Mandible Segmentation of Dental CBCT Scans Affected by Metal Artifacts Using Coarse-to-Fine Learning Model
Abstract
1. Introduction
- First, we apply the concept of curriculum learning to split the mandible segmentation into two sub-tasks. We extract the mandible-like organ using a 3D Unet in the coarse stage and then apply the mandible-like organ into the recurrent segmentation network in the fine stage. In comparison with other CNN approaches, the proposed segmentation approach is robust against metal artifacts.
- Second, the proposed model achieves promising performance on the dataset of CBCT scans of dental braces. Furthermore, the proposed model achieves a promising performance on the conventional CT dataset and Public Domain Database of the Computational Anatomy (PDDCA) dataset.
2. Methodology
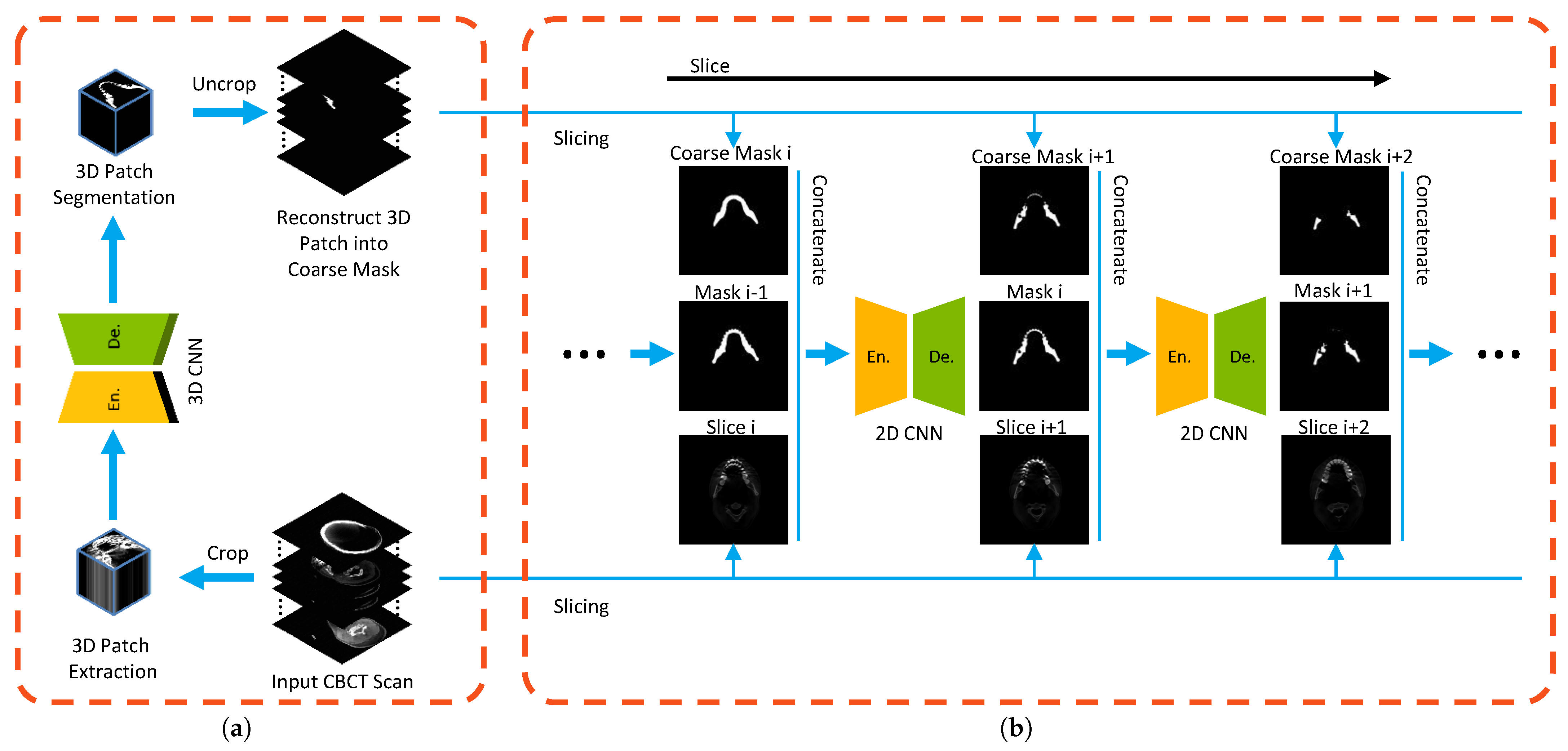
2.1. Curriculum Learning in Mandible Segmentation
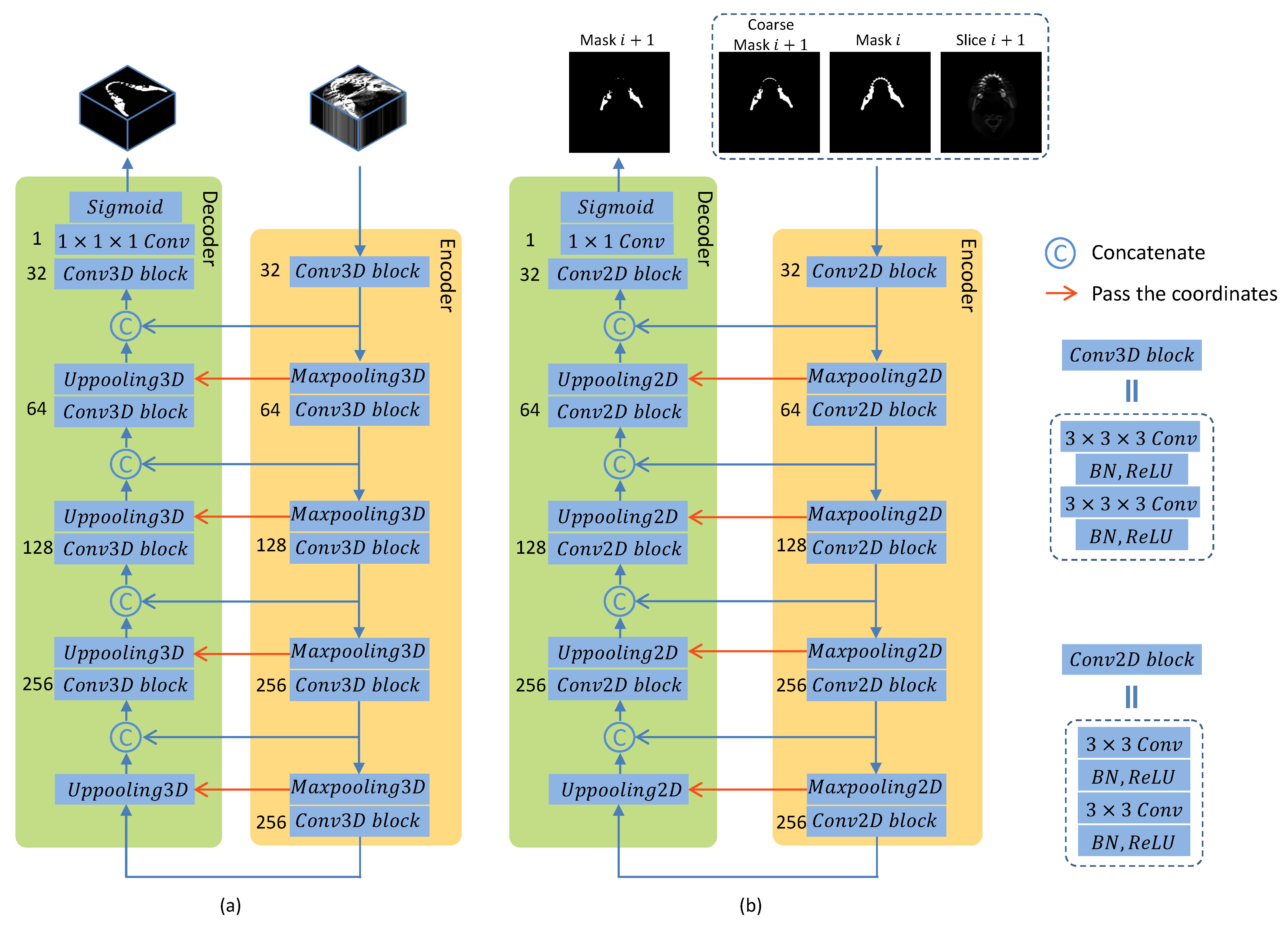
2.2. Coarse Stage: Mandible-Like Organ Prediction
2.3. Fine Stage: False Positive Reduction
2.4. Loss
2.5. Evaluation Metrics
3. Experiments
3.1. Datasets
3.1.1. CBCT Dataset
3.1.2. CT Dataset
3.2. Implementation Details
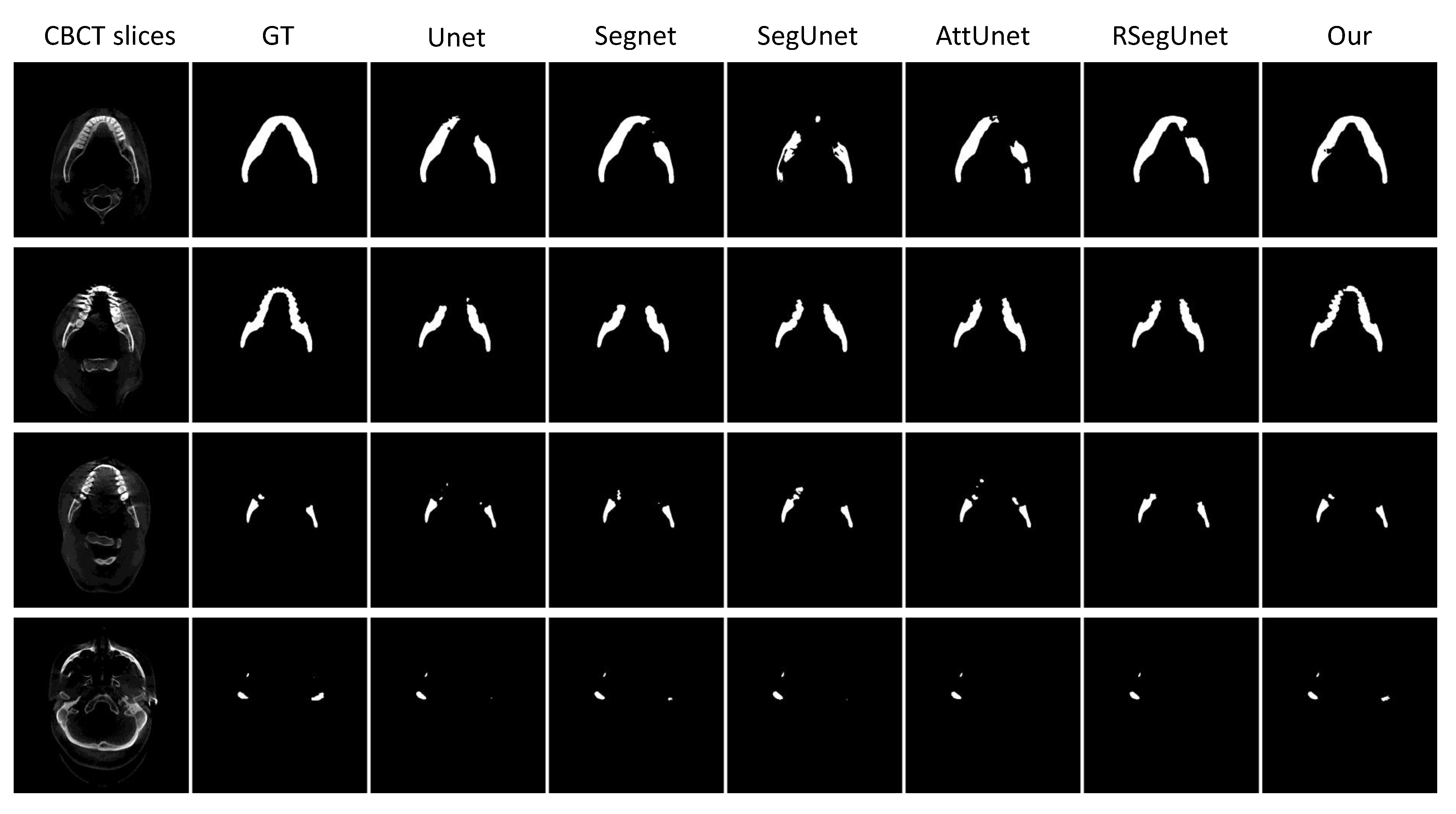
3.3. Results
3.3.1. Experiments on the CBCT Dataset
3.3.2. Experiments on the CT Dataset
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kraeima, J. Three Dimensional Virtual Surgical Planning for Patient Specific Osteosynthesis and Devices in Oral and Maxillofacial Surgery. A New Era. Ph.D. Thesis, University of Groningen, Groningen, The Netherlands, 2019. [Google Scholar]
- Gollmer, S.T.; Buzug, T.M. Fully automatic shape constrained mandible segmentation from cone-beam CT data. In Proceedings of the 2012 9th IEEE International Symposium on Biomedical Imaging (ISBI), Barcelona, Spain, 2–5 May 2012; pp. 1272–1275. [Google Scholar]
- Wang, L.; Gao, Y.; Shi, F.; Li, G.; Chen, K.C.; Tang, Z.; Xia, J.J.; Shen, D. Automated segmentation of dental CBCT image with prior-guided sequential random forests. Med. Phys. 2016, 43, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Indraswari, R.; Arifin, A.Z.; Suciati, N.; Astuti, E.R.; Kurita, T. Automatic segmentation of mandibular cortical bone on cone-beam CT images based on histogram thresholding and polynomial fitting. Int. J. Intell. Eng. Syst. 2019, 12, 130–141. [Google Scholar] [CrossRef]
- Linares, O.C.; Bianchi, J.; Raveli, D.; Neto, J.B.; Hamann, B. Mandible and skull segmentation in cone beam computed tomography using super-voxels and graph clustering. Vis. Comput. 2019, 35, 1461–1474. [Google Scholar]
- Fan, Y.; Beare, R.; Matthews, H.; Schneider, P.; Kilpatrick, N.; Clement, J.; Claes, P.; Penington, A.; Adamson, C. Marker-based watershed transform method for fully automatic mandibular segmentation from CBCT images. Dentomaxillofac. Radiol. 2019, 48, 20180261. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Guo, J.; Kraeima, J.; Glas, H.H.; Borra, R.J.; Witjes, M.J.; van Ooijen, P.M. Automatic segmentation of the mandible from computed tomography scans for 3D virtual surgical planning using the convolutional neural network. Phys. Med. Biol. 2019, 64, 175020. [Google Scholar] [CrossRef]
- Ibragimov, B.; Xing, L. Segmentation of organs-at-risks in head and neck CT images using convolutional neural networks. Med. Phys. 2017, 44, 547–557. [Google Scholar] [CrossRef]
- Zhu, W.; Huang, Y.; Tang, H.; Qian, Z.; Du, N.; Fan, W.; Xie, X. AnatomyNet: Deep 3D Squeeze-and-excitation U-Nets for fast and fully automated whole-volume anatomical segmentation. arXiv 2018, arXiv:1808.05238. [Google Scholar]
- Tong, N.; Gou, S.; Yang, S.; Ruan, D.; Sheng, K. Fully automatic multi-organ segmentation for head and neck cancer radiotherapy using shape representation model constrained fully convolutional neural networks. Med. Phys. 2018, 45, 4558–4567. [Google Scholar] [CrossRef]
- Qiu, B.; Guo, J.; Kraeima, J.; Glas, H.H.; Borra, R.J.; Witjes, M.J.; van Ooijen, P.M. Recurrent convolutional neural networks for mandible segmentation from computed tomography. arXiv 2020, arXiv:2003.06486. [Google Scholar]
- Bengio, Y.; Louradour, J.; Collobert, R.; Weston, J. Curriculum learning. In Proceedings of the 26th Annual International Conference on Machine Learning, Montreal, QC, Canada, 14–18 June 2009; pp. 41–48. [Google Scholar]
- Taghanaki, S.A.; Zheng, Y.; Zhou, S.K.; Georgescu, B.; Sharma, P.; Xu, D.; Comaniciu, D.; Hamarneh, G. Combo loss: Handling input and output imbalance in multi-organ segmentation. Comput. Med. Imaging Graph. 2019, 75, 24–33. [Google Scholar] [CrossRef]
- Milletari, F.; Navab, N.; Ahmadi, S.A. V-net: Fully convolutional neural networks for volumetric medical image segmentation. In Proceedings of the 2016 Fourth International Conference on 3D Vision (3DV), Stanford, CA, USA, 25–28 October 2016; pp. 565–571. [Google Scholar]
- Kamal, U.; Tonmoy, T.I.; Das, S.; Hasan, M.K. Automatic traffic sign detection and recognition using SegU-Net and a modified Tversky loss function with L1-constraint. IEEE Trans. Intell. Transp. Syst. 2019, 21, 1467–1479. [Google Scholar] [CrossRef]
- Ioffe, S.; Szegedy, C. Batch normalization: Accelerating deep network training by reducing internal covariate shift. arXiv 2015, arXiv:1502.03167. [Google Scholar]
- Nair, V.; Hinton, G.E. Rectified linear units improve restricted boltzmann machines. In Proceedings of the 27th International Conference on Machine Learning (ICML-10), Haifa, Israel, 21–24 June 2010; pp. 807–814. [Google Scholar]
- Ronneberger, O.; Fischer, P.; Brox, T. U-net: Convolutional networks for biomedical image segmentation. In Proceedings of the International Conference on Medical Image Computing and Computer-ASSISTED Intervention, Munich, Germany, 5–9 October 2015; Springer: Berlin/Heidelberg, Germany, 2015; pp. 234–241. [Google Scholar]
- Glorot, X.; Bengio, Y. Understanding the difficulty of training deep feedforward neural networks. In Proceedings of the Thirteenth International Conference on Artificial Intelligence and Statistics, JMLR Workshop and Conference Proceedings, Sardinia, Italy, 13–15 May 2010; pp. 249–256. [Google Scholar]
- Abulnaga, S.M.; Rubin, J. Ischemic stroke lesion segmentation in ct perfusion scans using pyramid pooling and focal loss. In Proceedings of the International MICCAI Brainlesion Workshop, Granada, Spain, 16 September 2018; Springer: Berlin/Heidelberg, Germany, 2018; pp. 352–363. [Google Scholar] [CrossRef]
- Sudre, C.H.; Li, W.; Vercauteren, T.; Ourselin, S.; Cardoso, M.J. Generalised dice overlap as a deep learning loss function for highly unbalanced segmentations. In Deep Learning in Medical Image Analysis and Multimodal Learning for Clinical Decision Support; Springer: Berlin/Heidelberg, Germany, 2017; pp. 240–248. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436. [Google Scholar] [CrossRef]
- Ghafoorian, M.; Karssemeijer, N.; Heskes, T.; Uden, I.W.; Sanchez, C.I.; Litjens, G.; Leeuw, F.E.; Ginneken, B.; Marchiori, E.; Platel, B. Location sensitive deep convolutional neural networks for segmentation of white matter hyperintensities. Sci. Rep. 2017, 7, 5110. [Google Scholar] [CrossRef] [PubMed]
- Huttenlocher, D.P.; Rucklidge, W.J.; Klanderman, G.A. Comparing images using the Hausdorff distance under translation. In Proceedings of the 1992 IEEE Computer Society Conference on Computer Vision and Pattern Recognition, Champaign, IL, USA, 15–18 June 1992; pp. 654–656. [Google Scholar] [CrossRef]
- Raudaschl, P.F.; Zaffino, P.; Sharp, G.C.; Spadea, M.F.; Chen, A.; Dawant, B.M.; Albrecht, T.; Gass, T.; Langguth, C.; Lüthi, M.; et al. Evaluation of segmentation methods on head and neck CT: Auto-segmentation challenge 2015. Med. Phys. 2017, 44, 2020–2036. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.A.; Hanbury, A. Metrics for evaluating 3D medical image segmentation: Analysis, selection, and tool. BMC Med. Imaging 2015, 15, 29. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Xiang, L.; Nie, D.; Shao, Y.; Zhang, H.; Shen, D.; Wang, Q. Interleaved 3D-CNN s for joint segmentation of small-volume structures in head and neck CT images. Med. Phys. 2018, 45, 2063–2075. [Google Scholar] [CrossRef]
- Paszke, A.; Gross, S.; Massa, F.; Lerer, A.; Bradbury, J.; Chanan, G.; Killeen, T.; Lin, Z.; Gimelshein, N.; Antiga, L.; et al. PyTorch: An Imperative Style, High-Performance Deep Learning Library. In Proceedings of the Advances in Neural Information Processing Systems, Vancouver, BC, Canada, 8–14 December 2019; pp. 8024–8035. [Google Scholar]
- Badrinarayanan, V.; Kendall, A.; Cipolla, R. Segnet: A deep convolutional encoder-decoder architecture for image segmentation. IEEE Trans. Pattern Anal. Mach. Intell. 2017, 39, 2481–2495. [Google Scholar] [CrossRef]
- Oktay, O.; Schlemper, J.; Folgoc, L.L.; Lee, M.; Heinrich, M.; Misawa, K.; Mori, K.; McDonagh, S.; Hammerla, N.Y.; Kainz, B.; et al. Attention U-Net: Learning where to look for the pancreas. arXiv 2018, arXiv:1804.03999. [Google Scholar]
- Chen, A.; Dawant, B. A multi-atlas approach for the automatic segmentation of multiple structures in head and neck CT images. In Proceedings of the Head Neck Auto-Segmentation Challenge (MICCAI), Munich, Germany, 5–9 October 2015. [Google Scholar]
- Mannion-Haworth, R.; Bowes, M.; Ashman, A.; Guillard, G.; Brett, A.; Vincent, G. Fully automatic segmentation of head and neck organs using active appearance models. In Proceedings of the Head Neck Auto-Segmentation Challenge (MICCAI), Munich, Germany, 5–9 October 2015. [Google Scholar]
- Albrecht, T.; Gass, T.; Langguth, C.; Lüthi, M. Multi atlas segmentation with active shape model refinement for multi-organ segmentation in head and neck cancer radiotherapy planning. In Proceedings of the Head Neck Auto-Segmentation Challenge (MICCAI), Munich, Germany, 5–9 October 2015. [Google Scholar]
- Orbes-Arteaga, M.; Pea, D.; Dominguez, G. Head and neck auto segmentation challenge based on non-local generative models. In Proceedings of the Head Neck Auto-Segmentation Challenge (MICCAI), Munich, Germany, 5–9 October 2015. [Google Scholar]
- Kodym, O.; Španěl, M.; Herout, A. Segmentation of Head and Neck Organs at Risk Using CNN with Batch Dice Loss. arXiv 2018, arXiv:1812.02427. [Google Scholar]
- Wang, Z.; Wei, L.; Wang, L.; Gao, Y.; Chen, W.; Shen, D. Hierarchical vertex regression-based segmentation of head and neck CT images for radiotherapy planning. IEEE Trans. Image Process. 2017, 27, 923–937. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, L.; Song, Z.; Wang, M. Organ at Risk Segmentation in Head and Neck CT Images by Using a Two-Stage Segmentation Framework Based on 3D U-Net. arXiv 2018, arXiv:1809.00960. [Google Scholar] [CrossRef]
- Liang, S.; Thung, K.; Nie, D.; Zhang, Y.; Shen, D. Multi-view Spatial Aggregation Framework for Joint Localization and Segmentation of Organs at risk in Head and Neck CT Images. IEEE Trans. Med. Imaging 2020, 39, 2794–2805. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; van der Wel, H.; Kraeima, J.; Glas, H.H.; Guo, J.; Borra, R.J.; Witjes, M.J.H.; van Ooijen, P. Robust and Accurate Mandible Segmentation on Dental CBCT Scans Affected by Metal Artifacts Using a Prior Shape Model. J. Pers. Med. 2021, 11, 364. [Google Scholar] [CrossRef]
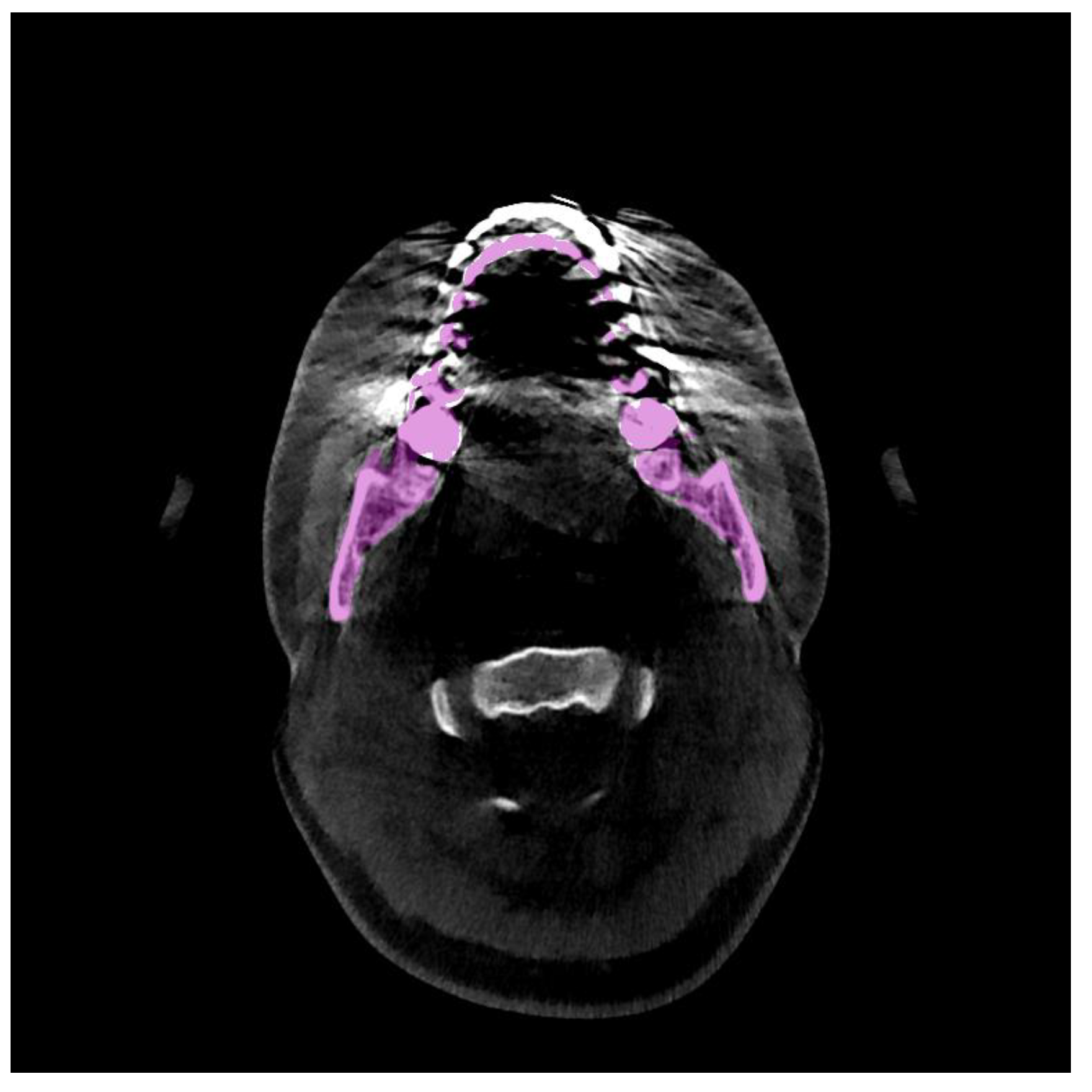
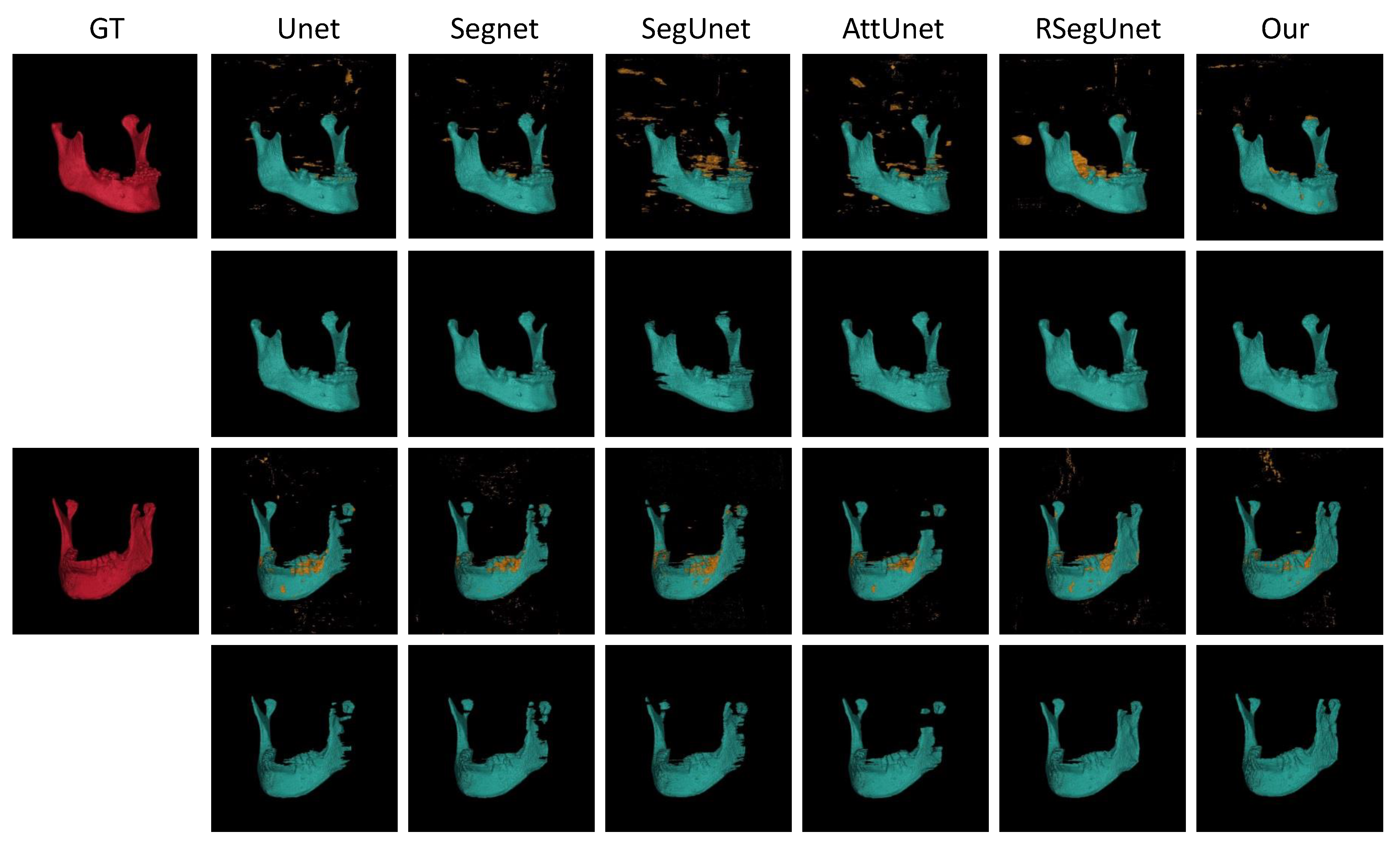
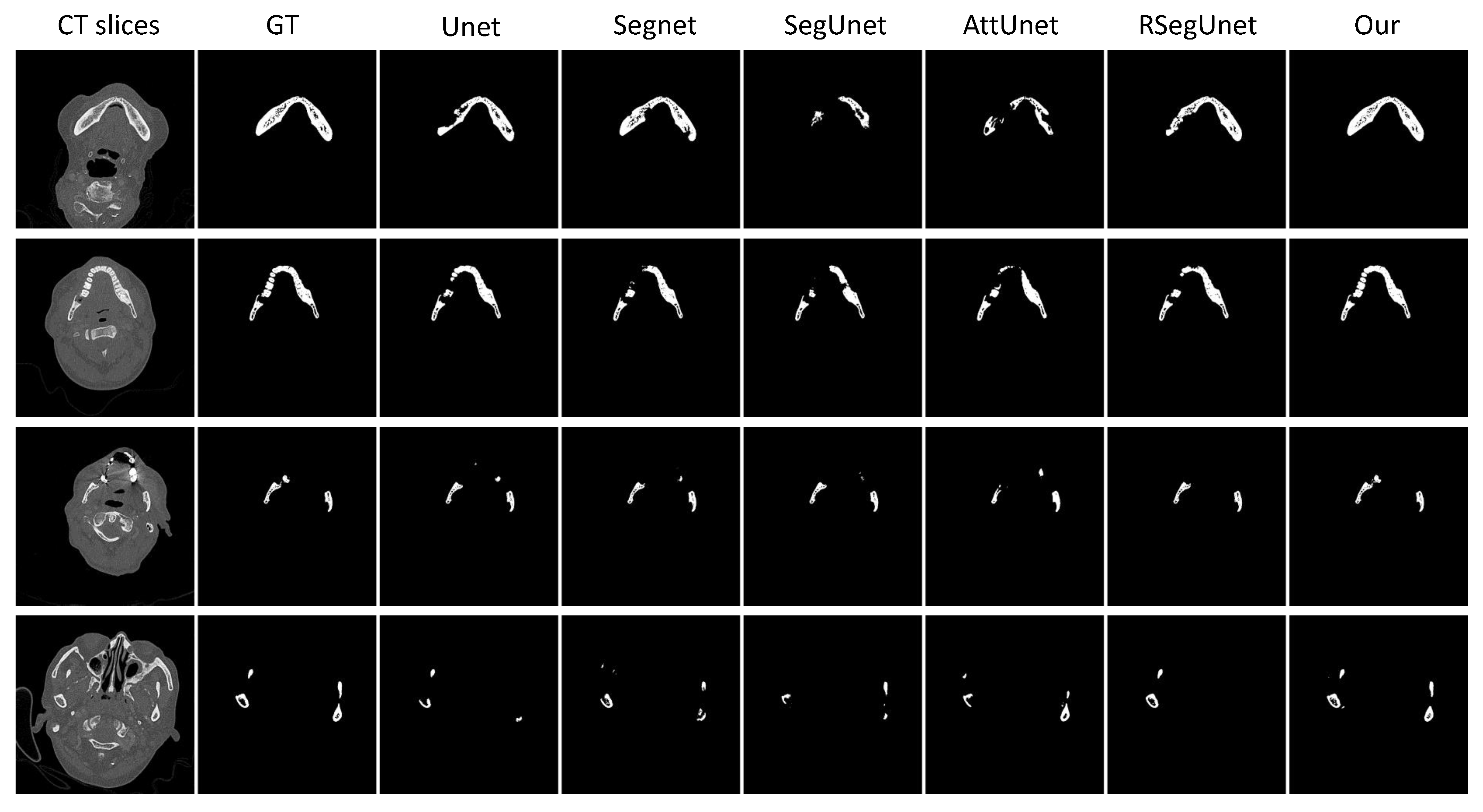
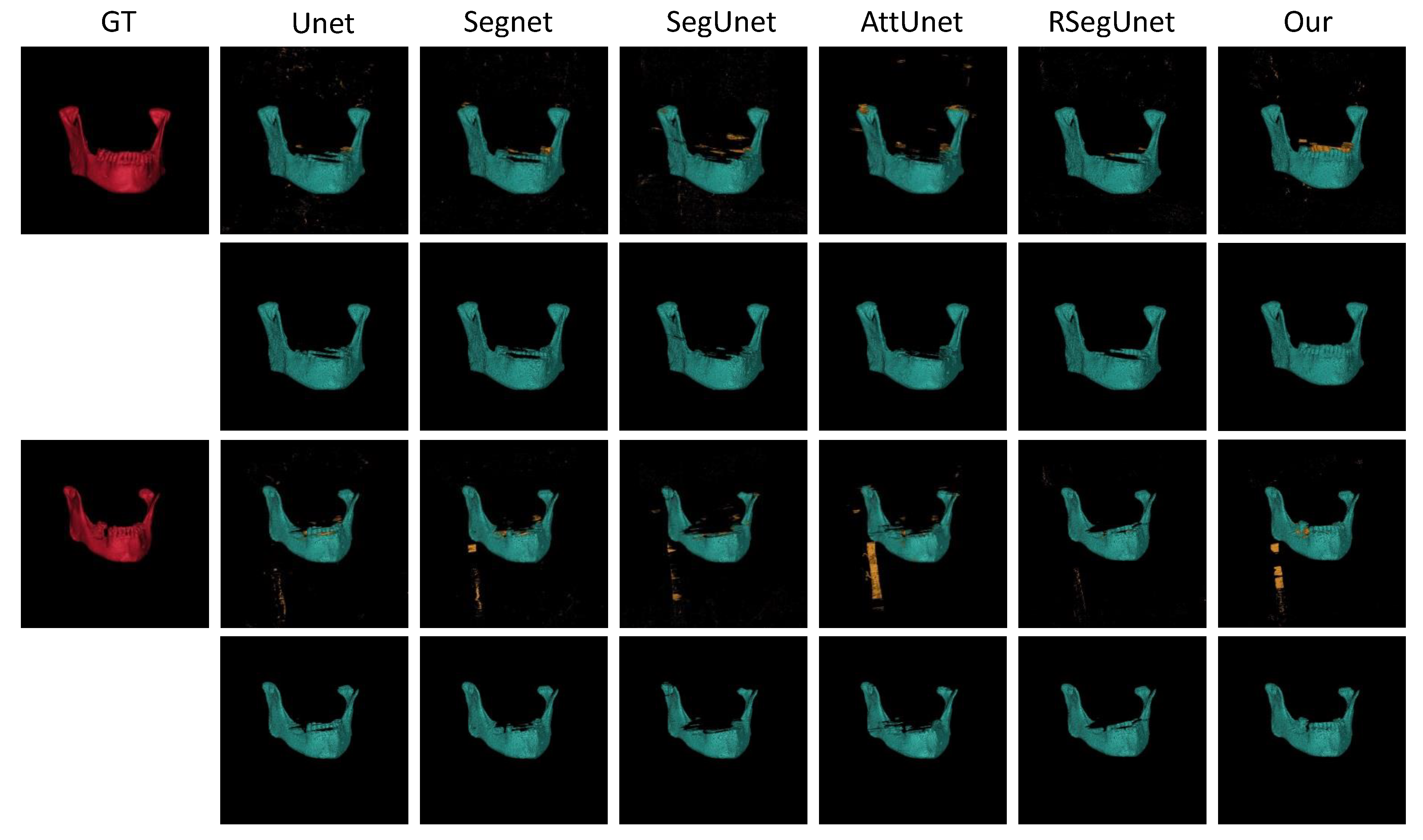
| Methods | (%) | (mm) | (mm) |
|---|---|---|---|
| Unet [18] | 94.79 (±1.77) | 2.0698 (±0.6137) | 32.6401 (±22.0779) |
| SegNet [29] | 94.93 (±1.74 ) | 1.7762 (±1.5937) | 15.9851 (±26.5286) |
| SegUnet [15] | 91.27 (±5.13) | 3.1436 (±3.6049) | 26.3569 (±34.9539) |
| AttUnet [30] | 93.34 (±3.79) | 3.9705 (±4.6460) | 35.1859 (±42.3474) |
| RSegUnet [11] | 92.26 (±5.66) | 1.3133 (±0.7276) | 7.2442 (±8.9275) |
| Ours | 95.31 (±1.11) | 1.2827 (±0.2780) | 3.1258 (±3.2311) |
| Methods | (%) | (mm) | (mm) |
|---|---|---|---|
| Unet [18] | 87.61 (±5.13) | 1.8779 (±0.7407) | 9.2152 (±17.0825) |
| SegNet [29] | 86.11 (±7.69) | 1.6028 (±0.7194) | 7.6235 (±15.1696) |
| SegUnet [15] | 83.14 (±12.65) | 2.4753 (±1.9507) | 15.4372 (±25.1890) |
| AttUnet [30] | 86.11 (±11.63) | 1.6033 (±1.4386) | 16.7041 (±24.2038) |
| RSegUnet [11] | 86.48 (± 7.98) | 1.3907 (± 0.7566 ) | 7.6591 (±16.7968 ) |
| Ours | 88.62 (±4.98) | 1.2582 (±0.4102) | 4.9668 (±5.0592) |
| Methods | (%) | (mm) | (mm) |
|---|---|---|---|
| Multi-atlas [31] | 91.7 (±2.34) | - | 2.4887 (±0.7610) |
| AAM [32] | 92.67 (±1) | - | 1.9767 (±0.5945) |
| ASM [33] | 88.13 (±5.55) | - | 2.832 (±1.1772) |
| CNN [8] | 89.5 (±3.6) | - | - |
| NLGM [34] | 93.08 (±2.36) | - | - |
| AnatomyNet [9] | 92.51 (±2) | - | 6.28 (±2.21) |
| FCNN [10] | 92.07 (±1.15) | 0.51 (±0.12) | 2.01 (±0.83) |
| FCNN+SRM [10] | 93.6 (±1.21) | 0.371 (±0.11) | 1.5 (±0.32) |
| CNN+BD [35] | 94.6 (±0.7) | 0.29 (±0.03) | - |
| HVR [36] | 94.4 (± 1.3) | 0.43 (± 0.12) | - |
| Cascade 3D Unet [37] | 93 (±1.9) | - | 1.26 (±0.5) |
| Multi-plana r [7] | 93.28 (±1.44) | - | 1.4333 (±0.5564) |
| Multi-view [38] | 94.1 (±0.7) | 0.28 (±0.14) | - |
| RSegUnet [11] | 95.10 (±1.21) | 0.1367 (±0.0382) | 1.3560 (±0.4487) |
| SASeg [39] | 95.29 (±1.16) | 0.1353 (±0.0481) | 1.3054 (±0.3195) |
| Our | 94.57 (±1.21) | 0.1252 (±0.0275) | 1.1813 (±0.4028) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, B.; van der Wel, H.; Kraeima, J.; Glas, H.H.; Guo, J.; Borra, R.J.H.; Witjes, M.J.H.; van Ooijen, P.M.A. Mandible Segmentation of Dental CBCT Scans Affected by Metal Artifacts Using Coarse-to-Fine Learning Model. J. Pers. Med. 2021, 11, 560. https://doi.org/10.3390/jpm11060560
Qiu B, van der Wel H, Kraeima J, Glas HH, Guo J, Borra RJH, Witjes MJH, van Ooijen PMA. Mandible Segmentation of Dental CBCT Scans Affected by Metal Artifacts Using Coarse-to-Fine Learning Model. Journal of Personalized Medicine. 2021; 11(6):560. https://doi.org/10.3390/jpm11060560
Chicago/Turabian StyleQiu, Bingjiang, Hylke van der Wel, Joep Kraeima, Haye Hendrik Glas, Jiapan Guo, Ronald J. H. Borra, Max Johannes Hendrikus Witjes, and Peter M. A. van Ooijen. 2021. "Mandible Segmentation of Dental CBCT Scans Affected by Metal Artifacts Using Coarse-to-Fine Learning Model" Journal of Personalized Medicine 11, no. 6: 560. https://doi.org/10.3390/jpm11060560
APA StyleQiu, B., van der Wel, H., Kraeima, J., Glas, H. H., Guo, J., Borra, R. J. H., Witjes, M. J. H., & van Ooijen, P. M. A. (2021). Mandible Segmentation of Dental CBCT Scans Affected by Metal Artifacts Using Coarse-to-Fine Learning Model. Journal of Personalized Medicine, 11(6), 560. https://doi.org/10.3390/jpm11060560








