The Effects of Dimethylsulfoxide and Oxygen on DNA Damage Induction and Repair Outcomes for Cells Irradiated by 62 MeV Proton and 3.31 MeV Helium Ions
Abstract
1. Introduction
2. Materials and Methods
2.1. Monte Carlo Damage Simulation (MCDS)
2.2. Monte Carlo Excision Repair (MCER) Simulation
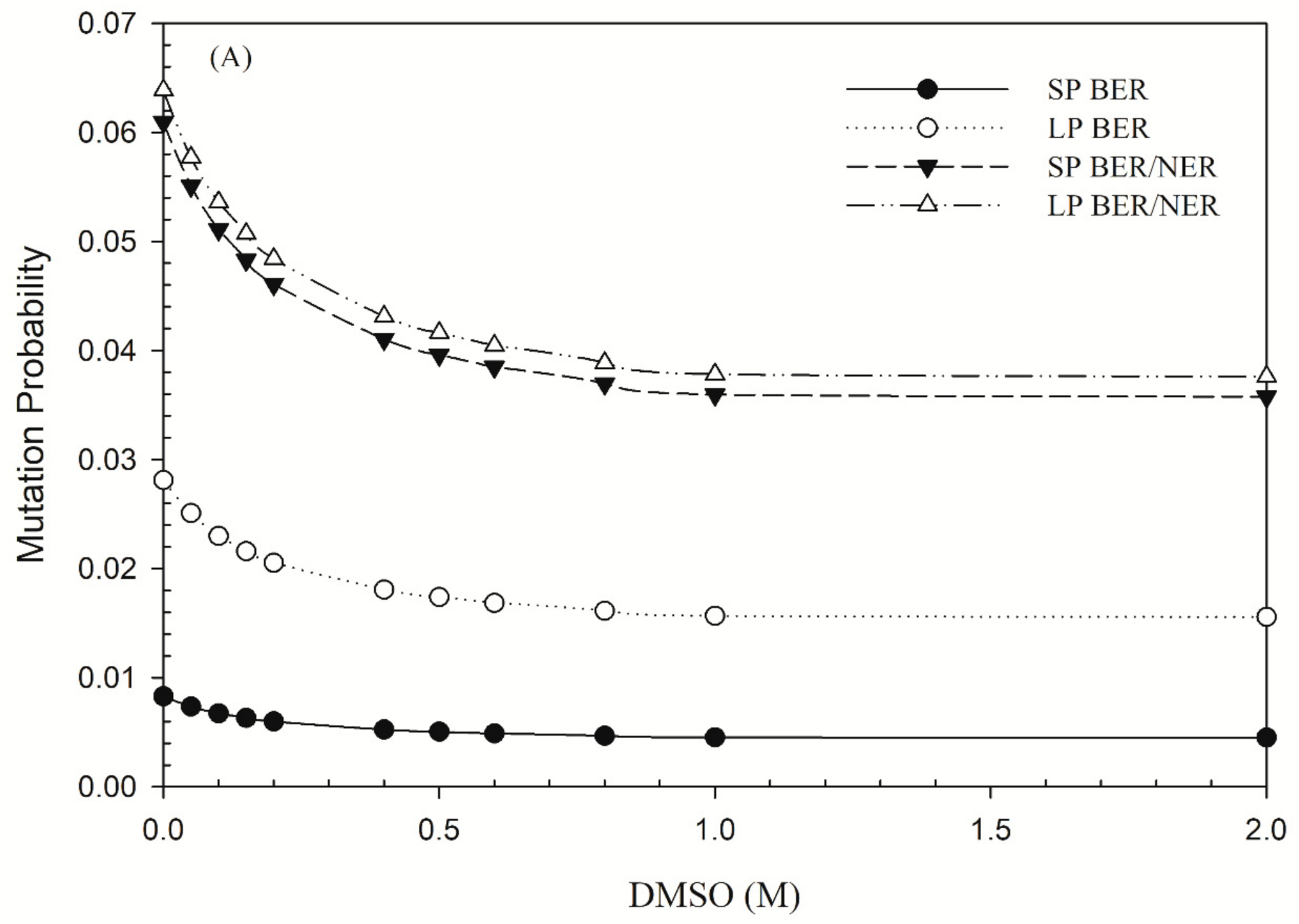
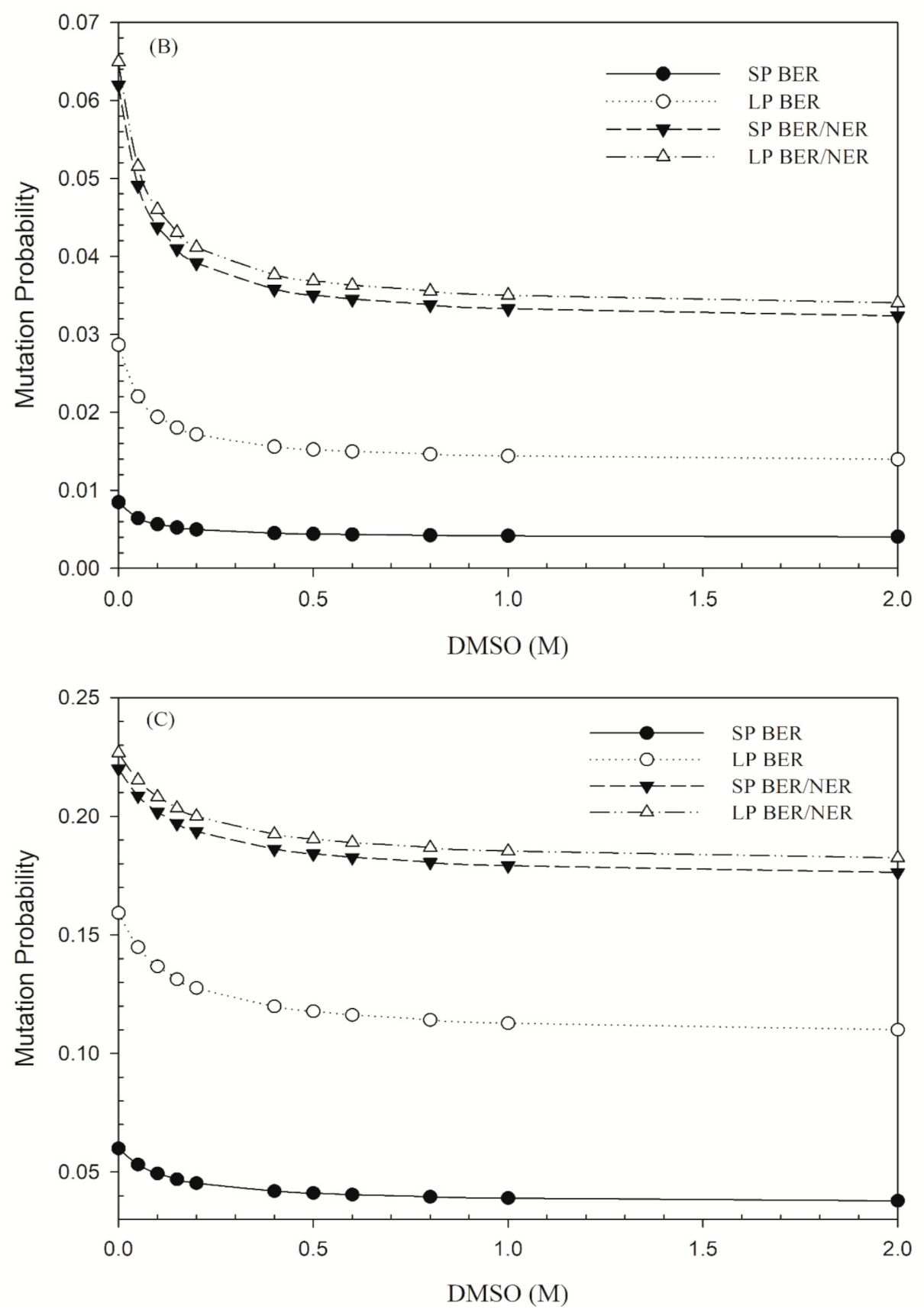
2.3. Calculation of DSB Induction and Mutation Frequency
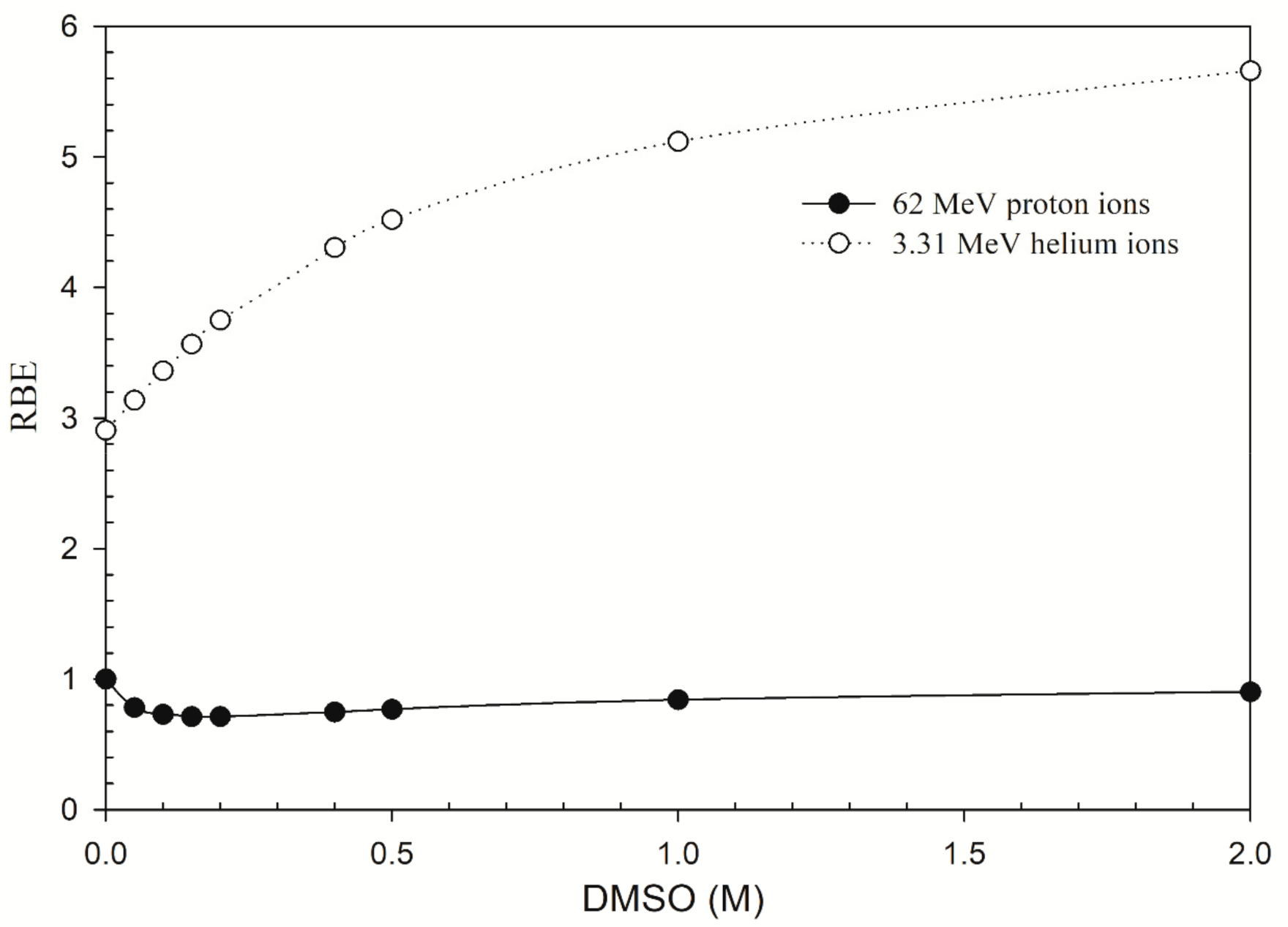
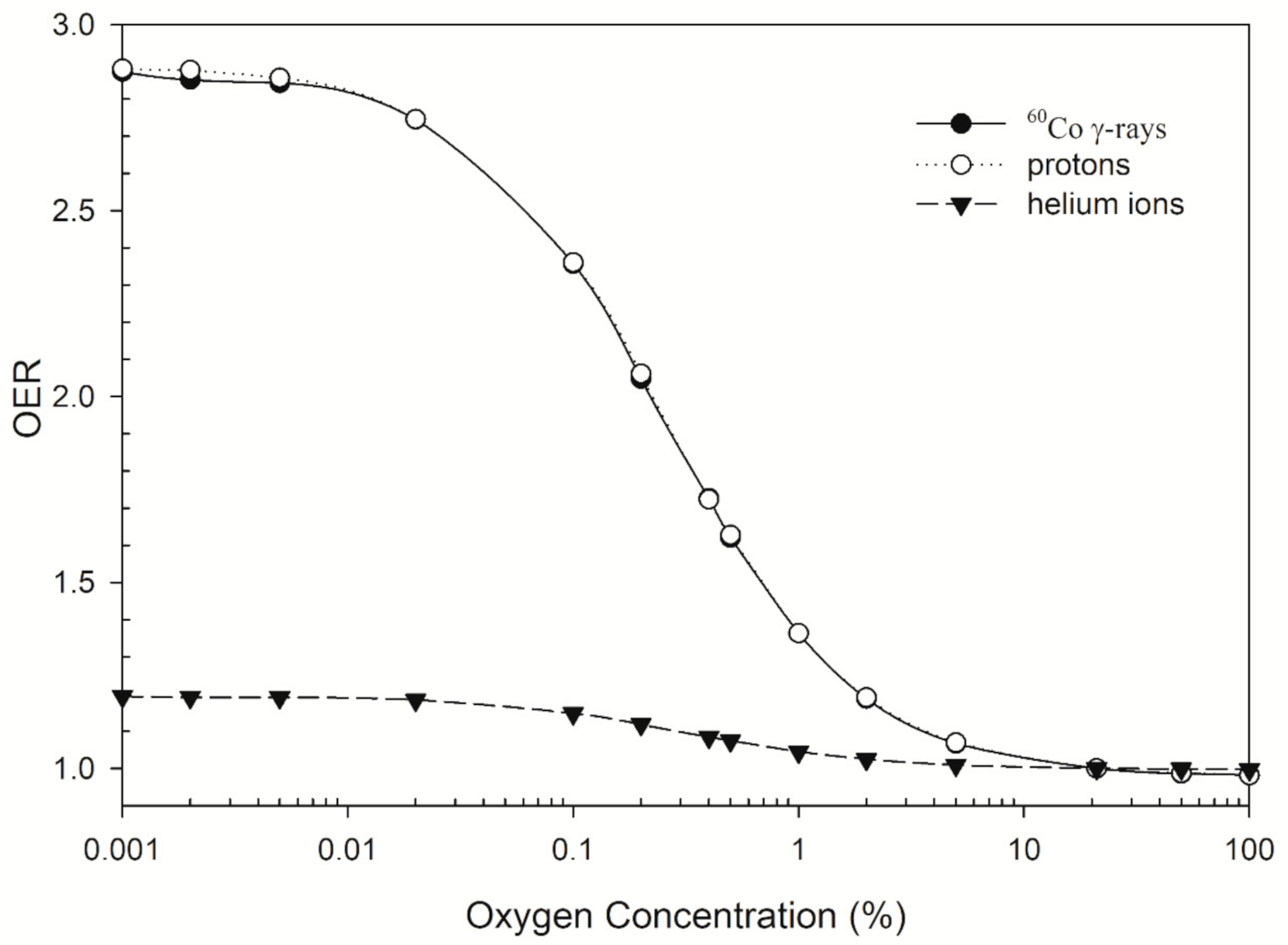
2.4. OER and RBE
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Karger, C.P.; Jäkel, O. Current status and new developments in ion therapy. Strahlenther. Onkol. 2007, 183, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Krämer, M.; Scifoni, E.; Schuy, C.; Rovituso, M.; Tinganelli, W.; Maier, A.; Kaderka, R.; Kraft-Weyrather, W.; Brons, S.; Tessonnier, T.; et al. Helium ions for radiotherapy? Physical and biological verifications of a novel treatment modality. Med. Phys. 2016, 43, 1995–2004. [Google Scholar] [CrossRef] [PubMed]
- Palmans, H.; Rabus, H.; Belchior, A.L.; Bug, M.U.; Galer, S.; Giesen, U.; Gonon, G.; Gruel, G.; Hilgers, G.; Moro, D.; et al. Future development of biologically relevant dosimetry. Br. J. Radiol. 2015, 88, 20140392. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.J.; Giaccia, A.J. Radiobiology for the Radiologist, 7th ed.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012; pp. 16–29. [Google Scholar]
- Holley, A.K.; Miao, L.; Clair, D.K.S.; Clair, W.H.S. Redox-modulated phenomena and radiation therapy: The central role of superoxide dismutases. Antioxid. Redox Signal. 2014, 20, 1567–1589. [Google Scholar] [CrossRef]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef]
- Mitteer, R.A.; Wang, Y.; Shah, J.; Gordon, S.; Fager, M.; Butter, P.-P.; Kim, H.J.; Guardiola-Salmeron, C.; Carabe-Fernandez, A.; Fan, Y. Proton beam radiation induces DNA damage and cell apoptosis in glioma stem cells through reactive oxygen species. Sci. Rep. 2015, 5, srep13961. [Google Scholar] [CrossRef]
- Sharma, V.; Collins, L.B.; Chen, T.-H.; Herr, N.; Takeda, S.; Sun, W.; Swenberg, J.A.; Nakamura, J. Oxidative stress at low levels can induce clustered DNA lesions leading to NHEJ mediated mutations. Oncotarget 2016, 7, 25377–25390. [Google Scholar] [CrossRef]
- Srinivas, U.S.; Tan, B.W.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Hasegawa, T.; Takahashi, J.; Nagasawa, S.; Doi, M.; Moriyama, A.; Iwahashi, H. DNA Strand break properties of protoporphyrin IX by X-ray irradiation against melanoma. Int. J. Mol. Sci. 2020, 21, 2302. [Google Scholar] [CrossRef]
- Asaithamby, A.; Chen, D.J. Mechanism of cluster DNA damage repair in response to high-atomic number and energy particles radiation. Mutat. Res. Mol. Mech. Mutagen. 2011, 711, 87–99. [Google Scholar] [CrossRef]
- Sutherland, B.M.; Bennett, P.V.; Sidorkina, O.; Laval, J. Clustered DNA damages induced in isolated DNA and in human cells by low doses of ionizing radiation. Proc. Natl. Acad. Sci. USA 2000, 97, 103–108. [Google Scholar] [CrossRef]
- Fokas, E.; Kraft, G.; An, H.; Engenhart-Cabillic, R. Ion beam radiobiology and cancer: Time to update ourselves. Biochim. Biophys. Acta (BBA) Bioenerg. 2009, 1796, 216–229. [Google Scholar] [CrossRef]
- Ward, J. DNA Damage produced by ionizing radiation in mammalian cells: Identities, mechanisms of formation, and reparability. Progr. Nucleic Acid Res. Mol. Biol. 1988, 35, 95–125. [Google Scholar] [CrossRef]
- Shikazono, N.; Noguchi, M.; Fujii, K.; Urushibara, A.; Yokoya, A. The yield, processing, and biological consequences of clustered dna damage induced by ionizing radiation. J. Radiat. Res. 2009, 50, 27–36. [Google Scholar] [CrossRef]
- Nickoloff, J.A.; Sharma, N.; Taylor, L. Clustered DNA double-strand breaks: Biological effects and relevance to cancer radiotherapy. Genes 2020, 11, 99. [Google Scholar] [CrossRef]
- Huang, J.C.; Hsu, D.S.; Kazantsev, A.; Sancar, A. Substrate spectrum of human excinuclease: Repair of abasic sites, methylated bases, mismatches, and bulky adducts. Proc. Natl. Acad. Sci. USA 1994, 91, 12213–12217. [Google Scholar] [CrossRef]
- Reardon, J.T.; Bessho, T.; Kung, H.C.; Bolton, P.H.; Sancar, A. In vitro repair of oxidative DNA damage by human nucleotide excision repair system: Possible explanation for neurodegeneration in Xeroderma pigmentosum patients. Proc. Natl. Acad. Sci. USA 1997, 94, 9463–9468. [Google Scholar] [CrossRef]
- Semenenko, V.A.; Stewart, R.D. Monte Carlo simulation of base and nucleotide excision repair of clustered DNA damage sites. II. Comparisons of model predictions to measured data. Radiat. Res. 2005, 164, 194–201. [Google Scholar] [CrossRef]
- Hemnani, T.; Parihar, M.S. Reactive oxygen species and oxidative DNA damage. Indian J. Physiol. Pharmacol. 1998, 42, 440–452. [Google Scholar]
- Delara, C.M.; Jenner, T.J.; Townsend, K.M.S.; Marsden, S.J.; O’Neill, P. The effect of dimethyl sulfoxide on the induction of DNA double-strand breaks in v79-4 mammalian cells by alpha particles. Radiat. Res. 1995, 144, 43. [Google Scholar] [CrossRef]
- Zwicker, F.; Hauswald, H.; Debus, J.; Huber, P.E.; Weber, K.-J. Impact of dimethyl sulfoxide on irradiation-related DNA double-strand-break induction, -repair and cell survival. Radiat. Environ. Biophys. 2019, 58, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.D.; Doern, S.D.; Reuvers, A.P.; Gillespie, C.J.; Chatterjee, A.; Blakely, E.A.; Smith, K.C.; Tobias, C.A. Radioprotection by DMSO of mammalian cells exposed to X-rays and to heavy charged-particle beams. Radiat. Environ. Biophys. 1979, 16, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.-Y.; Chen, F.-H.; Hsieh, T.-Y.; Hsiao, Y.-Y. Effects of indirect actions and oxygen on relative biological effectiveness: Estimate of DSB induction and conversion induced by gamma rays and helium ions. J. Radiat. Res. 2015, 56, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.-R.; Chen, F.-H.; Huang, R.-J.; Chen, Y.-P.; Hsiao, Y.-Y.; Chen, F.-H. Effects of indirect actions and oxygen on relative biological effectiveness: Estimate of DSB inductions and conversions induced by therapeutic proton beams. Int. J. Radiat. Biol. 2019, 96, 187–196. [Google Scholar] [CrossRef]
- Yang, C.; Tang, H.; Wang, L.; Peng, R.; Bai, F.; Shan, Y.; Yu, Z.; Zhou, P.; Cong, Y. Dimethyl sulfoxide prevents radiation-induced oral mucositis through facilitating dna double-strand break repair in epithelial stem cells. Int. J. Radiat. Oncol. 2018, 102, 1577–1589. [Google Scholar] [CrossRef]
- von Sonntag, C. Free-Radical-Induced DNA Damage and Its Repair, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 357–482. [Google Scholar]
- Lomax, M.E.; Folkes, L.K.; O’Neill, P. Biological consequences of radiation-induced DNA damage: Relevance to radiotherapy. Clin. Oncol. 2013, 25, 578–585. [Google Scholar] [CrossRef]
- Khoshinani, H.M.; Afshar, S.; Najafi, R. Hypoxia: A double-edged sword in cancer therapy. Cancer Investig. 2016, 34, 536–545. [Google Scholar] [CrossRef]
- Bader, S.B.; Dewhirst, M.W.; Hammond, E.M. Cyclic hypoxia: An update on its characteristics, methods to measure it and biological implications in cancer. Cancers 2020, 13, 23. [Google Scholar] [CrossRef]
- Chan, N.; Ali, M.; McCallum, G.P.; Kumareswaran, R.; Koritzinsky, M.; Wouters, B.G.; Wells, P.G.; Gallinger, S.; Bristow, R.G. Hypoxia provokes base excision repair changes and a repair-deficient, mutator phenotype in colorectal cancer cells. Mol. Cancer Res. 2014, 12, 1407–1415. [Google Scholar] [CrossRef]
- Hammond, E.; Asselin, M.-C.; Forster, D.; O’Connor, J.; Senra, J.; Williams, K. The meaning, measurement and modification of hypoxia in the laboratory and the clinic. Clin. Oncol. 2014, 26, 277–288. [Google Scholar] [CrossRef]
- Oshikawa, T.; Okamoto, M.; Ahmed, S.U.; Furuichi, S.; Tano, T.; Sasai, A.; Kan, S.; Kasai, S.; Uto, Y.; Nagasawa, H.; et al. TX-1877, a bifunctional hypoxic cell radiosensitizer, enhances anticancer host response: Immune cell migration and nitric oxide production. Int. J. Cancer 2005, 116, 571–578. [Google Scholar] [CrossRef]
- Samec, M.; Liskova, A.; Hushmandi, K.; Ashrafizadeh, M.; Saso, L.; Brockmueller, A.; Shakibaei, M.; Büsselberg, D.; Kubatka, P.; Koklesova, L.; et al. Flavonoids targeting HIF-1: Implications on cancer metabolism. Cancers 2021, 13, 130. [Google Scholar] [CrossRef]
- Moen, I.; Stuhr, L.E.B. Hyperbaric oxygen therapy and cancer—A review. Target. Oncol. 2012, 7, 233–242. [Google Scholar] [CrossRef]
- Linam, J.; Yang, L.-X. Recent developments in radiosensitization. Anticancer. Res. 2015, 35, 2479. [Google Scholar]
- Gong, L.; Zhang, Y.; Liu, C.; Zhang, M.; Han, S. Application of radiosensitizers in cancer radiotherapy. Int. J. Nanomed. 2021, 2021, 1083–1102. [Google Scholar] [CrossRef]
- Semenenko, V.A.; Stewart, R.D. A fast Monte Carlo algorithm to simulate the spectrum of DNA damages formed by ionizing radiation. Radiat. Res. 2004, 161, 451–457. [Google Scholar] [CrossRef]
- Semenenko, V.A.; Stewart, R.D. Fast Monte Carlo simulation of DNA damage formed by electrons and light ions. Phys. Med. Biol. 2006, 51, 1693–1706. [Google Scholar] [CrossRef]
- Stewart, R.D.; Yu, V.K.; Georgakilas, A.G.; Koumenis, C.; Park, J.H.; Carlson, D.J. Effects of radiation quality and oxygen on clustered DNA lesions and cell death. Radiat. Res. 2011, 176, 587–602. [Google Scholar] [CrossRef]
- Chaudhary, P.; Gwynne, D.; Doria, D.; Romagnani, L.; Maiorino, C.; Padda, H.; Alejo, A.; Booth, N.; Carroll, D.; Kar, S.; et al. Laser accelerated ultra high dose rate protons induced DNA damage under hypoxic conditions. Radiother. Oncol. 2016, 118, S24–S25. [Google Scholar] [CrossRef]
- Chaudhary, P.; Gwynne, D.; Doria, D.; Romagnani, L.; Maiorino, C.; Padda, H.; Alejo, A.; Booth, N.; Carroll, D.; Kar, S.; et al. Effectiveness of laser accelerated ultra high dose rate protons in DNA DSB damage induction under hypoxic conditions. In Proceedings of the 44th EPS Conference on Plasma Physics, EPS 2017, Belfast, UK, 20–26 June 2017; Bret, A., Fajardo, M., Westerhof, E., Melzer, A., Dromey, B., Riconda, C., Eds.; European Physical Society: Mulhouse, France, 2017. [Google Scholar]
- Chaudhary, P.; Marshall, T.I.; Prise, K.M.; Schettino, G.; Perozziello, F.M.; Manti, L.; Currell, F.J.; Hanton, F.; McMahon, S.J.; Kavanagh, J.N.; et al. Relative biological effectiveness variation along monoenergetic and modulated bragg peaks of a 62-mev therapeutic proton beam: A preclinical assessment. Int. J. Radiat. Oncol. 2014, 90, 27–35. [Google Scholar] [CrossRef]
- Semenenko, V.A.; Stewart, R.D.; Ackerman, E.J. Monte Carlo simulation of base and nucleotide excision repair of clustered DNA damage sites. I. Model properties and predicted trends. Radiat. Res. 2005, 164, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.; Stewart, R.D. Monte Carlo simulation of DNA damage induction by x-rays and selected radioisotopes. Phys. Med. Biol. 2007, 53, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Carlson, D.J.; Stewart, R.D.; Semenenko, V.A. Effects of oxygen on intrinsic radiation sensitivity: A test of the relationship between aerobic and hypoxic linear-quadratic (LQ) model parameters. Med. Phys. 2006, 33, 3105. [Google Scholar] [CrossRef] [PubMed]
- Pater, P.; Bäckstöm, G.; Villegas, F.; Ahnesjö, A.; Enger, S.A.; Seuntjens, J.; El Naqa, I. Proton and light ion RBE for the induction of direct DNA double strand breaks. Med. Phys. 2016, 43, 2131–2140. [Google Scholar] [CrossRef]
- Stewart, R.D.; Carlson, D.J.; Butkus, M.P.; Hawkins, R.; Friedrich, T.; Scholz, M. A comparison of mechanism-inspired models for particle relative biological effectiveness (RBE). Med. Phys. 2018, 45, e925–e952. [Google Scholar] [CrossRef]
- Kashino, G.; Liu, Y.; Suzuki, M.; Masunaga, S.-I.; Kinashi, Y.; Ono, K.; Tano, K.; Watanabe, M. An alternative mechanism for radioprotection by dimethyl sulfoxide; possible facilitation of DNA double-strand break repair. J. Radiat. Res. 2010, 51, 733–740. [Google Scholar] [CrossRef]
- Mognato, M.; Bortoletto, E.; Ferraro, P.; Baggio, L.; Cherubini, R.; Canova, S.; Russo, A.; Celotti, L. Genetic damage induced by in vitro irradiation of human G0 lymphocytes with low-energy protons (28 keV/μm): HPRT mutations and chromosome aberrations. Radiat. Res. 2003, 160, 52–60. [Google Scholar] [CrossRef]
- Hashimoto, S.; Sugie, C.; Iwata, H.; Ogino, H.; Omachi, C.; Yasui, K.; Mizoe, J.E.; Shibamoto, Y. Recovery from sublethal damage and potentially lethal damage: Proton beam irradiation vs. Xray irradiation. Strahlenther Onkol. 2018, 194, 343–351. [Google Scholar] [CrossRef]
- Ito, A.; Nakano, H.; Kusano, Y.; Hirayama, R.; Furusawa, Y.; Murayama, C.; Mori, T.; Katsumura, Y.; Shinohara, K. Contribution of indirect action to radiation-induced mammalian cell inactivation: Dependence on photon energy and heavy-ion LET. Radiat. Res. 2006, 165, 703–712. [Google Scholar] [CrossRef]
- Sapora, O.; Barone, F.; Belli, M.; Maggi, A.; Quintiliani, M.; Tabocchini, M. Relationships between cell killing, mutation induction and DNA damage in X-irradiated v79 cells: The influence of oxygen and DMSO. Int. J. Radiat. Biol. 1991, 60, 467–482. [Google Scholar] [CrossRef]
- Hirayama, R.; Furusawa, Y.; Fukawa, T.; Ando, K. Repair kinetics of DNA-DSB induced by X-rays or carbon ions under oxic and hypoxic conditions. J. Radiat. Res. 2005, 46, 325–332. [Google Scholar] [CrossRef]
- Jacob, S.; Torre, J. Dimethyl Sulfoxide (DMSO) in Trauma and Disease; CRC Press: Boca Raton, FL, USA, 2015; pp. 1–242. [Google Scholar]
- Kollerup Madsen, B.; Hilscher, M.; Zetner, D.; Rosenberg, J. Adverse reactions of dimethyl sulfoxide in humans: A systematic review. F1000Res 2018, 7, 1746. [Google Scholar] [CrossRef]
- Tunçer, S.; Gurbanov, R.; Sheraj, I.; Solel, E.; Esenturk, O.; Banerjee, S. Low dose dimethyl sulfoxide driven gross molecular changes have the potential to interfere with various cellular processes. Sci. Rep. 2018, 8, 14828. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, R.; Ito, A.; Furusawa, Y.; Tomita, M.; Tsukada, T.; Yatagai, F.; Noguchi, M.; Matsumoto, Y.; Kase, Y.; Ando, K.; et al. Contributions of direct and indirect actions in cell killing by high-let radiations. Radiat. Res. 2009, 171, 212–218. [Google Scholar] [CrossRef]
- Bajinskis, A.; Natarajan, A.T.; Erixon, K.; Harms-Ringdahl, M. DNA double strand breaks induced by the indirect effect of radiation are more efficiently repaired by non-homologous end joining compared to homologous recombination repair. Mutat. Res. Toxicol. Environ. Mutagen. 2013, 756, 21–29. [Google Scholar] [CrossRef]
- Petkovic, V.D.; Keta, O.D.; Vidosavljevic, M.Z.; Incerti, S.; Ristic Fira, A.M.; Petrovic, I.M. Biological outcomes of gamma-radiation induced DNA damages in breast and lung cancer cells pretreated with free radical scavengers. Int. J. Radiat. Biol. 2019, 95, 274–285. [Google Scholar] [CrossRef]
- Yuan, C.; Gao, J.; Guo, J.; Bai, L.; Marshall, C.; Cai, Z.; Wang, L.; Xiao, M. Dimethyl sulfoxide damages mitochondrial integrity and membrane potential in cultured astrocytes. PLoS ONE 2014, 9, e107447. [Google Scholar] [CrossRef]
- Galvao, J.; Davis, B.; Tilley, M.; Normando, E.; Duchen, M.R.; Cordeiro, M.F. Unexpected low-dose toxicity of the universal solvent DMSO. FASEB J. 2014, 28, 1317–1330. [Google Scholar] [CrossRef]
- Martin, W.B.; Sicard, R.; Namin, S.M.; Ganey, T. Methods of cryoprotectant preservation: Allogeneic cellular bone grafts and potential effects. BioMed Res. Int. 2019, 2019, 1–7. [Google Scholar] [CrossRef]
- Milligan, J.R.; Ng, J.Y.-Y.; Wu, C.C.L.; Aguilera, J.A.; Ward, J.F.; Kow, Y.W.; Wallace, S.S.; Cunningham, R.P. Methylperoxyl radicals as intermediates in the damage to DNA irradiated in aqueous dimethyl sulfoxide with gamma rays. Radiat. Res. 1996, 146, 436. [Google Scholar] [CrossRef] [PubMed]
- Milligan, J.R.; Ward, J.F. Yield of single-strand breaks due to attack on dna by scavenger-derived radicals. Radiat. Res. 1994, 137, 295. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.F. Ionizing Radiation Damage to DNA: A Challenge to Repair System. In Advances in DNA Damage & Repair: Oxygen Radical Effects, Cellular Protection and Biological Consequences, 1st ed.; Miral, D., Karakaya, A.E., Eds.; Springer: New York, NY, USA, 1999; Volume 302, pp. 431–439. [Google Scholar]
- Bajinskis, A.; Olsson, G.; Harms-Ringdahl, M. The indirect effect of radiation reduces the repair fidelity of NHEJ as verified in repair deficient CHO cell lines exposed to different radiation qualities and potassium bromate. Mutat. Res. Mol. Mech. Mutagen. 2012, 731, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Jonathan-Moyal, É.; Vendrely, V.; Motte, L.; Balosso, J.; Thariat, J. Radioresistant tumours: From identification to targeting. Cancer/Radiothér. 2020, 24, 699–705. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Jiang, X.; Wang, X.; Zhou, X.; Wan, L.; Zhao, H.; Zhou, Z.; Gao, L.; Huang, G.; et al. Preparation and validation of cyclodextrin-based excipients for radioiodinated hypericin applied in a targeted cancer radiotherapy. Int. J. Pharm. 2021, 599, 120393. [Google Scholar] [CrossRef]
- Capriotti, K.; Capriotti, J.A. Dimethyl sulfoxide: History, chemistry, and clinical utility in dermatology. J. Clin. Aesthet. Dermatol. 2012, 5, 24–26. [Google Scholar]
- Brien, S.; Prescott, P.; Bashir, N.; Lewith, H.; Lewith, G. Systematic review of the nutritional supplements dimethyl sulfoxide (DMSO) and methylsulfonylmethane (MSM) in the treatment of osteoarthritis. Osteoarthr. Cartil. 2008, 16, 1277–1288. [Google Scholar] [CrossRef]
- Hoang, B.X.; Le, B.T.; Shaw, D.G.; Tran, H.D.; Hoang, C.; Tran, H.Q.; Tran, D.M.; Pham, C.Q.; Pham, T.D.; Ha, T.V.; et al. Dimethyl sulfoxide–sodium bicarbonate infusion for palliative care and pain relief in patients with metastatic prostate cancer. J. Pain Palliat. Care Pharmacother. 2011, 25, 350–355. [Google Scholar] [CrossRef]
- Wang, J.Z.; Li, X.A. Impact of tumor repopulation on radiotherapy planning. Int. J. Radiat. Oncol. 2005, 61, 220–227. [Google Scholar] [CrossRef]
- McKeown, S.R. Defining normoxia, physoxia and hypoxia in tumours—implications for treatment response. Br. J. Radiol. 2014, 87, 20130676. [Google Scholar] [CrossRef]
- Colucci, M.; Maione, F.; Bonito, M.C.; Piscopo, A.; Di Giannuario, A.; Pieretti, S. New insights of dimethyl sulphoxide effects (DMSO) on experimental in vivo models of nociception and inflammation. Pharmacol. Res. 2008, 57, 419–425. [Google Scholar] [CrossRef]
| Absolute Yields (per Gy per Gbp) | BD a | SSB a | SSB+ a | 2SSB a | DSB a | DSB+ a | DSB++ a | Total SSB a | Total DSB a | Total Damage |
|---|---|---|---|---|---|---|---|---|---|---|
| proton b | 421.03 ± 0.04 | 177.77 ± 0.02 | 8.04 ± 0.00 | 1.01 ± 0.00 | 7.19 ± 0.01 | 0.99 ± 0.00 | 0.12 ± 0.00 | 186.83 ± 0.02 | 8.29 ± 0.01 | 616.15 ± 0.05 |
| proton + 0.1 M DMSO | 341.22 ± 0.04 | 135.00 ± 0.02 | 4.20 ± 0.00 | 0.39 ± 0.00 | 3.95 ± 0.01 | 0.38 ± 0.00 | 0.03 ± 0.00 | 139.59 ± 0.02 | 4.36 ± 0.01 | 485.17 ± 0.04 |
| (19%↓) | (24%↓) | (48%↓) | (62%↓) | (45%↓) | (61%↓) | (75%↓) | (25%↓) | (47%↓) | (21%↓) | |
| proton + 0.5 M DMSO | 292.05 ± 0.03 | 111.85 ± 0.02 | 2.77 ± 0.00 | 0.20 ± 0.00 | 2.64 ± 0.01 | 0.20 ± 0.00 | 0.01 ± 0.00 | 114.83 ± 0.02 | 2.86 ± 0.01 | 409.74 ± 0.03 |
| (31%↓) | (37%↓) | (65%↓) | (80%↓) | (63%↓) | (79%↓) | (90%↓) | (39%↓) | (66%↓) | (33%↓) | |
| proton + 1 M DMSO b | 281.33 ± 0.03 | 107.09 ± 0.01 | 2.54 ± 0.00 | 0.18 ± 0.00 | 2.45 ± 0.01 | 0.17 ± 0.00 | 0.00 ± 0.00 | 109.80 ± 0.01 | 2.62 ± 0.01 | 393.75 ± 0.03 |
| (33%↓) | (40%↓) | (68%↓) | (82%↓) | (66%↓) | (82%↓) | (100%↓) | (41%↓) | (68%↓) | (36%↓) |
| Absolute Yields (per Gy per Gbp) | BD | SSB | SSB+ | 2SSB | DSB | DSB+ | DSB++ | Total SSB | Total DSB | Total Damage |
|---|---|---|---|---|---|---|---|---|---|---|
| proton a | 421.03 ± 0.04 | 177.77 ± 0.02 | 8.04 ± 0.00 | 1.01 ± 0.00 | 7.19 ± 0.01 | 0.99 ± 0.00 | 0.12 ± 0.00 | 186.83 ± 0.02 | 8.29 ± 0.01 | 616.15 ± 0.05 |
| proton + 0.1 M DMSO + 21% O2 | 341.22 ± 0.04 | 135.00 ± 0.02 | 4.20 ± 0.00 | 0.39 ± 0.00 | 3.95 ± 0.01 | 0.38 ± 0.00 | 0.03 ± 0.00 | 139.59 ± 0.02 | 4.36 ± 0.01 | 485.17 ± 0.04 |
| (19%↓) | (24%↓) | (48%↓) | (62%↓) | (45%↓) | (61%↓) | (75%↓) | (25%↓) | (47%↓) | (21%↓) | |
| proton + 0.1 M DMSO + 2% O2 | 320.19 ± 0.03 | 124.91 ± 0.02 | 3.53 ± 0.00 | 0.30 ± 0.00 | 3.34 ± 0.01 | 0.29 ± 0.00 | 0.02 ± 0.00 | 128.73 ± 0.02 | 3.66 ± 0.01 | 452.58 ± 0.04 |
| (24%↓) | (30%↓) | (56%↓) | (70%↓) | (53%↓) | (70%↓) | (81%↓) | (31%↓) | (56%↓) | (27%↓) | |
| proton + 0.1 M DMSO + 0.1% O2 | 244.96 ± 0.03 | 91.24 ± 0.01 | 1.76 ± 0.00 | 0.11 ± 0.00 | 1.74 ± 0.01 | 0.10 ± 0.00 | 0.00 ± 0.00 | 93.12 ± 0.01 | 1.85 ± 0.01 | 339.92 ± 0.03 |
| (42%↓) | (49%↓) | (78%↓) | (89%↓) | (76%↓) | (90%↓) | (97%↓) | (50%↓) | (78%↓) | (45%↓) |
| Absolute Yields (per Gy per Gbp) | BD | SSB | SSB+ | 2SSB | DSB | DSB+ | DSB++ | Total SSB | Total DSB | Total Damage |
|---|---|---|---|---|---|---|---|---|---|---|
| proton a | 421.03 ± 0.04 | 177.77 ± 0.02 | 8.04 ± 0.00 | 1.01 ± 0.00 | 7.19 ± 0.01 | 0.99 ± 0.00 | 0.12 ± 0.00 | 186.83 ± 0.02 | 8.29 ± 0.01 | 616.15 ± 0.05 |
| proton + 2% a | 399.55 ± 0.04 | 165.47 ± 0.02 | 6.78 ± 0.00 | 0.78 ± 0.00 | 6.14 ± 0.01 | 0.76 ± 0.00 | 0.08 ± 0.00 | 173.03 ± 0.02 | 6.99 ± 0.01 | 579.57 ± 0.04 |
| (5%↓) | (7%↓) | (16%↓) | (23%↓) | (15%↓) | (23%↓) | (31%↓) | (7%↓) | (16%↓) | (6%↓) | |
| proton + 0.1 M DMSO +2% | 320.19 ± 0.03 | 124.91 ± 0.02 | 3.53 ± 0.00 | 0.30 ± 0.00 | 3.34 ± 0.01 | 0.29 ± 0.00 | 0.02 ± 0.00 | 128.73 ± 0.02 | 3.66 ± 0.01 | 452.58 ± 0.04 |
| (24%↓) | (30%↓) | (56%↓) | (70%↓) | (53%↓) | (70%↓) | (81%↓) | (31%↓) | (56%↓) | (27%↓) | |
| proton + 1 M DMSO +2% | 262.45 | 98.73 | 2.12 | 0.14 | 2.06 | 0.13 | 0.01 | 100.99 | 2.20 | 365.65 |
| (38%↓) | (44%↓) | (74%↓) | (87%↓) | (71%↓) | (87%↓) | (94%↓) | (46%↓) | (73%↓) | (41%↓) |
| Absolute Yields (per Gy per Gbp) | BD | SSB | SSB+ | 2SSB | DSB | DSB+ | DSB++ | Total SSB | Total DSB | Total Damage |
|---|---|---|---|---|---|---|---|---|---|---|
| proton a | 421.03 ± 0.04 | 177.77 ± 0.02 | 8.04 ± 0.00 | 1.01 ± 0.00 | 7.19 ± 0.01 | 0.99 ± 0.00 | 0.12 ± 0.00 | 186.83 ± 0.02 | 8.29 ± 0.01 | 616.15 ± 0.05 |
| proton + 0.1% | 316.26 ± 0.03 | 123.07 ± 0.02 | 3.42 ± 0.00 | 0.28 ± 0.00 | 3.25 ± 0.01 | 0.28 ± 0.00 | 0.02 ± 0.00 | 126.76 ± 0.02 | 3.55 ± 0.01 | 446.58 ± 0.04 |
| (25%↓) | (31%↓) | (57%↓) | (73%↓) | (55%↓) | (72%↓) | (85%↓) | (32%↓) | (57%↓) | (28%↓) | |
| proton + 0.1 M DMSO +0.1% | 244.96 ± 0.03 | 91.24 ± 0.01 | 1.76 ± 0.00 | 0.11 ± 0.00 | 1.74 ± 0.01 | 0.10 ± 0.00 | 0.00 ± 0.00 | 93.12 ± 0.01 | 1.85 ± 0.01 | 339.92 ± 0.03 |
| (42%↓) | (49%↓) | (78%↓) | (89%↓) | (76%↓) | (90%↓) | (97%↓) | (50%↓) | (78%↓) | (45%↓) | |
| proton + 1 M DMSO +0.1% | 196.90 ± 0.02 | 71.52 ± 0.01 | 1.06 ± 0.00 | 0.05 ± 0.00 | 1.05 ± 0.00 | 0.05 ± 0.00 | 0.00 ± 0.00 | 72.63 ± 0.01 | 1.10 ± 0.00 | 270.63 ± 0.02 |
| (53%↓) | (60%↓) | (87%↓) | (95%↓) | (85%↓) | (95%↓) | (98%↓) | (61%↓) | (87%↓) | (56%↓) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, C.-C.; Hsiao, Y.-Y. The Effects of Dimethylsulfoxide and Oxygen on DNA Damage Induction and Repair Outcomes for Cells Irradiated by 62 MeV Proton and 3.31 MeV Helium Ions. J. Pers. Med. 2021, 11, 286. https://doi.org/10.3390/jpm11040286
Chan C-C, Hsiao Y-Y. The Effects of Dimethylsulfoxide and Oxygen on DNA Damage Induction and Repair Outcomes for Cells Irradiated by 62 MeV Proton and 3.31 MeV Helium Ions. Journal of Personalized Medicine. 2021; 11(4):286. https://doi.org/10.3390/jpm11040286
Chicago/Turabian StyleChan, Chun-Chieh, and Ya-Yun Hsiao. 2021. "The Effects of Dimethylsulfoxide and Oxygen on DNA Damage Induction and Repair Outcomes for Cells Irradiated by 62 MeV Proton and 3.31 MeV Helium Ions" Journal of Personalized Medicine 11, no. 4: 286. https://doi.org/10.3390/jpm11040286
APA StyleChan, C.-C., & Hsiao, Y.-Y. (2021). The Effects of Dimethylsulfoxide and Oxygen on DNA Damage Induction and Repair Outcomes for Cells Irradiated by 62 MeV Proton and 3.31 MeV Helium Ions. Journal of Personalized Medicine, 11(4), 286. https://doi.org/10.3390/jpm11040286





