Predicting 1-Year Mortality after Hip Fracture Surgery: An Evaluation of Multiple Machine Learning Approaches
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Descriptive Analysis
2.3. Predictive Models
2.4. Data Normalization and Model Construction
2.5. Metrics of Predictive Ability
3. Results
3.1. Patient Demographics
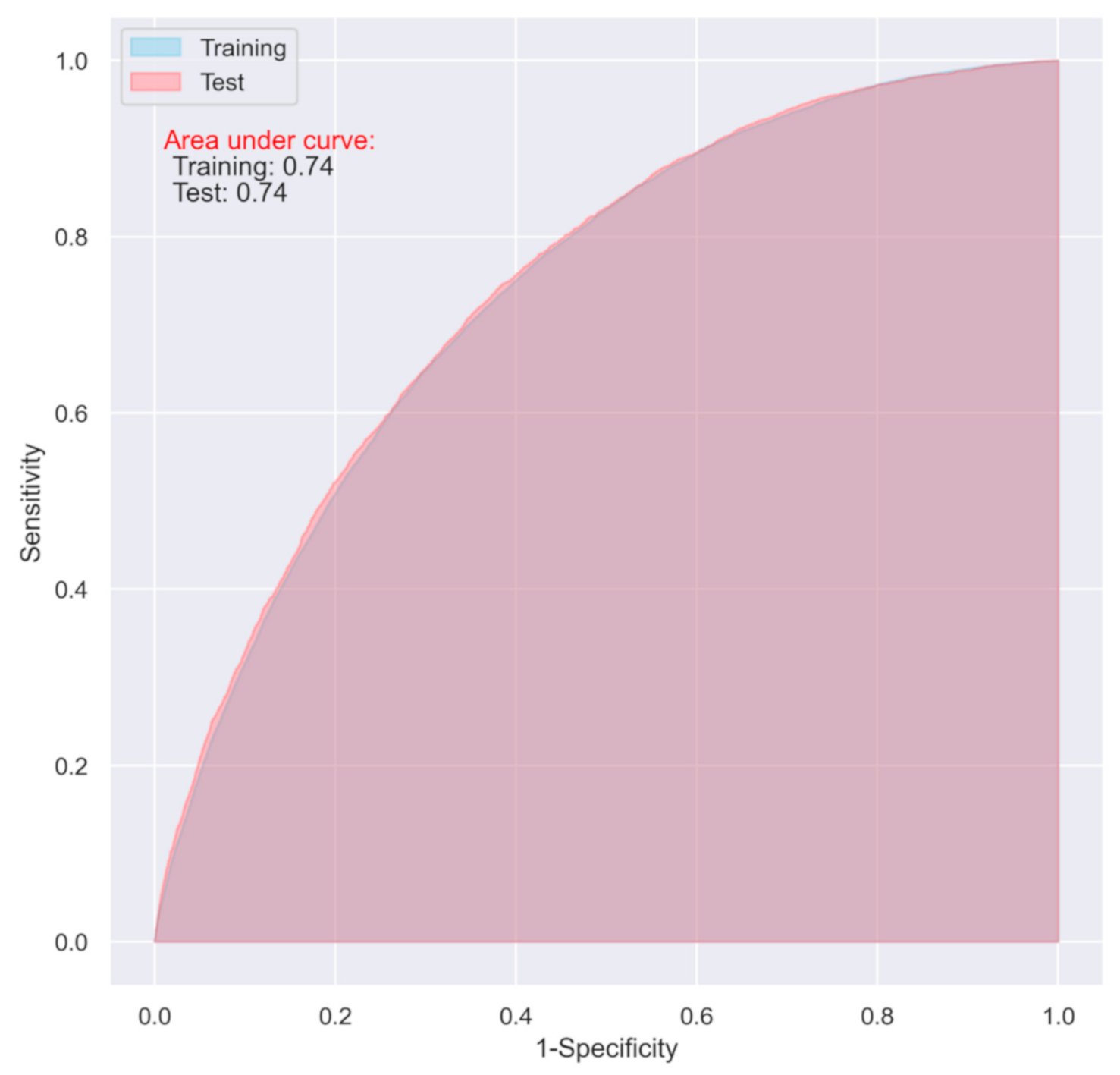
3.2. Predictive Performance of the LR Models
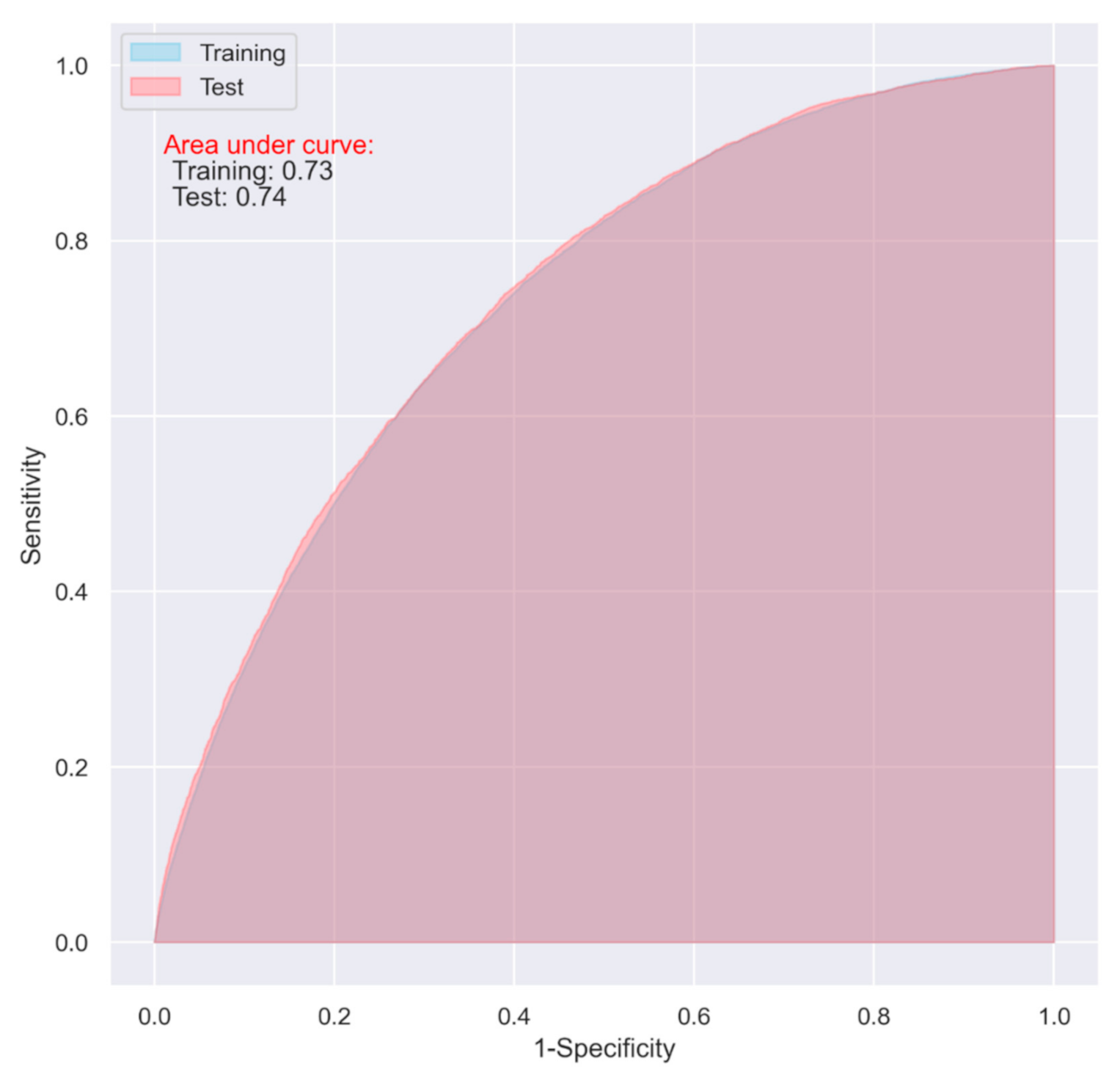
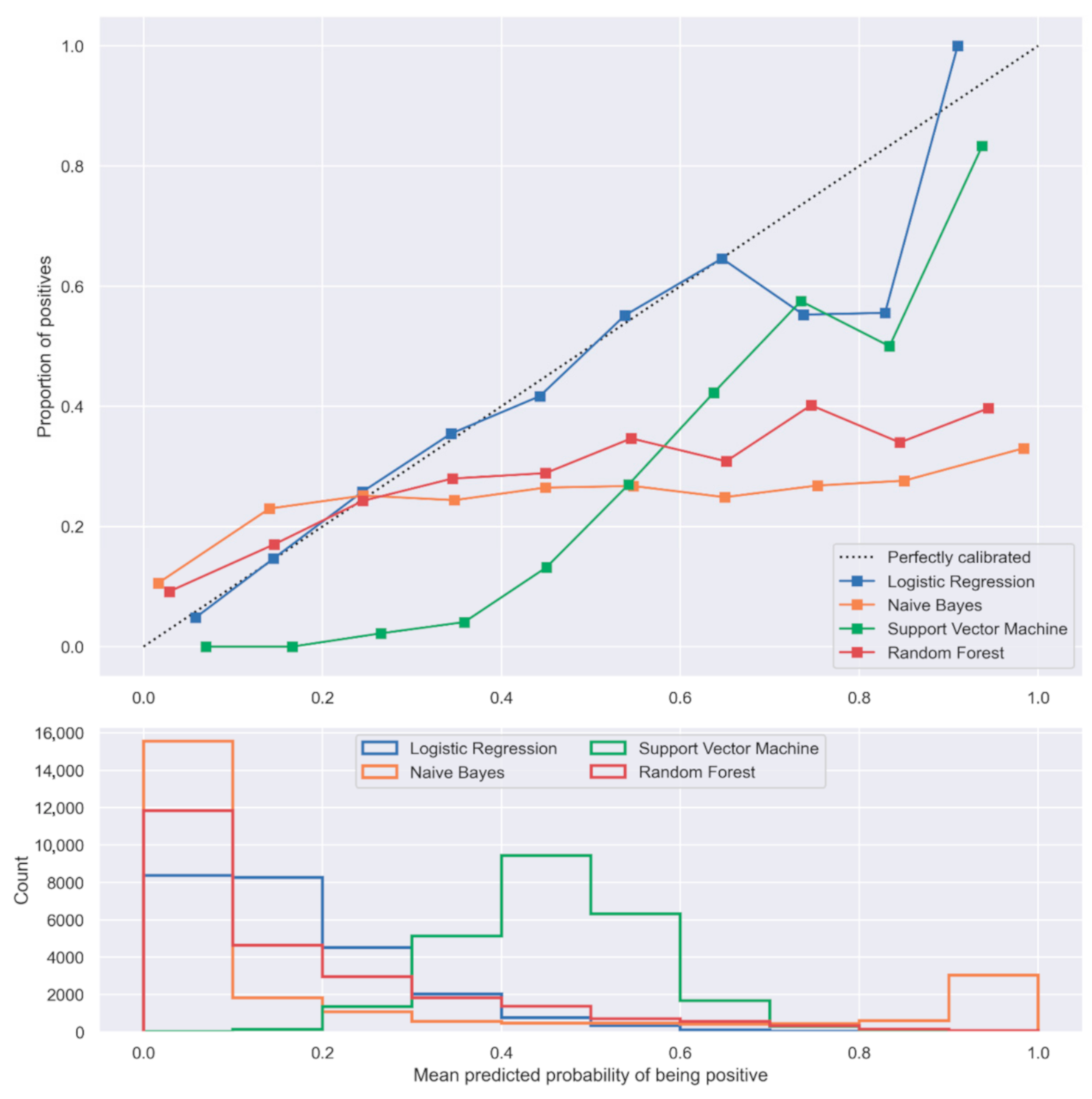
3.3. Comparison of LR with the Selected Machine Learning Algorithms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Forssten, M.P.; Mohammad Ismail, A.; Sjolin, G.; Ahl, R.; Wretenberg, P.; Borg, T.; Mohseni, S. The Association between the Revised Cardiac Risk Index and Short-Term Mortality after Hip Fracture Surgery. Eur. J. Trauma Emerg. Surg. 2020. [Google Scholar] [CrossRef]
- NHFD 2019 Annual Report. Available online: https://www.nhfd.co.uk/20/hipfractureR.nsf/docs/2019Report (accessed on 18 October 2020).
- Gundel, O.; Thygesen, L.C.; Gögenur, I.; Ekeloef, S. Postoperative Mortality after a Hip Fracture over a 15-Year Period in Denmark: A National Register Study. Acta Orthop. 2020, 91, 58–62. [Google Scholar] [CrossRef]
- Rikshoft Arsrapport. 2018. Available online: https://www.xn--rikshft-e1a.se/forsking-och-utv%C3%A4rdering (accessed on 2 October 2020).
- Thorngren, K.-G. Fractures in the Elderly. Acta Orthop. Scand. 1995, 66, 208–210. [Google Scholar] [CrossRef]
- Statens beredning för medicinsk utvärdering. Rehabilitering av Äldre Personer Med Höftfrakturer—Interdisciplinära Team: En Systematisk Litteraturöversikt; Statens beredning för medicinsk utvärdering (SBU): Stockholm, Sweden, 2015; ISBN 978-91-85413-79-9. [Google Scholar]
- Kanis, J.A.; Odén, A.; McCloskey, E.V.; Johansson, H.; Wahl, D.A.; Cooper, C. IOF Working Group on Epidemiology and Quality of Life A Systematic Review of Hip Fracture Incidence and Probability of Fracture Worldwide. Osteoporos Int. 2012, 23, 2239–2256. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Forssten, M.P.; Mohammad Ismail, A.; Borg, T.; Ioannidis, I.; Montgomery, S.; Mohseni, S. Predictive Values of Preoperative Characteristics for 30-Day Mortality in Traumatic Hip Fracture Patients. J. Pers. Med. 2021, 11, 353. [Google Scholar] [CrossRef] [PubMed]
- Salazar, D.A.; Vélez, J.I.; Salazar, J.C. Comparison between SVM and Logistic Regression: Which One Is Better to Discriminate? Rev. Colomb. Estadística 2012, 35, 223–237. [Google Scholar]
- Ng, A.Y.; Jordan, M.I. On Discriminative vs. Generative Classifiers: A Comparison of Logistic Regression and Naive Bayes. In Proceedings of the 14th International Conference on Neural Information Processing Systems: Natural and Synthetic, Vancouver, BC, Canada, 3–8 December 2001; MIT Press: Cambridge, MA, USA, 2001; pp. 841–848. [Google Scholar]
- Couronné, R.; Probst, P.; Boulesteix, A.-L. Random Forest versus Logistic Regression: A Large-Scale Benchmark Experiment. BMC Bioinform. 2018, 19, 270. [Google Scholar] [CrossRef]
- Om Rikshöft—RIKSHÖFT. Available online: https://www.xn--rikshft-e1a.se/om-oss (accessed on 20 April 2021).
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Lee, T.H.; Marcantonio, E.R.; Mangione, C.M.; Thomas, E.J.; Polanczyk, C.A.; Cook, E.F.; Sugarbaker, D.J.; Donaldson, M.C.; Poss, R.; Ho, K.K.; et al. Derivation and Prospective Validation of a Simple Index for Prediction of Cardiac Risk of Major Noncardiac Surgery. Circulation 1999, 100, 1043–1049. [Google Scholar] [CrossRef] [Green Version]
- Pearson, K. On the Criterion that a Given System of Deviations from the Probable in the Case of a Correlated System of Variables is Such that it Can be Reasonably Supposed to have Arisen from Random Sampling. In Breakthroughs in Statistics: Methodology and Distribution; Kotz, S., Johnson, N.L., Eds.; Springer Series in Statistics; Springer: New York, NY, USA, 1992; pp. 11–28. ISBN 978-1-4612-4380-9. [Google Scholar]
- Student. The Probable Error of a Mean. Biometrika 1908, 6, 1–25. [Google Scholar] [CrossRef]
- Mann, H.B.; Whitney, D.R. On a Test of Whether One of Two Random Variables Is Stochastically Larger than the Other. Ann. Math. Stat. 1947, 18, 50–60. [Google Scholar] [CrossRef]
- Azur, M.J.; Stuart, E.A.; Frangakis, C.; Leaf, P.J. Multiple Imputation by Chained Equations: What Is It and How Does It Work? Int J. Methods Psychiatr. Res. 2011, 20, 40–49. [Google Scholar] [CrossRef]
- Fix, E.; Hodges, J.L. Discriminatory Analysis. Nonparametric Discrimination: Consistency Properties. Int. Stat. Rev. Rev. Int. De Stat. 1989, 57, 238–247. [Google Scholar] [CrossRef]
- Cox, D.R. The Regression Analysis of Binary Sequences. J. R. Stat. Society. Ser. B 1958, 20, 215–242. [Google Scholar] [CrossRef]
- Zou, H.; Hastie, T. Regularization and Variable Selection via the Elastic Net. J. R. Stat. Soc. Ser. B 2005, 67, 301–320. [Google Scholar] [CrossRef] [Green Version]
- Altmann, A.; Toloşi, L.; Sander, O.; Lengauer, T. Permutation Importance: A Corrected Feature Importance Measure. Bioinformatics 2010, 26, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Youden, W.J. Index for Rating Diagnostic Tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Hand, D.J.; Yu, K. Idiot’s Bayes: Not So Stupid after All? Int. Stat. Rev. Rev. Int. Stat. 2001, 69, 385–398. [Google Scholar] [CrossRef]
- Cortes, C.; Vapnik, V. Support-Vector Networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Dormann, C.F. Calibration of Probability Predictions from Machine-Learning and Statistical Models. Glob. Ecol. Biogeogr. 2020, 29, 760–765. [Google Scholar] [CrossRef]
- Obaid, H.S.; Dheyab, S.A.; Sabry, S.S. The Impact of Data Pre-Processing Techniques and Dimensionality Reduction on the Accuracy of Machine Learning. In Proceedings of the 2019 9th Annual Information Technology, Electromechanical Engineering and Microelectronics Conference (IEMECON), Jaipur, India, 13–15 March 2019; pp. 279–283. [Google Scholar]
- Wong, T.T.; Yeh, P.Y. Reliable Accuracy Estimates from K-Fold Cross Validation. IEEE Trans. Knowl. Data Eng. 2020, 32, 1586–1594. [Google Scholar] [CrossRef]
- Bergstra, J.; Bengio, Y. Random Search for Hyper-Parameter Optimization. J. Mach. Learn. Res. 2012, 13, 281–305. [Google Scholar]
- Lawson, C.R.; Hodgson, J.A.; Wilson, R.J.; Richards, S.A. Prevalence, Thresholds and the Performance of Presence–Absence Models. Methods Ecol. Evol. 2014, 5, 54–64. [Google Scholar] [CrossRef] [Green Version]
- Fluss, R.; Faraggi, D.; Reiser, B. Estimation of the Youden Index and Its Associated Cutoff Point. Biom. J. 2005, 47, 458–472. [Google Scholar] [CrossRef] [Green Version]
- Mandrekar, J.N. Receiver Operating Characteristic Curve in Diagnostic Test Assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef] [Green Version]
- Mohammad Ismail, A.; Borg, T.; Sjolin, G.; Pourlotfi, A.; Holm, S.; Cao, Y.; Wretenberg, P.; Ahl, R.; Mohseni, S. β-Adrenergic Blockade Is Associated with a Reduced Risk of 90-Day Mortality after Surgery for Hip Fractures. Trauma Surg. Acute Care Open 2020, 5, e000533. [Google Scholar] [CrossRef]
- Ahl, R.; Mohammad Ismail, A.; Borg, T.; Sjölin, G.; Forssten, M.P.; Cao, Y.; Wretenberg, P.; Mohseni, S. A Nationwide Observational Cohort Study of the Relationship between Beta-Blockade and Survival after Hip Fracture Surgery. Eur J. Trauma Emerg. Surg. 2021. [Google Scholar] [CrossRef]
- Webb, G.I. Naïve Bayes. In Encyclopedia of Machine Learning; Sammut, C., Webb, G.I., Eds.; Springer US: Boston, MA, USA, 2010; pp. 713–714. ISBN 978-0-387-30164-8. [Google Scholar]
- Barker, L.; Brown, C. Logistic Regression When Binary Predictor Variables Are Highly Correlated. Stat. Med. 2001, 20, 1431–1442. [Google Scholar] [CrossRef]
- Mears, S. Fixing Hip Fractures. Available online: https://www.hopkinsmedicine.org/gec/series/fixing_hip_fractures (accessed on 7 June 2021).
- Raaymakers, E.; Schipper, I.; Simmermacher, R.; van der Werken, C. Treatment of Femoral Neck Fracture, Subcapital, Undisplaced or Impacted. Available online: https://surgeryreference.aofoundation.org/orthopedic-trauma/adult-trauma/proximal-femur/femoral-neck-fracture-subcapital-undisplaced-or-impacted (accessed on 7 June 2021).
- Lee, C.H.; Yoon, H.-J. Medical Big Data: Promise and Challenges. Kidney Res. Clin. Pract. 2017, 36, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Bennett, K.; Bredensteiner, E. Duality and Geometry in SVM Classifiers, ICML ’00. In Proceedings of the Seventeenth International Conference on Machine Learning, Stanford University, Stanford, CA, USA, 29 June–2 July 2000; Morgan Kaufmann Publishers: San Francisco, CA, USA, 2000; pp. 57–64. [Google Scholar]
- Zhang, X. Support Vector Machines. In Encyclopedia of Machine Learning; Sammut, C., Webb, G.I., Eds.; Springer US: Boston, MA, USA, 2010; pp. 941–946. ISBN 978-0-387-30164-8. [Google Scholar]
- Random Forests. In Encyclopedia of Machine Learning; Sammut, C.; Webb, G.I. (Eds.) Springer US: Boston, MA, USA, 2010; p. 828. ISBN 978-0-387-30164-8. [Google Scholar]
- Kirasich, K.; Smith, T.; Sadler, B. Random Forest vs Logistic Regression: Binary Classification for Heterogeneous Datasets. SMU Data Sci. Rev. 2018, 1, 9. [Google Scholar]
- Penrod, J.D.; Litke, A.; Hawkes, W.G.; Magaziner, J.; Koval, K.J.; Doucette, J.T.; Silberzweig, S.B.; Siu, A.L. Heterogeneity in Hip Fracture Patients: Age, Functional Status, and Comorbidity. J. Am. Geriatr. Soc. 2007, 55, 407–413. [Google Scholar] [CrossRef]
- Duceppe, E.; Parlow, J.; MacDonald, P.; Lyons, K.; McMullen, M.; Srinathan, S.; Graham, M.; Tandon, V.; Styles, K.; Bessissow, A.; et al. Canadian Cardiovascular Society Guidelines on Perioperative Cardiac Risk Assessment and Management for Patients Who Undergo Noncardiac Surgery. Can. J. Cardiol. 2017, 33, 17–32. [Google Scholar] [CrossRef] [Green Version]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.-M.; Sundararajan, V. Updating and Validating the Charlson Comorbidity Index and Score for Risk Adjustment in Hospital Discharge Abstracts Using Data from 6 Countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Radovanovic, D.; Seifert, B.; Urban, P.; Eberli, F.R.; Rickli, H.; Bertel, O.; Puhan, M.A.; Erne, P. AMIS Plus Investigators Validity of Charlson Comorbidity Index in Patients Hospitalised with Acute Coronary Syndrome. Insights from the Nationwide AMIS Plus Registry 2002–2012. Heart 2014, 100, 288–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ioannidis, I.; Mohammad Ismail, A.; Forssten, M.P.; Ahl, R.; Cao, Y.; Borg, T.; Mohseni, S. Surgical Management of Displaced Femoral Neck Fractures in Patients with Dementia: A Comparison in Mortality between Hemiarthroplasty and Pins/Screws. Eur. J. Trauma Emerg. Surg. 2021. [Google Scholar] [CrossRef]
- Rogmark, C.; Carlsson, A.; Johnell, O. Sernbo I Primary Hemiarthroplasty in Old Patients with Displaced Femoral Neck Fracture: A 1-Year Follow-up of 103 Patients Aged 80 Years or More. Acta Orthop. Scand. 2002, 73, 605–610. [Google Scholar] [CrossRef]
- Rogmark, C.; Carlsson, å.; Johnell, O.; Sernbo, I. A Prospective Randomised Trial of Internal Fixation versus Arthroplasty for Displaced Fractures of the Neck of the Femur. J. Bone Jt. Surg. Br. Vol. 2002, 84-B, 183–188. [Google Scholar] [CrossRef]
- Groff, H.; Kheir, M.M.; George, J.; Azboy, I.; Higuera, C.A.; Parvizi, J. Causes of In-Hospital Mortality after Hip Fractures in the Elderly. Hip Int. 2020, 30, 204–209. [Google Scholar] [CrossRef]
- Bass, G.A.; Forssten, M.; Pourlotfi, A.; Ahl Hulme, R.; Cao, Y.; Matthiessen, P.; Mohseni, S. Cardiac Risk Stratification in Emergency Resection for Colonic Tumours. BJS Open 2021, 5, zrab057. [Google Scholar] [CrossRef]
- Forssten, M.P.; Mohammad Ismail, A.; Borg, T.; Ahl, R.; Wretenberg, P.; Cao, Y.; Peden, C.J.; Mohseni, S. Postoperative Mortality in Hip Fracture Patients Stratified by The Revised Cardiac Risk Index: A Swedish Nationwide Retrospective Cohort Study. Trauma Surg. Acute Care Open 2021, 6, e000778. [Google Scholar] [CrossRef]
- Fleisher, L.A.; Beckman, J.A.; Brown, K.A.; Calkins, H.; Chaikof, E.; Fleischmann, K.E.; Freeman, W.K.; Froehlich, J.B.; Kasper, E.K.; Kersten, J.R.; et al. ACC/AHA 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): Developed in Collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation 2007, 116, e418–e499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismail, A.M.; Ahl, R.; Forssten, M.P.; Cao, Y.; Wretenberg, P.; Borg, T.; Mohseni, S. The Interaction Between Per-Admission β-Blocker Therapy, The Revised Cardiac Risk Index, and Mortality in Geriatric Hip Fracture Patients. J. Trauma Acute Care Surg. 2021. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.C.; Hedström, M.; Modig, K. The Swedish Hip Fracture Register and National Patient Register Were Valuable for Research on Hip Fractures: Comparison of Two Registers. J. Clin. Epidemiol. 2020, 125, 91–99. [Google Scholar] [CrossRef] [PubMed]

| Variable | Total (n = 124,707) | Alive (n = 103,662) | Dead (n = 21,045) | p-Value |
|---|---|---|---|---|
| Age, mean (SD) | 81.55 (10.10) | 80.75 (10.29) | 85.46 (8.01) | <0.001 |
| Sex, n (%) | <0.001 | |||
| Female | 86,379 (69.3) | 73,166 (70.6) | 13,213 (62.8) | |
| Male | 38,314 (30.7) | 30,483 (29.4) | 7831 (37.2) | |
| Missing | 14 (0.0) | 13 (0.0) | 1 (0.0) | |
| ASA classification, n (%) | <0.001 | |||
| 1 | 6356 (5.3) | 6216 (6.1) | 320 (1.6) | |
| 2 | 46,507 (38.0) | 41,626 (40.9) | 4878 (23.6) | |
| 3 | 60,857 (49.7) | 48,278 (47.4) | 12,579 (60.9) | |
| 4 | 8459 (6.9) | 5606 (5.5) | 2853 (13.8) | |
| 5 | 79 (0.1) | 44 (0.1) | 35 (0.2) | |
| CCI, n (%) | <0.001 | |||
| ≤4 | 57,634 (46.2) | 52,281 (50.4) | 5353 (25.4) | |
| 5–6 | 46,733 (36.7) | 36,604 (35.3) | 9129 (43.4) | |
| ≥7 | 21,340 (17.1) | 14,777 (14.3) | 6563 (31.2) | |
| RCRI, n (%) | <0.001 | |||
| 0 | 75,864 (60.8) | 65,476 (63.2) | 10,388 (49.4) | |
| 1 | 33,476 (26.8) | 27,135 (26.2) | 6341 (30.1) | |
| 2 | 11,284 (9.1) | 8352 (8.1) | 2932 (13.9) | |
| 3 | 3245 (2.6) | 2174 (2.1) | 1071 (5.1) | |
| ≥4 | 838 (0.7) | 525 (0.5) | 313 (1.5) | |
| Arrhythmia, n (%) | 22,305 (17.9) | 17,223 (16.6) | 5082 (24.1) | <0.001 |
| Hypertension, n (%) | 47,990 (38.5) | 39,849 (38.4) | 8141 (38.7) | 0.515 |
| Myocardial infarction, n (%) | 6789 (5.4) | 5065 (4.9) | 1724 (8.2) | <0.001 |
| Congestive heart failure, n (%) | 17,475 (14.0) | 12,276 (11.8) | 5199 (24.7) | <0.001 |
| Cerebrovascular disease, n (%) | 21,036 (16.9) | 16,669 (16.1) | 4367 (20.8) | <0.001 |
| COPD, n (%) | 13,933 (11.2) | 11,094 (10.7) | 2839 (13.5) | <0.001 |
| Connective tissue disease, n (%) | 6036 (4.8) | 5017 (4.8) | 1019 (4.8) | 1.000 |
| Dementia, n (%) | 23,789 (19.1) | 17,283 (16.7) | 6506 (30.9) | <0.001 |
| Diabetes mellitus, n (%) | 18,166 (14.6) | 14,588 (14.1) | 3578 (17.0) | <0.001 |
| Liver disease, n (%) | 1232 (1.0) | 985 (1.0) | 247 (1.2) | 0.003 |
| Hemiplegia, n (%) | 2715 (2.2) | 2302 (2.2) | 413 (2.0) | 0.021 |
| Chronic kidney disease, n (%) | 5774 (4.6) | 3920 (3.8) | 1854 (8.8) | <0.001 |
| Local tumor, n (%) | 13,108 (10.5) | 10,037 (9.7) | 3071 (14.6) | <0.001 |
| Metastatic carcinoma, n (%) | 2498 (2.0) | 1357 (1.3) | 1141 (5.4) | <0.001 |
| Peptic ulcer disease, n (%) | 3918 (3.1) | 3036 (2.9) | 882 (4.2) | <0.001 |
| Type of fracture, n (%) | <0.001 | |||
| Non-displaced cervical (Garden 1–2) | 16,840 (13.5) | 14,138 (13.6) | 2702 (12.8) | |
| Displaced cervical (Garden 3–4) | 46,248 (37.1) | 38,824 (37.5) | 7424 (35.3) | |
| Basicervical | 4126 (3.3) | 3337 (3.2) | 789 (3.7) | |
| Peritrochanteric (two fragments) | 24,775 (19.9) | 20,314 (19.6) | 4461 (21.2) | |
| Peritrochanteric (multiple fragments) | 22,487 (18.0) | 18,502 (17.8) | 3985 (18.9) | |
| Subtrochanteric | 10,178 (8.2) | 8501 (8.2) | 1677 (8.0) | |
| Missing | 53 (0.0) | 46 (0.0) | 7 (0.0) | |
| Type of surgery, n (%) | <0.001 | |||
| Pins or screws | 21,849 (17.5) | 18,026 (17.4) | 3823 (18.2) | |
| Screws or pins with sideplate | 32,146 (25.8) | 26,221 (25.3) | 5925 (28.2) | |
| Intramedullary nail | 29,496 (23.7) | 24,461 (23.6) | 5035 (23.9) | |
| Hemiarthroplasty | 31,473 (25.2) | 25,729 (24.8) | 5744 (27.3) | |
| Total hip replacement | 9676 (7.8) | 9170 (8.8) | 506 (2.4) | |
| Missing | 67 (0.1) | 55 (0.1) | 12 (0.1) |
| Model | Training | Test | ||||
|---|---|---|---|---|---|---|
| Specificity | Sensitivity | AUC (95% CI) | Specificity | Sensitivity | AUC (95% CI) | |
| LR | 0.64 | 0.72 | 0.74 (0.73–0.74) | 0.62 | 0.75 | 0.74 (0.74–0.75) |
| LR (including only the top ten variables) | 0.65 | 0.73 | 0.73 (0.73–0.75) | 0.61 | 0.74 | 0.74 (0.74–0.75) |
| NB | 0.62 | 0.67 | 0.69 (0.69–0.70) | 0.63 | 0.67 | 0.70 (0.69–0.70) |
| SVM | 0.62 | 0.74 | 0.74 (0.73–0.74) | 0.61 | 0.70 | 0.72 (0.72–0.73) |
| RF | 0.57 | 0.73 | 0.71 (0.70–0.71) | 0.66 | 0.67 | 0.72 (0.71–0.72) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forssten, M.P.; Bass, G.A.; Ismail, A.M.; Mohseni, S.; Cao, Y. Predicting 1-Year Mortality after Hip Fracture Surgery: An Evaluation of Multiple Machine Learning Approaches. J. Pers. Med. 2021, 11, 727. https://doi.org/10.3390/jpm11080727
Forssten MP, Bass GA, Ismail AM, Mohseni S, Cao Y. Predicting 1-Year Mortality after Hip Fracture Surgery: An Evaluation of Multiple Machine Learning Approaches. Journal of Personalized Medicine. 2021; 11(8):727. https://doi.org/10.3390/jpm11080727
Chicago/Turabian StyleForssten, Maximilian Peter, Gary Alan Bass, Ahmad Mohammad Ismail, Shahin Mohseni, and Yang Cao. 2021. "Predicting 1-Year Mortality after Hip Fracture Surgery: An Evaluation of Multiple Machine Learning Approaches" Journal of Personalized Medicine 11, no. 8: 727. https://doi.org/10.3390/jpm11080727
APA StyleForssten, M. P., Bass, G. A., Ismail, A. M., Mohseni, S., & Cao, Y. (2021). Predicting 1-Year Mortality after Hip Fracture Surgery: An Evaluation of Multiple Machine Learning Approaches. Journal of Personalized Medicine, 11(8), 727. https://doi.org/10.3390/jpm11080727






