Acceptability, Appropriateness, and Feasibility of Automated Screening Approaches and Family Communication Methods for Identification of Familial Hypercholesterolemia: Stakeholder Engagement Results from the IMPACT-FH Study
Abstract
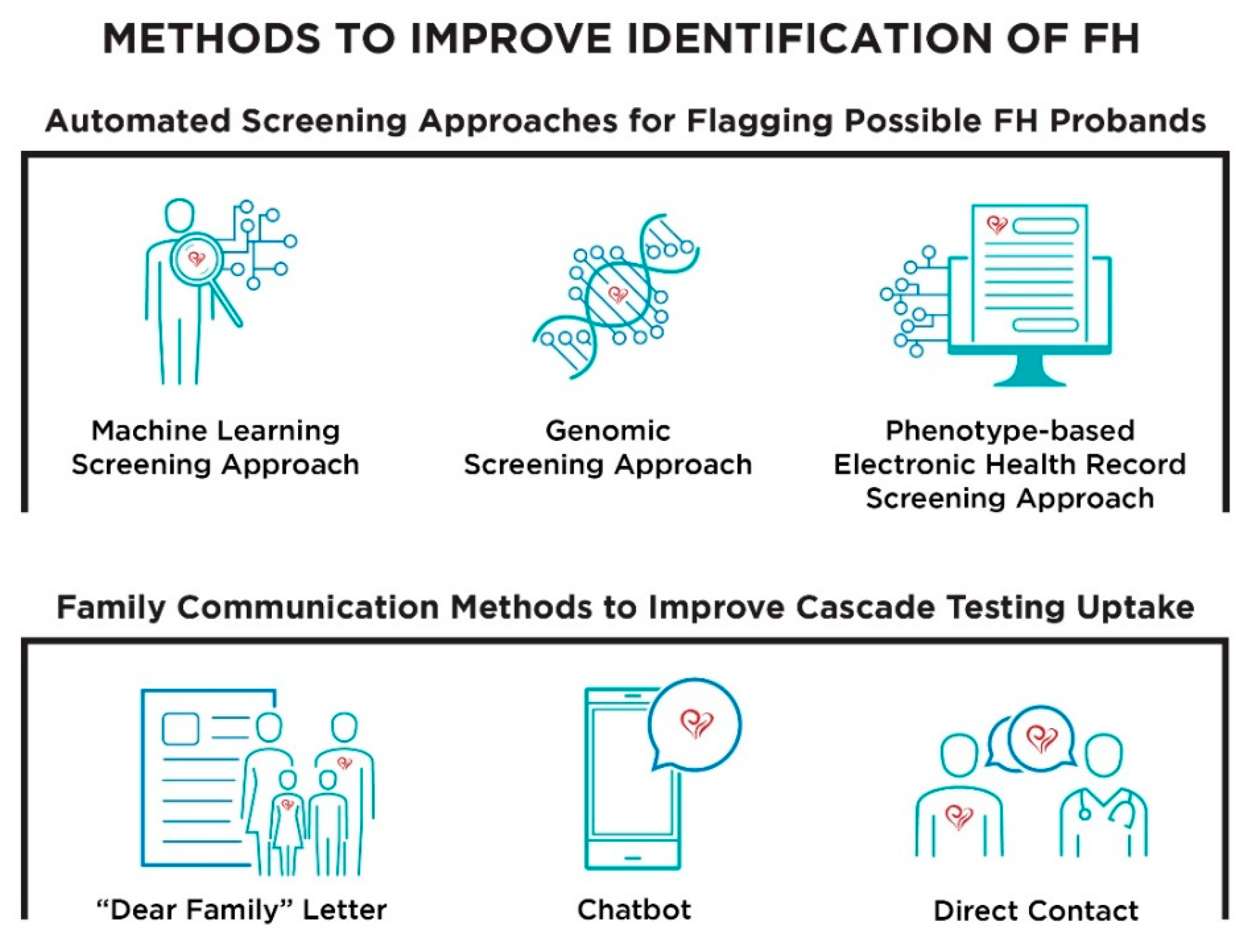
:1. Introduction
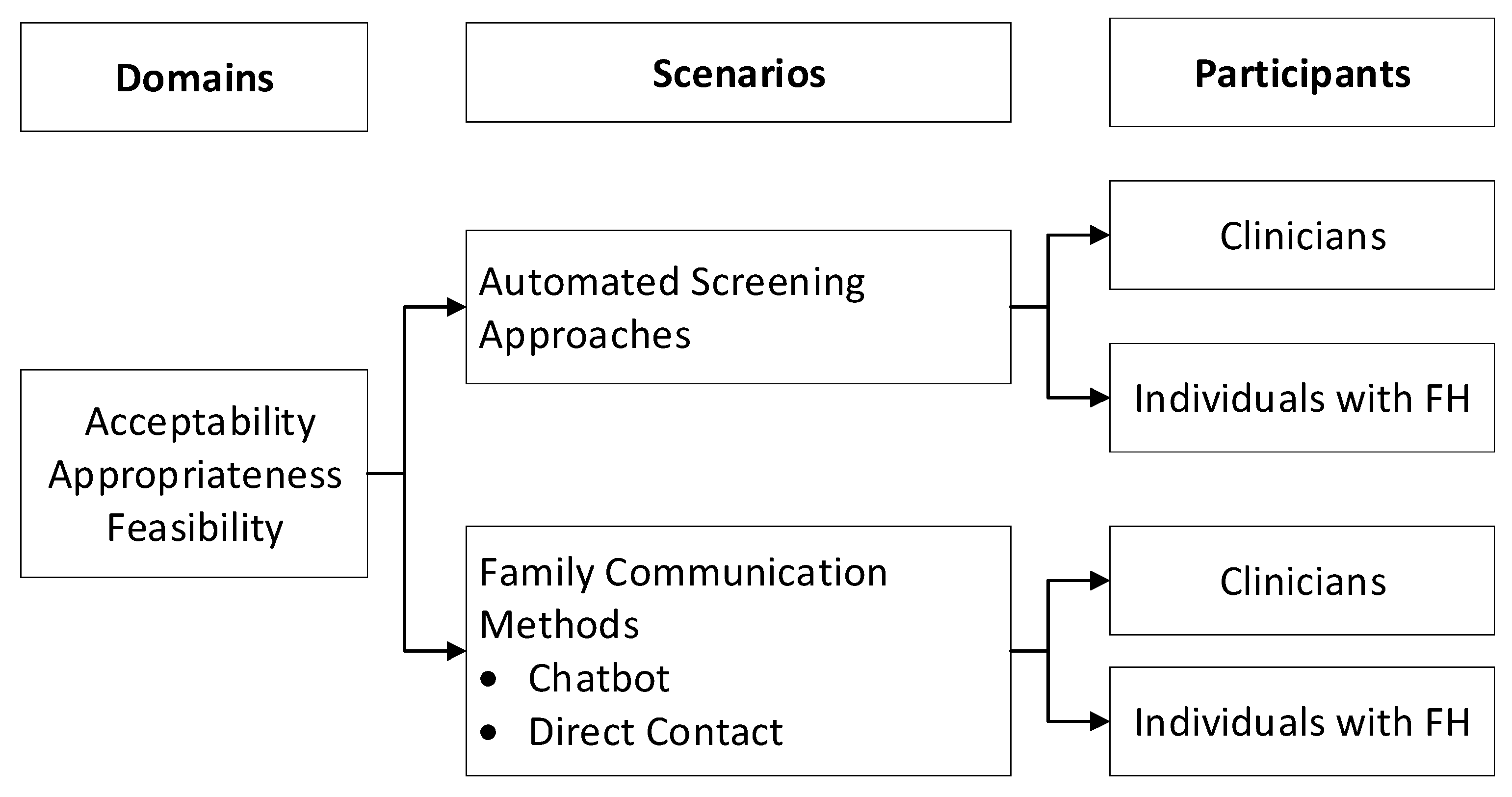
2. Materials and Methods
2.1. Study Population and Recruitment
2.2. Focus Group Procedures
2.3. Data Analysis
3. Results
3.1. Demographics
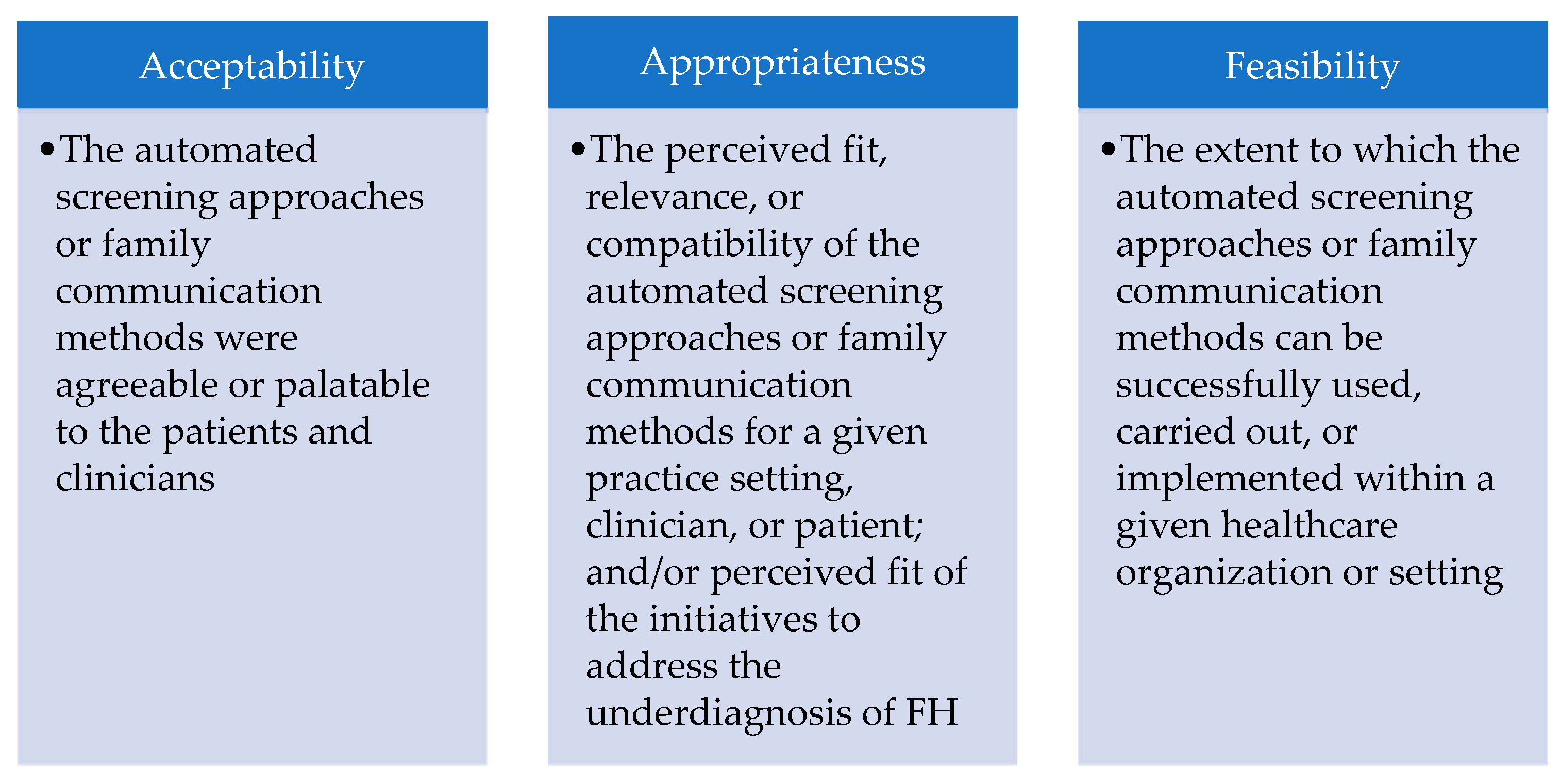
3.2. Acceptability of Automated Screening Approaches and Family Communication Methods
3.2.1. General Acceptability
3.2.2. Acceptability Specific to Automated Screening Approaches
3.2.3. Acceptability Specific to Family Communication Methods
3.3. Appropriateness of Automated Screening Approaches and Family Communication Methods
3.3.1. General Appropriateness
3.3.2. Appropriateness Specific to Automated Screening Approaches
3.3.3. Appropriateness Specific to Family Communication Methods
3.4. Feasibility of Automated Screening Approaches and Family Communication Methods
3.4.1. General Feasibility
3.4.2. Feasibility Specific to Automated Screening Approaches
3.4.3. Feasibility Specific to Family Communication Methods
3.5. Perceived Obstacles to Implementation of Automated Screening Approaches and Family Communication Methods
3.5.1. Perceived Obstacles Specific to Automated Screening Approaches
3.5.2. Perceived Obstacles Specific to Family Communication Methods
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 139, e1082–e1143. [Google Scholar] [CrossRef]
- Goldberg, A.C.; Hopkins, P.N.; Toth, P.P.; Ballantyne, C.M.; Rader, D.J.; Robinson, J.G.; Daniels, S.R.; Gidding, S.S.; de Ferranti, S.D.; Ito, M.K.; et al. Familial hypercholesterolemia: Screening, diagnosis and management of pediatric and adult patients: Clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J. Clin. Lipidol. 2011, 5, S1–S8. [Google Scholar] [CrossRef]
- Buchanan, A.H.; Kirchner, H.L.; Schwartz, M.L.B.; Kelly, M.A.; Schmidlen, T.; Jones, L.K.; Hallquist, M.L.G.; Rocha, H.; Betts, M.; Schwiter, R.; et al. Clinical outcomes of a genomic screening program for actionable genetic conditions. Genet. Med. 2020, 22, 1874–1882. [Google Scholar] [CrossRef] [PubMed]
- Knowles, J.W.; O’Brien, E.C.; Greendale, K.; Wilemon, K.; Genest, J.; Sperling, L.S.; Neal, W.A.; Rader, D.J.; Khoury, M.J. Reducing the burden of disease and death from familial hypercholesterolemia: A call to action. Am. Heart J. 2014, 168, 807–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmerman, J.; Duprez, D.; Veach, P.M.; Zierhut, H.A. Barriers to the identification of familial hypercholesterolemia among primary care providers. J. Community Genet. 2019, 10, 229–236. [Google Scholar] [CrossRef]
- Alonso, R.; de Isla, L.P.; Muñiz-Grijalvo, O.; Mata, P. Barriers to Early Diagnosis and Treatment of Familial Hypercholesterolemia: Current Perspectives on Improving Patient Care. Vasc. Health Risk Manag. 2020, 16, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, S.; Reeskamp, L.F.; Stroes, E.S.G.; Watts, G.F. Advances, gaps and opportunities in the detection of familial hypercholesterolemia: Overview of current and future screening and detection methods. Curr. Opin. Lipidol. 2020, 31, 347–355. [Google Scholar] [CrossRef]
- Foody, J.M. Familial hypercholesterolemia: An under-recognized but significant concern in cardiology practice. Clin. Cardiol. 2014, 37, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Safarova, M.S.; Liu, H.; Kullo, I.J. Rapid identification of familial hypercholesterolemia from electronic health records: The SEARCH study. J. Clin. Lipidol. 2016, 10, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Myers, K.D.; Knowles, J.W.; Staszak, D.; Shapiro, M.D.; Howard, W.; Yadava, M.; Zuzick, D.; Williamson, L.; Shah, N.H.; Banda, J.M.; et al. Precision screening for familial hypercholesterolaemia: A machine learning study applied to electronic health encounter data. Lancet Digit. Health 2019, 1, e393–e402. [Google Scholar] [CrossRef] [Green Version]
- Schmidlen, T.; Schwartz, M.; DiLoreto, K.; Kirchner, H.L.; Sturm, A.C. Patient assessment of chatbots for the scalable delivery of genetic counseling. J. Genet. Couns. 2019, 28, 1166–1177. [Google Scholar] [CrossRef] [PubMed]
- Schwiter, R.; Brown, E.; Murray, B.; Kindt, I.; Van Enkevort, E.; Pollin, T.I.; Sturm, A.C. Perspectives from individuals with familial hypercholesterolemia on direct contact in cascade screening. J. Genet. Couns. 2020, 29, 1142–1150. [Google Scholar] [CrossRef]
- Schwiter, R.; Rahm, A.K.; Williams, J.L.; Sturm, A.C. How Can We Reach At-Risk Relatives? Efforts to Enhance Communication and Cascade Testing Uptake: A Mini-Review. Curr. Genet. Med. Rep. 2018, 6, 21–27. [Google Scholar] [CrossRef]
- Sturm, A.C. Cardiovascular Cascade Genetic Testing: Exploring the Role of Direct Contact and Technology. Front. Cardiovasc. Med. 2016, 3, 11. [Google Scholar] [CrossRef] [Green Version]
- Kavakiotis, I.; Tsave, O.; Salifoglou, A.; Maglaveras, N.; Vlahavas, I.; Chouvarda, I. Machine Learning and Data Mining Methods in Diabetes Research. Comput. Struct. Biotechnol. J. 2017, 15, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Rahimian, F.; Salimi-Khorshidi, G.; Payberah, A.H.; Tran, J.; Solares, R.A.; Raimondi, F.; Nazarzadeh, M.; Canoy, D.; Rahimi, K. Predicting the risk of emergency admission with machine learning: Development and validation using linked electronic health records. PLoS Med. 2018, 15, e1002695. [Google Scholar] [CrossRef] [PubMed]
- Petrov, I.; Postadzhiyan, A.; Vasilev, D.; Kasabov, R.; Tokmakova, M.; Nikolov, F.; Istatkov, V.; Zhao, B.; Mutafchiev, D.; Petkova, R. Familial Hypercholesterolemia Identification Algorithm in Patients with Acute Cardiovascular Events in A Large Hospital Electronic Database in Bulgaria: A Call for Implementation. Adv. Ther. 2021. [Google Scholar] [CrossRef]
- Banda, J.M.; Sarraju, A.; Abbasi, F.; Parizo, J.; Pariani, M.; Ison, H.; Briskin, E.; Wand, H.; Dubois, S.; Jung, K. Finding missed cases of familial hypercholesterolemia in health systems using machine learning. NPJ Digit. Med. 2019, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Paragh, G.; Harangi, M.; Karányi, Z.; Daróczy, B.; Németh, Á.; Fülöp, P. Identifying patients with familial hypercholesterolemia using data mining methods in the Northern Great Plain region of Hungary. Atherosclerosis 2018, 277, 262–266. [Google Scholar] [CrossRef] [Green Version]
- Pina, A.; Helgadottir, S.; Mancina, R.M.; Pavanello, C.; Pirazzi, C.; Montalcini, T.; Henriques, R.; Calabresi, L.; Wiklund, O.; Macedo, M.P.; et al. Virtual genetic diagnosis for familial hypercholesterolemia powered by machine learning. Eur. J. Prev. Cardiol. 2020, 27, 1639–1646. [Google Scholar] [CrossRef]
- Abul-Husn, N.; Manickam, K.; Jones, L.K.; Wright, E.A.; Hartzel, D.N.; Gonzaga-Jauregui, C.; O’Dushlaine, C.; Leader, J.B.; Kirchner, H.L.; Lindbuchler, D.A.; et al. Genetic identification of familial hypercholesterolemia within a single U.S. health care system. Science 2016, 354. [Google Scholar] [CrossRef] [PubMed]
- Gianfrancesco, M.A.; Tamang, S.; Yazdany, J.; Schmajuk, G. Potential Biases in Machine Learning Algorithms Using Electronic Health Record Data. JAMA Intern. Med. 2018, 178, 1544–1547. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Rivera-Valerio, M.; Bangash, H.; Prokop, L.; Kullo, I.J. New Case Detection by Cascade Testing in Familial Hypercholesterolemia: A Systematic Review of the Literature. Circ. Genom. Precis. Med. 2019, 12, e002723. [Google Scholar] [CrossRef]
- Umans-Eckenhausen, M.A.; Defesche, J.C.; Sijbrands, E.J.; Scheerder, R.L.; Kastelein, J.J. Review of first 5 years of screening for familial hypercholesterolaemia in the Netherlands. Lancet 2001, 357, 165–168. [Google Scholar] [CrossRef]
- Chaix, B.; Bibault, J.E.; Pienkowski, A.; Delamon, G.; Guillemassé, A.; Nectoux, P.; Brouard, B. When Chatbots Meet Patients: One-Year Prospective Study of Conversations Between Patients With Breast Cancer and a Chatbot. JMIR Cancer 2019, 5, e12856. [Google Scholar] [CrossRef]
- Pereira, J.; Díaz, Ó. Using Health Chatbots for Behavior Change: A Mapping Study. J. Med. Syst. 2019, 43, 135. [Google Scholar] [CrossRef] [PubMed]
- Pearson, N.; Naylor, P.-J.; Ashe, M.C.; Fernandez, M.; Yoong, S.L.; Wolfenden, L. Guidance for conducting feasibility and pilot studies for implementation trials. Pilot Feasibility Stud. 2020, 6, 167. [Google Scholar] [CrossRef]
- Campbell-Salome, G.; Jones, L.K.; Masnick, M.F.; Walton, N.A.; Ahmed, C.D.; Buchanan, A.H.; Brangan, A.; Esplin, E.D.; Kann, D.G.; Ladd, I.G.; et al. Developing and Optimizing Innovative Tools to Address Familial Hypercholesterolemia Underdiagnosis: Identification Methods, Patient Activation, and Cascade Testing for Familial Hypercholesterolemia (IMPACT-FH). Circ. Genom. Precis. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Carey, D.J.; Fetterolf, S.N.; Davis, F.D.; Faucett, W.A.; Kirchner, H.L.; Mirshahi, U.; Murray, M.F.; Smelser, D.T.; Gerhard, G.S.; Ledbetter, D.H. The Geisinger MyCode community health initiative: An electronic health record-linked biobank for precision medicine research. Genet. Med. 2016, 18, 906–913. [Google Scholar] [CrossRef] [Green Version]
- Proctor, E.; Silmere, H.; Raghavan, R.; Hovmand, P.; Aarons, G.; Bunger, A.; Griffey, R.; Hensley, M. Outcomes for implementation research: Conceptual distinctions, measurement challenges, and research agenda. Adm. Policy Ment. Health 2011, 38, 65–76. [Google Scholar] [CrossRef] [Green Version]
- Proctor, E.K.; Landsverk, J.; Aarons, G.; Chambers, D.; Glisson, C.; Mittman, B. Implementation research in mental health services: An emerging science with conceptual, methodological, and training challenges. Adm. Policy Ment. Health 2009, 36, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Gale, N.K.; Heath, G.; Cameron, E.; Rashid, S.; Redwood, S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med. Res. Methodol. 2013, 13, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patton, M.Q. Qualitative Evaluation and Research Methods; Sage Publications: Thousand Oaks, CA, USA, 1990. [Google Scholar]
- Strauss, A.; Corbin, J. Basics of Qualitative Research Techniques; Sage Publications: Thousand Oaks, CA, USA, 1998. [Google Scholar]
- Besseling, J.; Reitsma, J.B.; Gaudet, D.; Brisson, D.; Kastelein, J.J.; Hovingh, G.K.; Hutten, B.A. Selection of individuals for genetic testing for familial hypercholesterolaemia: Development and external validation of a prediction model for the presence of a mutation causing familial hypercholesterolaemia. Eur. Heart J. 2017, 38, 565–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatnagar, D.; Morgan, J.; Siddiq, S.; Mackness, M.I.; Miller, J.P.; Durrington, P.N. Outcome of case finding among relatives of patients with known heterozygous familial hypercholesterolaemia. BMJ 2000, 321, 1497–1500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGowan, M.P.; Cuchel, M.; Ahmed, C.D.; Khera, A.; Weintraub, W.S.; Wilemon, K.A.; Ahmad, Z. A proof-of-concept study of cascade screening for Familial Hypercholesterolemia in the US, adapted from the Dutch model. Am. J. Prev. Cardiol. 2021, 6, 100170. [Google Scholar] [CrossRef]
- Srinivasan, S.; Won, N.Y.; Dotson, W.D.; Wright, S.T.; Roberts, M.C. Barriers and facilitators for cascade testing in genetic conditions: A systematic review. Eur. J. Hum. Genet. 2020, 28, 1631–1644. [Google Scholar] [CrossRef]
- Neuner, J.; Dimmock, D.; Kirschner, A.L.P.; Beaudry, H.; Paradowski, J.; Orlando, L. Results and Lessons of a Pilot Study of Cascade Screening for Familial Hypercholesterolemia in US Primary Care Practices. J. Gen. Intern. Med. 2020, 35, 351–353. [Google Scholar] [CrossRef]
- Allison, M. Communicating risk with relatives in a familial hypercholesterolemia cascade screening program: A summary of the evidence. J. Cardiovasc. Nurs. 2015, 30, E1–E12. [Google Scholar] [CrossRef]
- Maxwell, S.J.; Molster, C.M.; Poke, S.J.; O’Leary, P. Communicating familial hypercholesterolemia genetic information within families. Genet. Test. Mol. Biomark. 2009, 13, 301–306. [Google Scholar] [CrossRef]
- Newson, A.J.; Humphries, S.E. Cascade testing in familial hypercholesterolaemia: How should family members be contacted? Eur. J. Hum. Genet. 2005, 13, 401–408. [Google Scholar] [CrossRef]
- Roberts, M.C.; Dotson, W.D.; DeVore, C.S.; Bednar, E.M.; Bowen, D.J.; Ganiats, T.G.; Green, R.F.; Hurst, G.M.; Philp, A.R.; Ricker, C.N.; et al. Delivery of Cascade Screening For Hereditary Conditions: A Scoping Review of the Literature. Health Aff. 2018, 37, 801–808. [Google Scholar] [CrossRef]
- Qureshi, N.; Weng, S.; Tranter, J.; El-Kadiki, A.; Kai, J. Feasibility of improving identification of familial hypercholesterolaemia in general practice: Intervention development study. BMJ Open 2016, 6, e011734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, L.K.; Tilberry, S.; Gregor, C.; Yaeger, L.H.; Hu, Y.; Sturm, A.C.; Seaton, T.L.; Waltz, T.J.; Rahm, A.K.; Goldberg, A.; et al. Implementation strategies to improve statin utilization in individuals with hypercholesterolemia: A systematic review and meta-analysis. Implement. Sci. 2021, 16, 40. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.C.; Kennedy, A.E.; Chambers, D.A.; Khoury, M.J. The current state of implementation science in genomic medicine: Opportunities for improvement. Genet. Med. 2017, 19, 858–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Focus Group | Stakeholder | Sample Representation | Invited | Participated |
|---|---|---|---|---|
| 1 | Individuals with FH | FH Foundation Advocates | 18 | 15 |
| 2 | Individuals with FH | Healthcare system | 66 | 7 |
| 3 | Clinician | Clinical lipid specialists | 28 | 9 |
| 4 | Clinician | Healthcare system | 203 | 5 |
| 5 | Clinician | Primary care practice | 7 | 6 |
| Demographics | Value | |
|---|---|---|
| Individuals with FH | 22 | |
| Female, n (%) | 19 (86) | |
| White, n (%) | 19 (86) | |
| Age range, years, n (%) | ||
| 28–34 | 6 (27) | |
| 35–54 | 7 (32) | |
| 55 or older | 9 (41) | |
| Higher educational obtainment, n (%) | ||
| Some college | 4 (18) | |
| College graduate | 12 (55) | |
| Post-graduate training | 6 (27) | |
| Health insurance status, yes, n (%) | 22 (100) | |
| Clinicians | 20 | |
| Type, n (%) | ||
| Physician | 15 (75) | |
| Advanced care providers (nurse practitioners, physician assistants, and pharmacists) | 5 (25) | |
| Female, n (%) | 7 (35) | |
| Race, n (%) * | ||
| White | 14 (74) | |
| Asian | 5 (26) | |
| Age range, years, n (%) | ||
| 18–34 | 2 (10) | |
| 35–54 | 6 (30) | |
| 55 or older | 12 (60) | |
| 20 or more years of experience, n (%) | 12 (60) | |
| Practice type, n (%) | ||
| Primary care | 13 (65) | |
| Cardiology | 4 (20) | |
| Others | 3 (15) | |
| Domain | Summary of Key Points | Exemplar Quotes |
|---|---|---|
| General |
| “[current standard] is one step better than asking the mortician to diagnose [FH]” (Clinician, FG3) “Ah, I don’t think [underdiagnosis of FH] is a lack of interest, but I think there is a lack of understanding of significance. (Clinician, FG3) “What we have missed the boat [on] is not diagnosing them with FH where we could impact their family members.” (Clinician, FG5) |
| Automated approaches |
| “Seems as though using this algorithm that will, it should help the doctors who aren’t specialists to call some attention to the possibility of FH. Because, when I think in my situation, yes, for years, I heard or every now and then that I had high cholesterol, but my primary care physician never knew I had it, was beating me up, saying I was eating the wrong things, only to find out later, after having an event, that the lipidologist said “Oh my gosh, you have FH.”” (Individual with FH, FG1) “I think in hindsight, have been very helpful in our journey would have been if I, if somehow this algorithm flagged me, or, and then they automatically got flagged so that they got their screening at age 2 instead of this whacky way that we went about it and all of a sudden we had it. Um, and that it would, it would encourage pediatricians to follow the guideline, oh “potential FH risk, blood test now” versus this, like, weird thing.” (Individual with FH, FG1) “If there are tools to help me make that decision, that would be extremely welcomed…I can just treat it as elevated LDL and need to get it under 100 and so on.” (Clinician, FG5) “…I would like to be aware of it, but actually maybe, you know, it could be done and get the patients interested and willing to come in and talk about it without me having to even acknowledge it, it’s fine with me.” (Clinician, FG5) |
| Family communication methods |
| “I think [chatbots are] a great idea. Because you can choose to send it, you can choose to open it. It’s another tool.” (Individual with FH, FG1) “…[Family communication is] really I think individually based, but knowing as a patient, knowing the options and just knowing what choices you have may be the best option.” (Individual with FH, FG2) “I think most of us would be more than willing to have a family meeting if they wanna have people in. … If they wanted to, I certainly would. Whether it was by, you know, phone, or if they wanted to come in and bring family members with them.” (Clinician, FG4) |
| Domain | Summary of Key Points | Exemplar Quotes |
|---|---|---|
| General |
| “They would realize that, and then the children, the relatives then should be recommended, and maybe that would be a part of this algorithm as well, is red flagging relatives that maybe haven’t gotten a–a lipid panel done, so when they go in for their next physical or so, to let the doctor know, ‘Hey this person has family risk of, uh, high cholesterol. Recommend a lipid panel to them.’” (Individual with FH, FG1) “I mean, I think you have a big advantage in that you’re basically asking people to get a blood test. Colonoscopies are, are a much harder thing to ask someone to do, a much bigger pitch. So, it…it’s something that most people have done, you know, yearly after a certain age and if they haven’t had it done for a couple years because they are younger and healthy, it’s generally not a big ask.” (Clinician, FG4) |
| Automated approaches |
| “I probably think it would make a lot of sense to use an algorithm because if it flagged it as FH specifically instead of just high cholesterol. If it was just high cholesterol, they would treat the patient as an individual. If it’s FH, they would treat the family.” (Individual with FH, FG1) “Build the algorithm into [electronic health record], so that when we are ready to mistakenly just click on dyslipidemia or elevated cholesterol, it will guide us to the correct… ‘Have you considered?’ and it will pop up. ‘Have you considered familial hypercholesterolemia?’” (Clinician, FG5) |
| Family communication methods |
| “I would like the opportunity to speak with my family first and then if I find some reluctancy from my family, then getting a, um, a doctor that is involved, but I would prefer it to be a lipidologist, because I feel–or a cardiologist–some type of specialty or genetic counselor, to put–I think that puts the fire under somebody’s butt.” (Individual with FH, FG1) “… I have a teenage sister, and she would not talk to somebody on the phone. She would absolutely text a bot over talking to somebody.” (Individual with FH, FG2) “If it’s just one or two family members then I would offer the patient that I would call them and explain that this is what they have and this is what they should do, but if it’s a big family then, then I would…if they wanna come in and have a family meeting or something then I would be willing to do that. But I’m not going to call, like, 20 different family members and explain them individually that this is something you’ve gotta do and this is something you need to be tested for.” (Clinician, FG4) |
| Domain | Summary of Key Points | Exemplar Quotes |
|---|---|---|
| General |
| “When I’ve had an unusual breast exam and colon... I got a letter, a phone call, and a letter. But they weren’t from my doctor. They were from where I went, clinic or whatever, to get the exam. And they said something very professional, ‘Your, uh, reading was abnormal. We’d like for you to schedule an appointment to come back in.’” (Individual with FH, FG1) “…we’re already doing [automated approaches] as [another participant] said with…with colon cancer.” (Clinician, FG4) “This sounds like an opportunity to educate the physicians … So, I guess this is an opportunity you can teach us and I guess help us reach other people that may not have access to or may not be involved with a physician.” (Clinician, FG5) |
| Automated approaches |
| “So, I say do it all. Send it by mail. Give them a call. Send an email because it’s just, each person receives it differently.” (Individual with FH, FG2) “We really should be approaching this from a team-based approach instead of trying to funnel every piece of information and every decision through the primary care physician. I mean, if you want to do that, you can go hang your shingle up down the street. But the whole idea of having a team-based approach is that we are improving the quality of care. At the same time, we’re letting our providers get home in time to have dinner with their family.” (Clinician, FG4) |
| Family communication methods |
| “Linking [the chatbot] to your [patient portal] I think would be…because that’s how I communicate with my doctors is through [the patient portal], so having that information in there I think would be beneficial.” (Individual with FH, FG2) “So, with the permission of the family member or the patient, to speak to others I mean, I don’t have any hesitation trying to, you know, convince them to go and get tested. So, um, if it was, you know, like the index patient, my patient had hesitation about giving me information or sharing anything with anyone else, that might be a different story.” (Clinician, FG5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, L.K.; Walters, N.; Brangan, A.; Ahmed, C.D.; Gatusky, M.; Campbell-Salome, G.; Ladd, I.G.; Sheldon, A.; Gidding, S.S.; McGowan, M.P.; et al. Acceptability, Appropriateness, and Feasibility of Automated Screening Approaches and Family Communication Methods for Identification of Familial Hypercholesterolemia: Stakeholder Engagement Results from the IMPACT-FH Study. J. Pers. Med. 2021, 11, 587. https://doi.org/10.3390/jpm11060587
Jones LK, Walters N, Brangan A, Ahmed CD, Gatusky M, Campbell-Salome G, Ladd IG, Sheldon A, Gidding SS, McGowan MP, et al. Acceptability, Appropriateness, and Feasibility of Automated Screening Approaches and Family Communication Methods for Identification of Familial Hypercholesterolemia: Stakeholder Engagement Results from the IMPACT-FH Study. Journal of Personalized Medicine. 2021; 11(6):587. https://doi.org/10.3390/jpm11060587
Chicago/Turabian StyleJones, Laney K., Nicole Walters, Andrew Brangan, Catherine D. Ahmed, Michael Gatusky, Gemme Campbell-Salome, Ilene G. Ladd, Amanda Sheldon, Samuel S. Gidding, Mary P. McGowan, and et al. 2021. "Acceptability, Appropriateness, and Feasibility of Automated Screening Approaches and Family Communication Methods for Identification of Familial Hypercholesterolemia: Stakeholder Engagement Results from the IMPACT-FH Study" Journal of Personalized Medicine 11, no. 6: 587. https://doi.org/10.3390/jpm11060587
APA StyleJones, L. K., Walters, N., Brangan, A., Ahmed, C. D., Gatusky, M., Campbell-Salome, G., Ladd, I. G., Sheldon, A., Gidding, S. S., McGowan, M. P., Rahm, A. K., & Sturm, A. C. (2021). Acceptability, Appropriateness, and Feasibility of Automated Screening Approaches and Family Communication Methods for Identification of Familial Hypercholesterolemia: Stakeholder Engagement Results from the IMPACT-FH Study. Journal of Personalized Medicine, 11(6), 587. https://doi.org/10.3390/jpm11060587






