 399–401. [Google Scholar] [CrossRef]
- Veideswar, P.B.J. Valvular Heart Diseases. In Cardiovascular; Pathology, B.M., Ed.; Elsevier: London, UK, 2016; pp. 485–526. [Google Scholar]
- Granowicz, E.; Chung, K. Improvement of Cardiac Vegetations in Antiphospholipid Syndrome with Enoxaparin and Corticosteroids after Rivaroxaban Failure. Case Rep. Hematol. 2018, 2018, 8097539. [Google Scholar] [CrossRef]
- Hojnik, M.; George, J.; Ziporen, L.; Shoenfeld, Y. Heart valve involvement (Libman-Sacks endocarditis) in the antiphospholipid syndrome. Circulation 1996, 93, 1579–1587. [Google Scholar] [CrossRef]
- Pinkney, J.A.; Nagassar, R.P.; Roye-Green, K.J.; Ferguson, T. Abiotrophia defectiva endocarditis. BMJ Case Rep. 2014, 2014, bcr2014207361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forde, G.; Lucey, M.; O’Shea, P.M.; Okiro, J.; Shatwan, R.; Mulkerrin, E.C. Atypical presentation of Abiotrophia defectiva infective endocarditis in an octogenarian. Clin. Case Rep. 2021, 9, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Rudrappa, M.; Kokatnur, L. Infective Endocarditis Due to Abiotrophia defectiva and Its Feared Complications in an Immunocompetent Person: Rare, But Real. J. Glob. Infect. Dis. 2017, 9, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, S.; Caccese, R.; Carfagna, P.; Vena, A.; Falcone, M.; Venditti, M. Endocarditis caused by nutritionally variant streptococci: A case report and literature review. Infez Med. 2012, 20, 67–74. [Google Scholar]
- Lepidi, H.; Houpikian, P.; Liang, Z.; Raoult, D. Cardiac valves in patients with Q fever endocarditis: Microbiological, molecular, and histologic studies. J. Infect. Dis. 2003, 187, 1097–1106. [Google Scholar] [CrossRef] [Green Version]
- Brouqui, P.; Dupont, H.T.; Drancourt, M.; Berland, Y.; Etienne, J.; Leport, C.; Goldstein, F.; Massip, P.; Micoud, M.; Bertrand, A.; et al. Chronic Q fever. Ninety-two cases from France, including 27 cases without endocarditis. Arch. Intern. Med. 1993, 153, 642–648. [Google Scholar] [CrossRef]
- Mendoza-Pinto, C.; García-Carrasco, M.; Cervera, R. Role of Infectious Diseases in the Antiphospholipid Syndrome (Including Its Catastrophic Variant). Curr. Rheumatol. Rep. 2018, 20, 62. [Google Scholar] [CrossRef] [PubMed]
- Chun, E.Y.; Park, S.G.; Cho, Y.H.; Lee, J.H.; Lee, K.H.; Bang, D.S.; Lee, E.S.; Lee, S.N. Antinuclear antibodies in patients with Behçet’s disease. Korean J. Dermatol. 2004, 42, 545–550. [Google Scholar]
- Ilhan, F.; Aksoy, R.; Tutkak, H. Are the Specific and Nonspecific ANA Staining Patterns of Behçet’s Disease Patients Important? Reum. Clin. 2020. [Google Scholar] [CrossRef] [PubMed]
- Im, J.H.; Chung, M.H.; Park, Y.K.; Kwon, H.Y.; Baek, J.H.; Lee, S.Y.; Lee, J.S. Antinuclear antibodies in infectious diseases. Infect. Dis. 2020, 52, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Lifshitz, Z.; Burstein, D.; Schwartz, K.; Shuman, H.A.; Pupko, T.; Segal, G. Identification of novel Coxiella burnetii Icm/Dot effectors and genetic analysis of their involvement in modulating a mitogen-activated protein kinase pathway. Infect. Immun. 2014, 82, 3740–3752. [Google Scholar] [CrossRef] [Green Version]
- Honstettre, A.; Imbert, G.; Ghigo, E.; Gouriet, F.; Capo, C.; Raoult, D.; Mege, J.L. Dysregulation of cytokines in acute Q fever: Role of interleukin-10 and tumor necrosis factor in chronic evolution of Q fever. J. Infect. Dis. 2003, 187, 956–962. [Google Scholar] [CrossRef] [Green Version]
- Jansen, A.F.M.; Schoffelen, T.; Bleeker-Rovers, C.P.; Wever, P.C.; Jaeger, M.; Oosting, M.; Adriaans, A.; Joosten, L.A.; Netea, M.G.; van Deuren, M.; et al. Genetic variations in innate immunity genes affect response to Coxiella burnetii and are associated with susceptibility to chronic Q fever. Clin. Microbiol. Infect. 2019, 25, e11–e631. [Google Scholar] [CrossRef]
- McGonagle, D.; Aydin, S.Z.; Gül, A.; Mahr, A.; Direskeneli, H. ‘MHC-I-opathy’-unified concept for spondyloarthritis and Behçet disease. Nat. Rev. Rheumatol. 2015, 11, 731–740. [Google Scholar] [CrossRef]
- Giza, M.; Koftori, D.; Chen, L.; Bowness, P. Is Behçet’s disease a ‘class 1-opathy’? The role of HLA-B*51 in the pathogenesis of Behçet’s disease. Clin. Exp. Immunol. 2018, 191, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Clemente, T.M.; Mulye, M.; Justis, A.V.; Nallandhighal, S.; Tran, T.M.; Gilk, S.D. Coxiella burnetii Blocks Intracellular Interleukin-17 Signaling in Macrophages. Infect. Immun. 2018, 86, e00532-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunha, L.D.; Ribeiro, J.M.; Fernandes, T.D.; Massis, L.M.; Khoo, C.A.; Moffatt, J.H.; Newton, H.J.; Roy, C.R.; Zamboni, D.S. Inhibition of inflammasome activation by Coxiella burnetii type IV secretion system effector IcaA. Nat. Commun. 2015, 6, 10205. [Google Scholar] [CrossRef] [PubMed]
- Nanke, Y.; Yago, T.; Kotake, S. The Role of Th17 Cells in the Pathogenesis of Behçet’s Disease. J. Clin. Med. 2017, 6, 74. [Google Scholar] [CrossRef] [Green Version]
- Stanford, M.; Whittall, T.; Bergmeier, L.A.; Lindblad, M.; Lundin, S.; Shinnick, T.; Mizushima, Y.; Holmgren, J.; Lehner, T. Oral tolerization with peptide 336-351 linked to cholera toxin B subunit in preventing relapses of uveitis in Behçet’s disease. Clin. Exp. Immunol. 2004, 137, 201–208. [Google Scholar] [CrossRef]
- Houpikian, P.; Raoult, D. Blood culture-negative endocarditis in a reference center: Etiologic diagnosis of 348 cases. Medicina 2005, 84, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Speth, C.; Löffler, J.; Krappmann, S.; Lass-Flörl, C.; Rambach, G. Platelets as immune cells in infectious diseases. Future Microbiol. 2013, 8, 1431–1451. [Google Scholar] [CrossRef]
- Gafter-Gvili, A.; Mansur, N.; Bivas, A.; Zemer-Wassercug, N.; Bishara, J.; Leibovici, L.; Paul, M. Thrombocytopenia in Staphylococcus aureus bacteremia: Risk factors and prognostic importance. Mayo Clin. Proc. 2011, 86, 389–396. [Google Scholar] [CrossRef] [Green Version]
- Kizilay, M.; Elbir, F.; Aglar, A.A.; Vural, U.; Balci, A.Y.; Yekeler, İ. An overlooked fact: Thrombocytopenia following bioprosthetic aortic valve replacement. Kardiochir Torakochirurgia Pol. 2019, 16, 19–26. [Google Scholar] [CrossRef]
- Ziogas, I.A.; Evangeliou, A.P.; Tsachouridou, O.; Arvanitaki, A.; Tsona, A.; Kamperidis, V.; Papagianni, M.; Panagiotidis, T.; Tourtounis, I.A.; Giannakoulas, G.; et al. Coxiella Endocarditis as the Cause of Recurrent Fever and Brain Abscess in a Patient with Complex Congenital Heart Disease: A Case Report and Literature Review. Case Rep. Infect. Dis. 2020, 2020, 7894574. [Google Scholar] [CrossRef]
- Molnar, A.; Beyer, R.; Florian, S.; Muresanu, D.F.; Trifan, C.; Muresan, I.; Sacui, D.; Scridon, T.; Balanescu, R.N. Drainage of cerebral abscesses prior to valve replacement in stable patients with acute left-sided infective endocarditis. CNS Neurol. Disord. Drug. Targets 2015, 14, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Molnar, A.; Săcui, D.; Scridon, T. Risk factors influencing the surgical outcome in 138 consecutive patients with infrarenal aortic aneurysm: Experience at the Cluj-Napoca Cardiovascular Surgery Center. Chirurgia 2014, 109, 223–228. [Google Scholar] [PubMed]
- Anchidin, O.I.N.A.; Molnar, A.; Rosianu, A.; Rosianu, S.H. Are cardiovascular rehabilitation programs implemented in young patients with acute coronary syndromes following revascularization procedures? Bal. Res. J. 2020, 11, 133–140. [Google Scholar] [CrossRef]

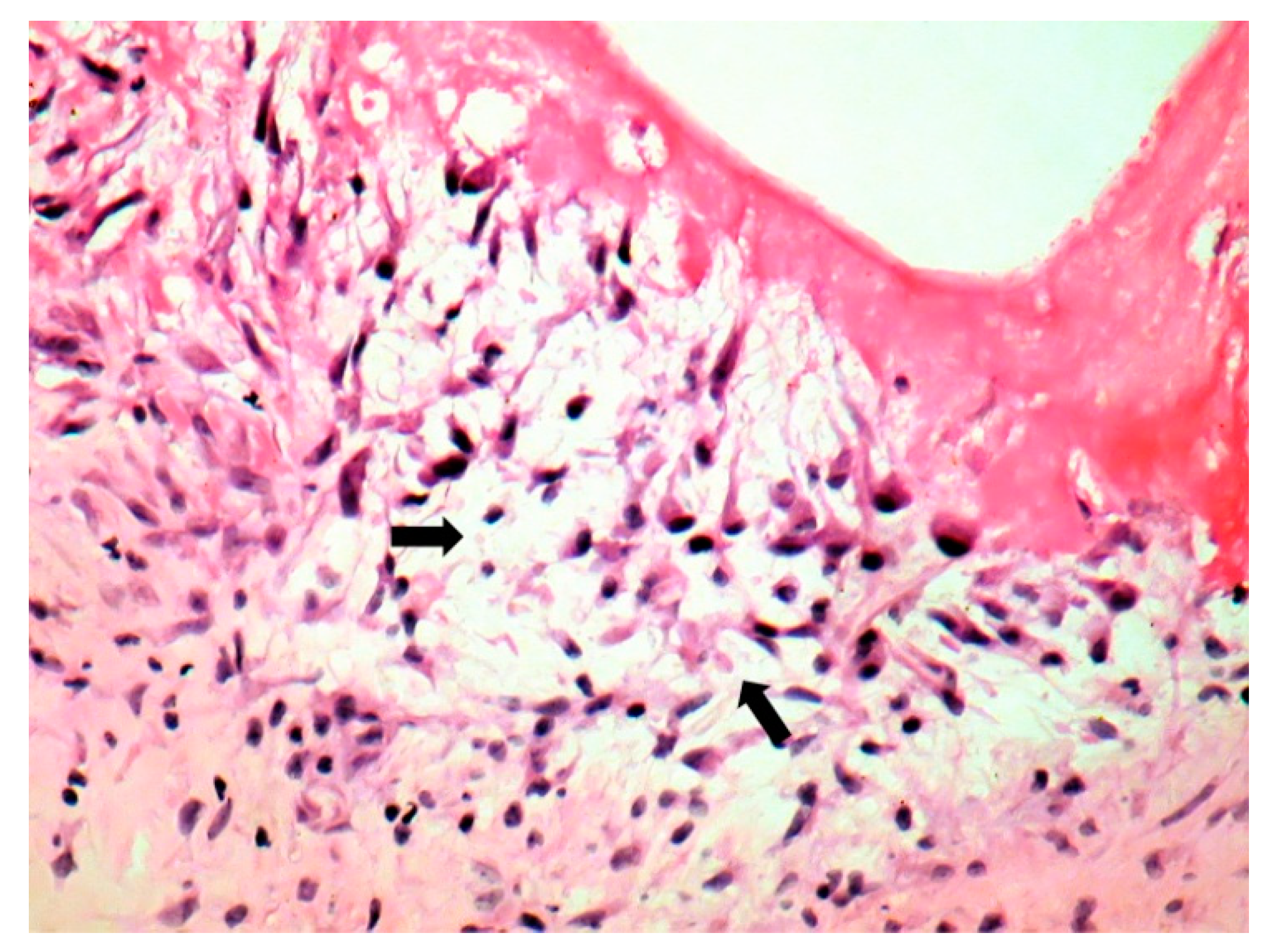
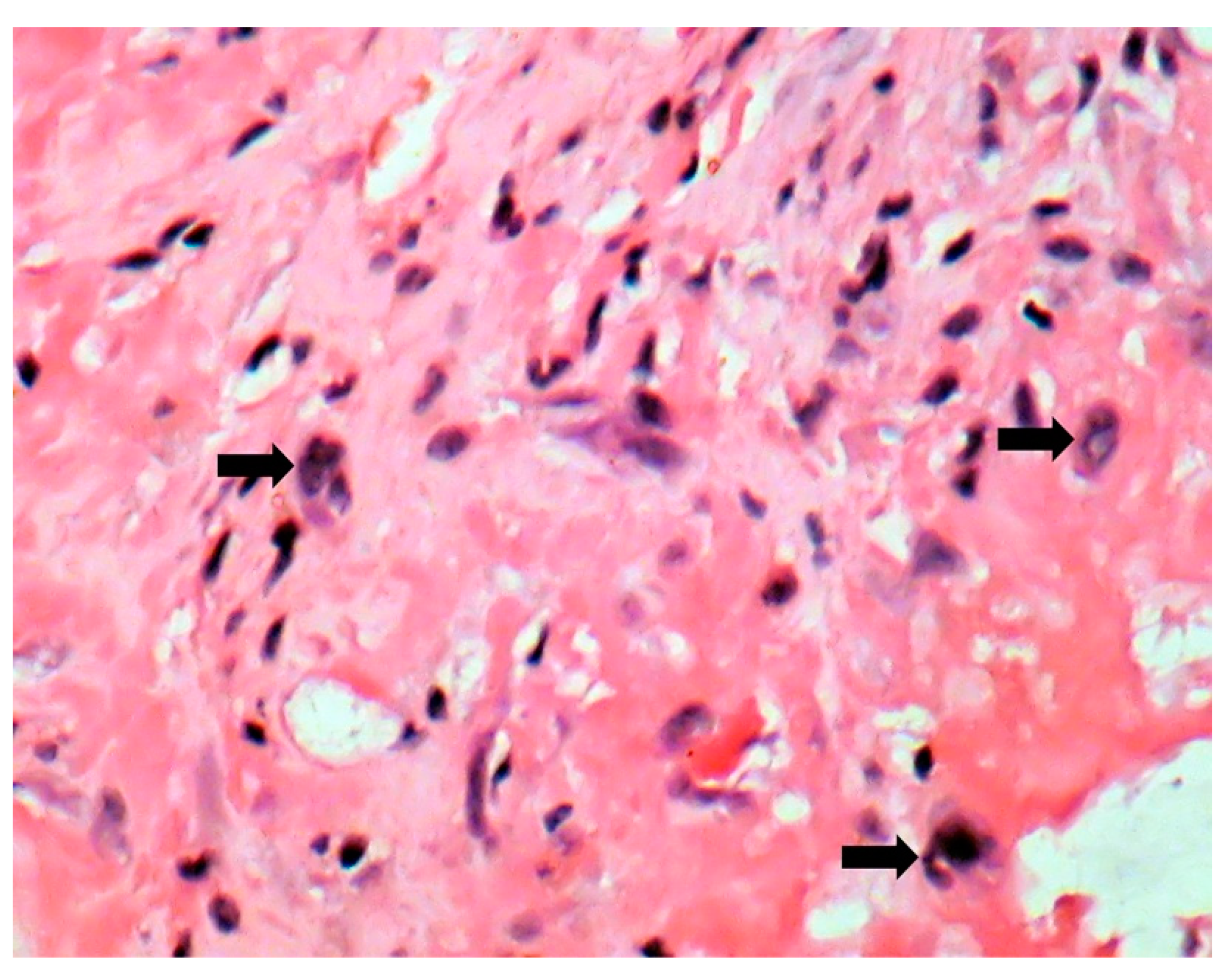
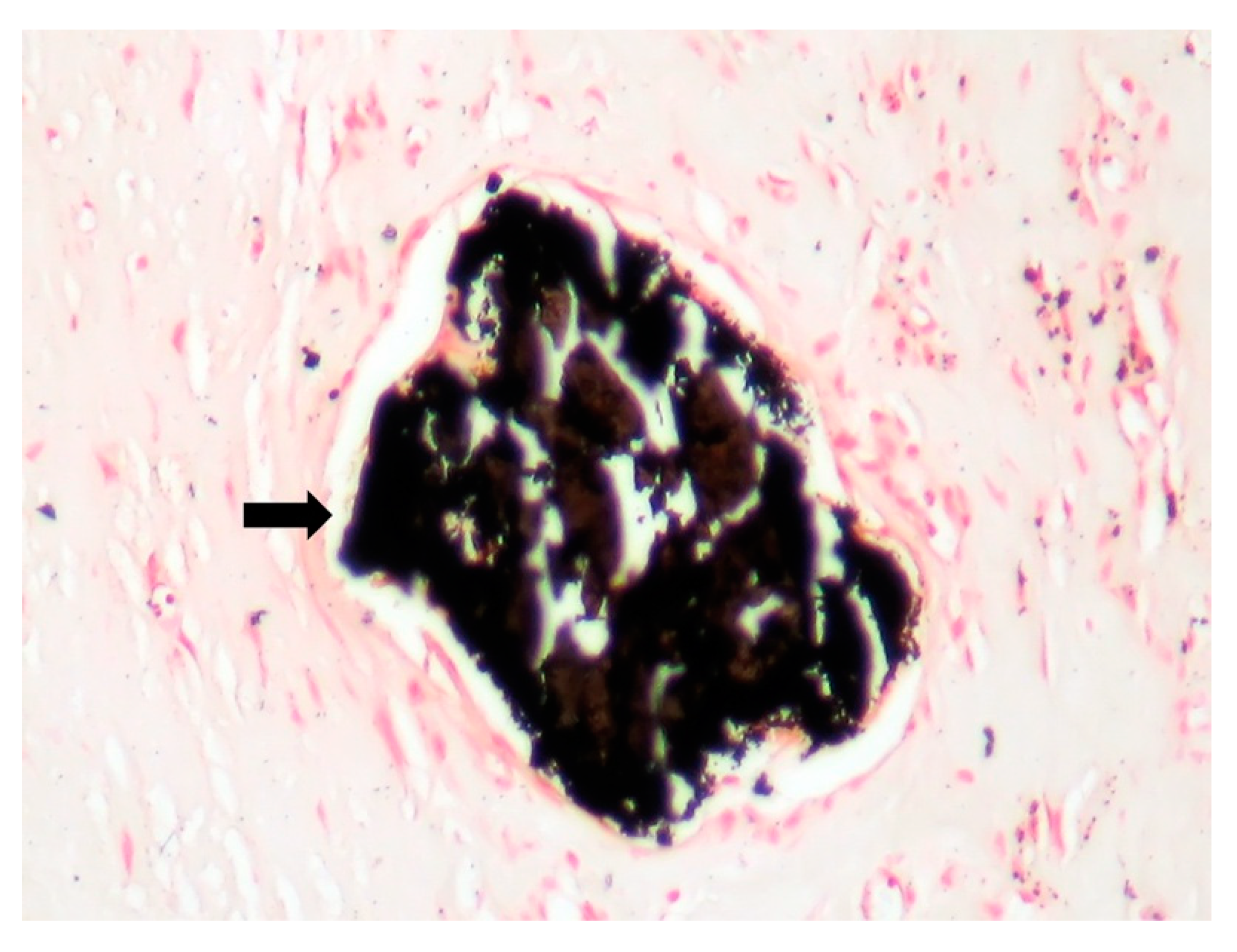
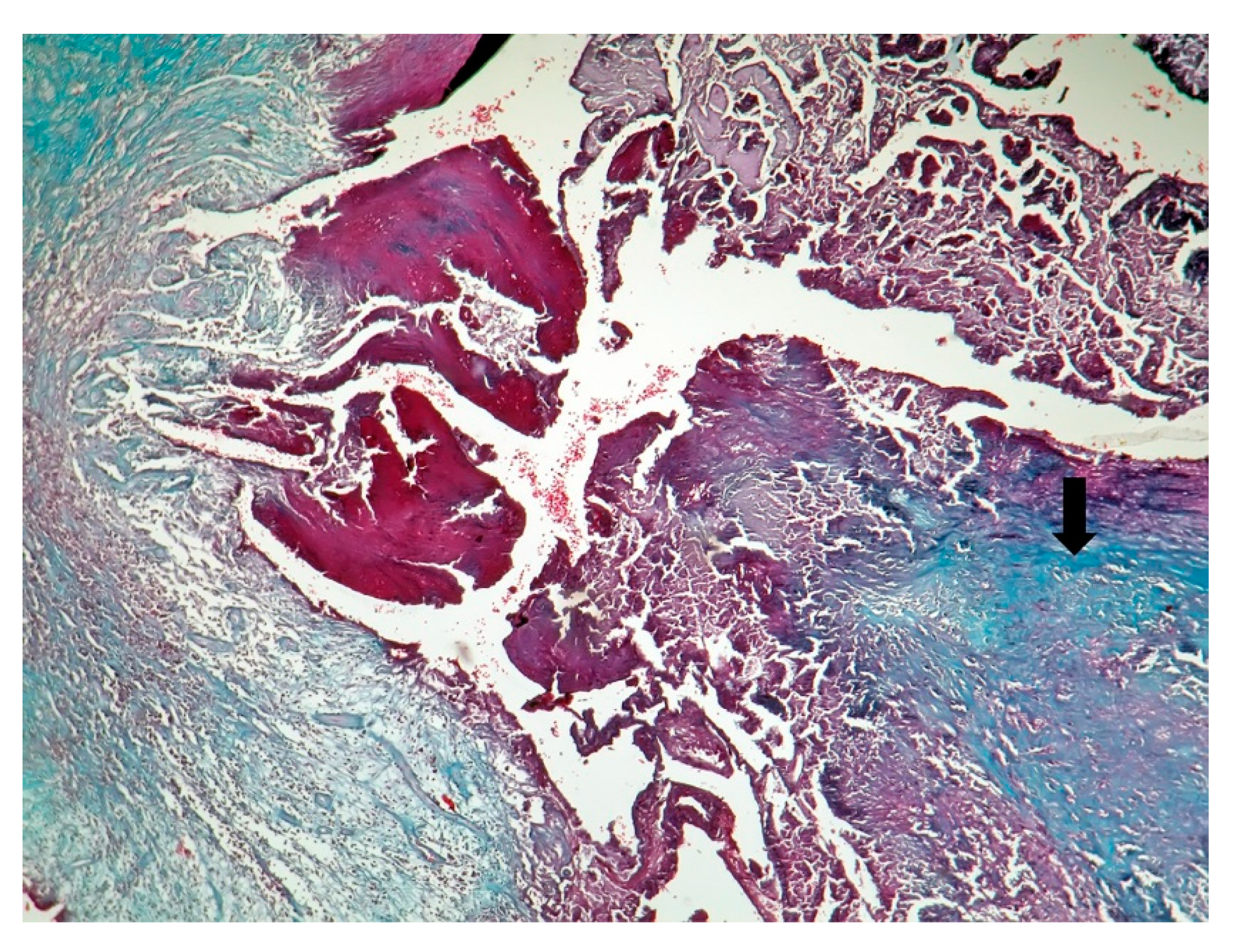
| Behçet’s Disease | Antiphospho Lipid Syndrome | A. defectiva | C. burnetii | |
|---|---|---|---|---|
| Macroscopic | Mural trombi, Endocardial fibrosis [23] Monovalvular involvement Mixoid degeneration Valve ulceration or fibrinous mass No fibrin valve deposits [24,25] | Small, warty, sessile vegetations on valve flow surface [26] | Large or small vegetations on valve flow surface [27] | Small or absent vegetations, with a smooth nodular aspect [13] |
| Microscopic | Mixoid degeneration, mixed inflammatory pattern, granulomas, thickening of the small vessels wall [23] | Platelets, fibrin, red blood cells Repetitive process with fibroblastic organization and neovascularization [28] | Fibrin, polymorphonuclear inflammation and some bacterial colonies [29] | Minimal mononuclear inflammation, histiocytes, foamy macrophages and mild vascularization [30] Granulomas [31] Microabscesses; usually no microorganism, sometimes coccoid inclusions [23] Fibrosis, thrombosis and calcifications, mimicking degenerative damage [32] |
| Predilection | Right heart [13] | Mitral, aortic, tricuspid valves [27] | Aortic, mitral valve [33] | Aortic, mitral valves [32] |
| Mechanism | Vasculitis, endothelial cells disruption, thrombo-inflammation, fibrinogen oxidation, hypercoagulability, APS induction, hyperhomocysteinemia, deficient fibrinolysis [34] | Valve endothelium initially normal [25] Fibrin-platelet, red blood cells thrombi [28] | Fibronectin adherence [35] | Vasculitis, APS induction [36] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moroșan, D.; Șerban, A.; Trifan, C.; Encica, S.; Pop, S.; Șerban, T.C.; Rednic, S.; Damian, L. Frenemies within: An Endocarditis Case in Behçet’s Disease. J. Pers. Med. 2021, 11, 728. https://doi.org/10.3390/jpm11080728
Moroșan D, Șerban A, Trifan C, Encica S, Pop S, Șerban TC, Rednic S, Damian L. Frenemies within: An Endocarditis Case in Behçet’s Disease. Journal of Personalized Medicine. 2021; 11(8):728. https://doi.org/10.3390/jpm11080728
Chicago/Turabian StyleMoroșan, Diana, Adela Șerban, Cătălin Trifan, Svetlana Encica, Sorin Pop, Tudor Costinel Șerban, Simona Rednic, and Laura Damian. 2021. "Frenemies within: An Endocarditis Case in Behçet’s Disease" Journal of Personalized Medicine 11, no. 8: 728. https://doi.org/10.3390/jpm11080728
APA StyleMoroșan, D., Șerban, A., Trifan, C., Encica, S., Pop, S., Șerban, T. C., Rednic, S., & Damian, L. (2021). Frenemies within: An Endocarditis Case in Behçet’s Disease. Journal of Personalized Medicine, 11(8), 728. https://doi.org/10.3390/jpm11080728






