Inter- and Intraobserver Repeatability of Myocardial Flow Reserve Values Determined with SPECT Study Using a Discovery NM530c Camera and Corridor 4DM Software
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Preparation and Data Acquisition
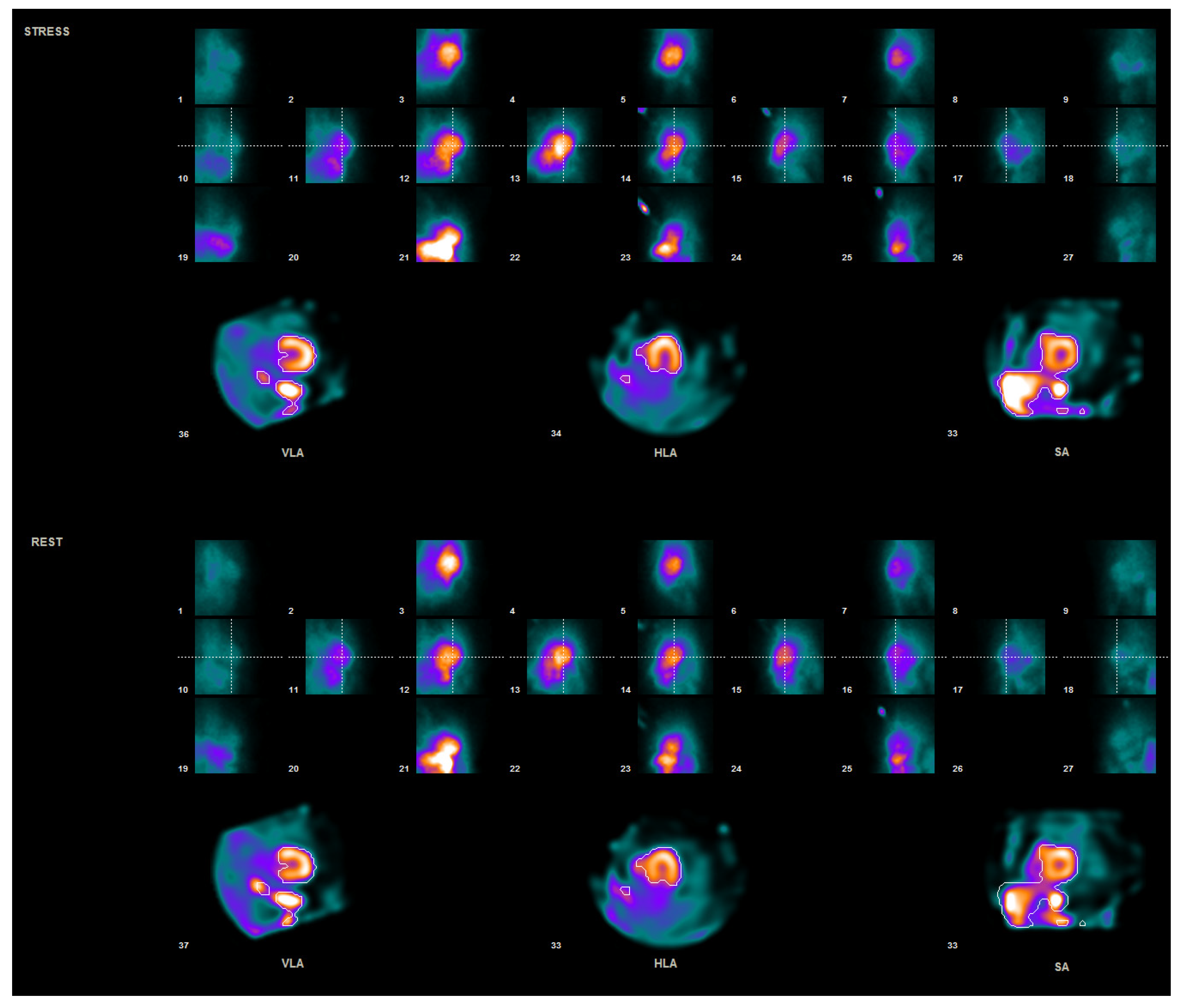
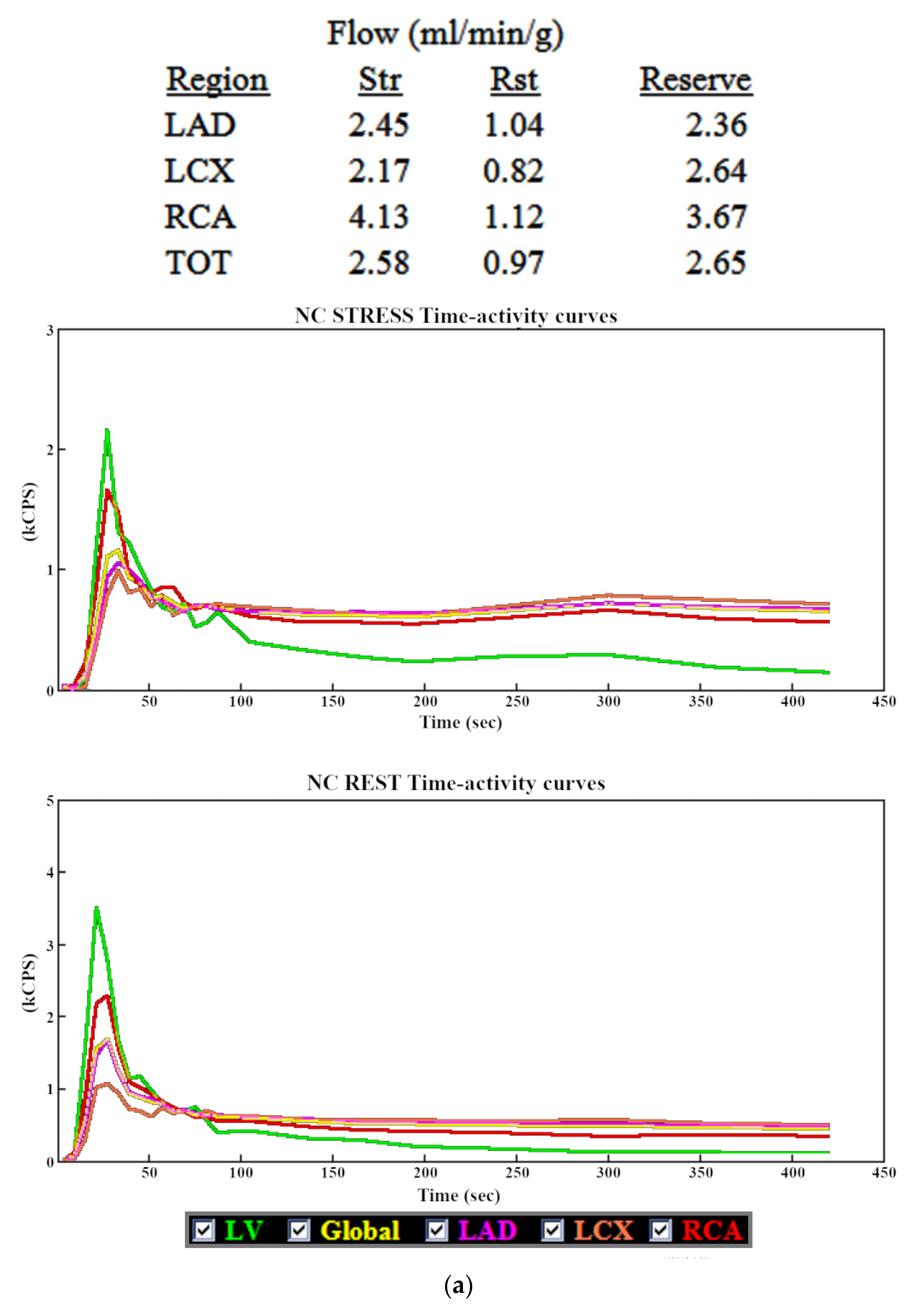
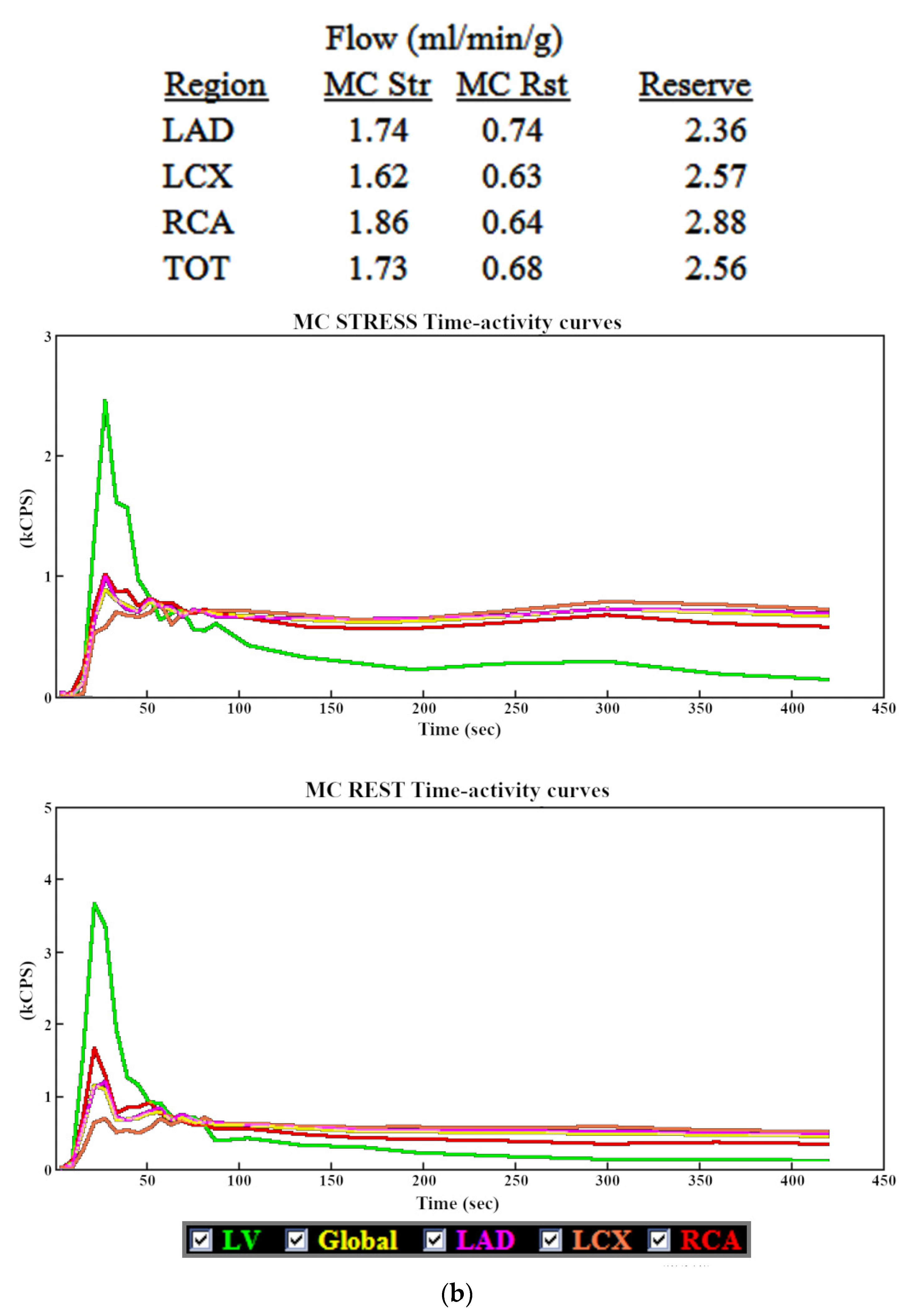
2.2. Data Processing
2.3. Statistical Analysis
3. Results
3.1. Myocardial Blood Flow
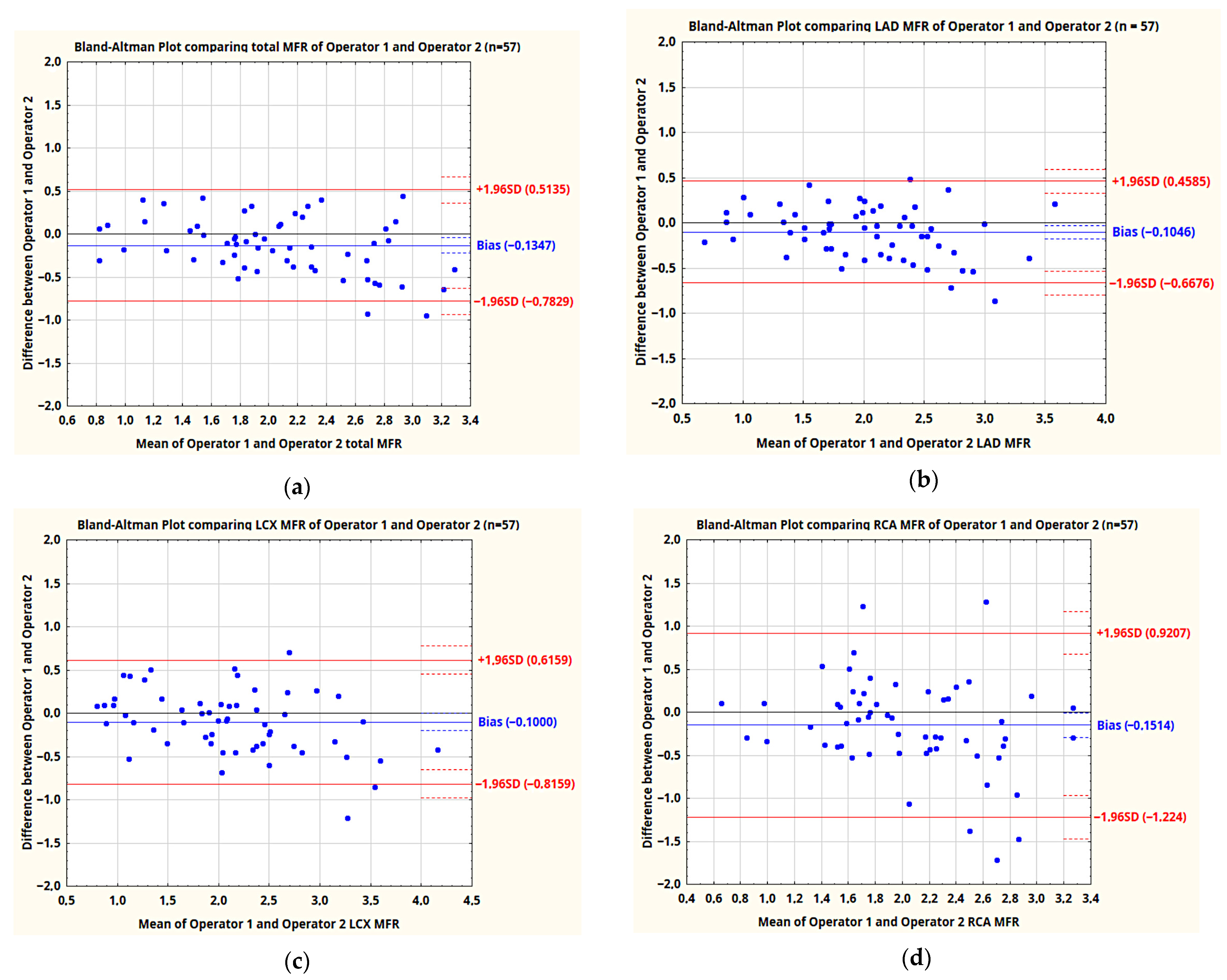
3.2. Myocardial Flow Reserve
4. Discussion
5. Conclusions
- MBF values in studies where automatic image orientation did not require significant manual corrections are characterized by good repeatability, both in assessments by the same and two independent operators.
- The repeatability of MBF values in case of incorrect automatic image orientation is significantly lower in the RCA vascular territory.
- The MFR values for the whole myocardium as well as LAD and LCX vascular territories, obtained by the same and two independent operators, are characterized by fairly good repeatability, although it is clearly lower than in PET studies.
- The repeatability of MFR values in RCA territory obtained by two operators processing studies in the current version of the software is unacceptably poor.
- Improving the reliability of automatic image orientation and introducing automatic motion correction is likely to significantly improve the repeatability of MFR values and is necessary for a reliable assessment of MFR in RCA territory. This will require further investigation with updated software.
- In the present situation, patients requiring the assessment of MFR in RCA territory should not undergo the MFR study using the CZT Discovery NM530c camera processed using the version of the Corridor 4DM software used in this paper. PET study results will be much more reliable for this group of patients.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murthy, V.L.; Bateman, T.M.; Beanlands, R.S.; Berman, D.S.; Borges-Neto, S.; Chareonthaitawee, P.; Cerqueira, M.D.; Dekemp, R.A.; DePuey, E.G.; Dilsizian, V.; et al. Clinical Quantification of Myocardial Blood Flow Using PET: Joint Position Paper of the SNMMI Cardiovascular Council and the ASNC. J. Nucl. Cardiol. 2018, 25, 269–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koenders, S.S.; Van Dijk, J.D.; Jager, P.L.; Ottervanger, J.P.; Slump, C.H.; Van Dalen, J.A. How to detect and correct myocardial creep in myocardial perfusion imaging using Rubidium-82 PET? J. Nucl. Cardiol. 2019, 26, 729–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koenders, S.S.; Van Dijk, J.D.; Jager, P.L.; Ottervanger, J.P.; Slump, C.H.; Van Dalen, J.A. Impact of regadenoson-induced myocardial creep on dynamic Rubidium-82 PET myocardial blood flow quantification. J. Nucl. Cardiol. 2019, 26, 719–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poitrasson-Rivière, A.; Moody, J.B.; Hagio, T.; Weinberg, R.L.; Corbett, J.R.; Murthy, V.L.; Ficaro, E.P. Reducing motion-correction-induced variability in 82rubidium myocardial blood-flow quantification. J. Nucl. Cardiol. 2020, 27, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Agostini, D.; Roule, V.; Nganoa, C.; Roth, N.; Baavour, R.; Parienti, J.J.; Beygui, F.; Manrique, A. First validation of myocardial flow reserve assessed by dynamic 99mTc-sestamibi CZT-SPECT camera: Head to head comparison with 15O-water PET and fractional flow reserve in patients with suspected coronary artery disease. The WATERDAY study. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1079–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouallègue, F.B.; Roubille, F.; Lattuca, B.; Cung, T.T.; Macia, J.C.; Gervasoni, R.; Leclercq, F.; Mariano-Goulart, D. SPECT Myocardial Perfusion Reserve in Patients with Multivessel Coronary Disease: Correlation with Angiographic Findings and Invasive Fractional Flow Reserve Measurements. J. Nucl. Med. 2015, 56, 1712–1717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cichocki, P.; Błaszczyk, M.; Cygulska, K.; Filipczak, K.; Kuśmierek, J.; Lipiec, P.; Kasprzak, J.; Płachcińska, A. Repeatability of Coronary Flow Reserve Values Determined with SPECT Technique Using a CZT Semiconductor Camera. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 536–537. [Google Scholar]
- Renaud, J.M.; Moody, J.B.; Ficaro, E.P. Optimization of 4DM SPECT MFR for the GE DNM 530c Cardiac Gamma Camera [White Paper]. INVIA Medical Imaging Solutions. 2021. Available online: https://www.inviasolutions.com/post/optimization-of-4dm-spect-mfr-for-the-ge-dnm-530c-cardiac-gamma-camera (accessed on 16 September 2021).
- van Dijk, J.D.; van Dalen, J.A.; Mouden, M.; Ottervanger, J.P.; Knollema, S.; Slump, C.H.; Jager, P.L. Value of automatic patient motion detection and correction in myocardial perfusion imaging using a CZT-based SPECT camera. J. Nucl. Cardiol. 2018, 25, 419–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redgate, S.; Barber, D.C.; Fenner, J.W.; Al-Mohammad, A.; Taylor, J.C.; Hanney, M.B.; Tindale, W.B. A study to quantify the effect of patient motion and develop methods to detect and correct for motion during myocardial perfusion imaging on a CZT solid-state dedicated cardiac camera. J. Nucl. Cardiol. 2016, 23, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Marvin, B.; Ruddy, T.; Wells, R.G. Patient motion on the GE Discovery CZT camera: Investigating the necessity of motion correction. J. Nucl. Med. 2010, 51, 2114. [Google Scholar]
- DePuey, E.G. Traditional gamma cameras are preferred. J. Nucl. Cardiol. 2016, 23, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Monroy-Gonzalez, A.G.; Juarez-Orozco, L.E.; Han, C.; Vedder, I.R.; García, D.V.; Borra, R.; Slomka, P.J.; Nesterov, S.V.; Knuuti, J.; Slart, R.H.J.A.; et al. Software reproducibility of myocardial blood flow and flow reserve quantification in ischemic heart disease: A 13N-ammonia PET study. J. Nucl. Cardiol. 2020, 27, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Celiker-Guler, E.; Rotstein, B.H.; Dekemp, R.A. PET and SPECT Tracers for Myocardial Perfusion Imaging. Semin. Nucl. Med. 2020, 50, 208–218. [Google Scholar] [CrossRef] [PubMed]


| Min | Max | Median | |
|---|---|---|---|
| Age | 48 | 84 | 64 |
| BMI | 17 | 38 | 27 |
| Sex | Post-Infarction | Prior Revascularization | Diabetes | ||
|---|---|---|---|---|---|
| Male | Female | PCI | CABG | ||
| 34 | 23 | 18 | 23 | 0 | 14 |
| Operators | TOT | LAD | LCX | RCA | RCA vs. LAD | RCA vs. LCX | RCA vs. TOT | |
|---|---|---|---|---|---|---|---|---|
| MBF | 1 | 0.97 | 0.97 | 0.97 | 0.94 | p = 0.0089 | p = 0.0089 | p = 0.0089 |
| 2 | 0.95 | 0.96 | 0.95 | 0.88 | p < 0.0001 | p = 0.0008 | p = 0.0008 |
| Automatic Heart Orientation Quality | Stress | Rest | Total |
|---|---|---|---|
| 0—little to no adjustments needed | 23 | 5 | 28 |
| 1—axis angle required correction | 19 | 20 | 39 |
| 2—center of axis placed outside of the heart | 15 | 32 | 47 |
| Vascular Territory | TOTAL MBF | LAD MBF | LCX MBF | RCA MBF | ||||
|---|---|---|---|---|---|---|---|---|
| Operators | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 |
| Good automatic orientation (0) n = 28 | 0.99 | 0.95 | 0.98 | 0.94 | 0.95 | 0.95 | 0.98 | 0.95 |
| Orientation required adjustments (1, 2) n = 86 | 0.95 | 0.94 | 0.95 | 0.95 | 0.96 | 0.93 | 0.92 | 0.84 |
| Statistical significance of difference (p) | 0.0005 | 0.68 | 0.04 | 0.68 | 0.62 | 0.45 | 0.0024 | 0.0086 |
| Operators | TOT | LAD | LCX | RCA | RCA vs. LAD | RCA vs. LCX | RCA vs. TOT | |
|---|---|---|---|---|---|---|---|---|
| MFR | 1 | 0.92 | 0.88 | 0.91 | 0.84 | p = 0.42 | p = 0.11 | p = 0.06 |
| 2 | 0.89 | 0.91 | 0.90 | 0.67 | p = 0.0003 | p = 0.0008 | p = 0.0019 | |
| Mean difference of MFR | 0.13 | 0.10 | 0.10 | 0.15 | ||||
| SD of difference | 0.33 | 0.29 | 0.37 | 0.55 | p < 0.0001 | p = 0.0036 | p = 0.0002 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cichocki, P.; Błaszczyk, M.; Cygulska, K.; Filipczak, K.; Adamczewski, Z.; Kuśmierek, J.; Lipiec, P.; Kasprzak, J.D.; Płachcińska, A. Inter- and Intraobserver Repeatability of Myocardial Flow Reserve Values Determined with SPECT Study Using a Discovery NM530c Camera and Corridor 4DM Software. J. Pers. Med. 2021, 11, 1164. https://doi.org/10.3390/jpm11111164
Cichocki P, Błaszczyk M, Cygulska K, Filipczak K, Adamczewski Z, Kuśmierek J, Lipiec P, Kasprzak JD, Płachcińska A. Inter- and Intraobserver Repeatability of Myocardial Flow Reserve Values Determined with SPECT Study Using a Discovery NM530c Camera and Corridor 4DM Software. Journal of Personalized Medicine. 2021; 11(11):1164. https://doi.org/10.3390/jpm11111164
Chicago/Turabian StyleCichocki, Paweł, Michał Błaszczyk, Kamila Cygulska, Krzysztof Filipczak, Zbigniew Adamczewski, Jacek Kuśmierek, Piotr Lipiec, Jarosław Damian Kasprzak, and Anna Płachcińska. 2021. "Inter- and Intraobserver Repeatability of Myocardial Flow Reserve Values Determined with SPECT Study Using a Discovery NM530c Camera and Corridor 4DM Software" Journal of Personalized Medicine 11, no. 11: 1164. https://doi.org/10.3390/jpm11111164
APA StyleCichocki, P., Błaszczyk, M., Cygulska, K., Filipczak, K., Adamczewski, Z., Kuśmierek, J., Lipiec, P., Kasprzak, J. D., & Płachcińska, A. (2021). Inter- and Intraobserver Repeatability of Myocardial Flow Reserve Values Determined with SPECT Study Using a Discovery NM530c Camera and Corridor 4DM Software. Journal of Personalized Medicine, 11(11), 1164. https://doi.org/10.3390/jpm11111164








