What Is the Optimal Duration of Adjuvant Mitotane Therapy in Adrenocortical Carcinoma? An Unanswered Question
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Methods
2.3. Statistical Analysis
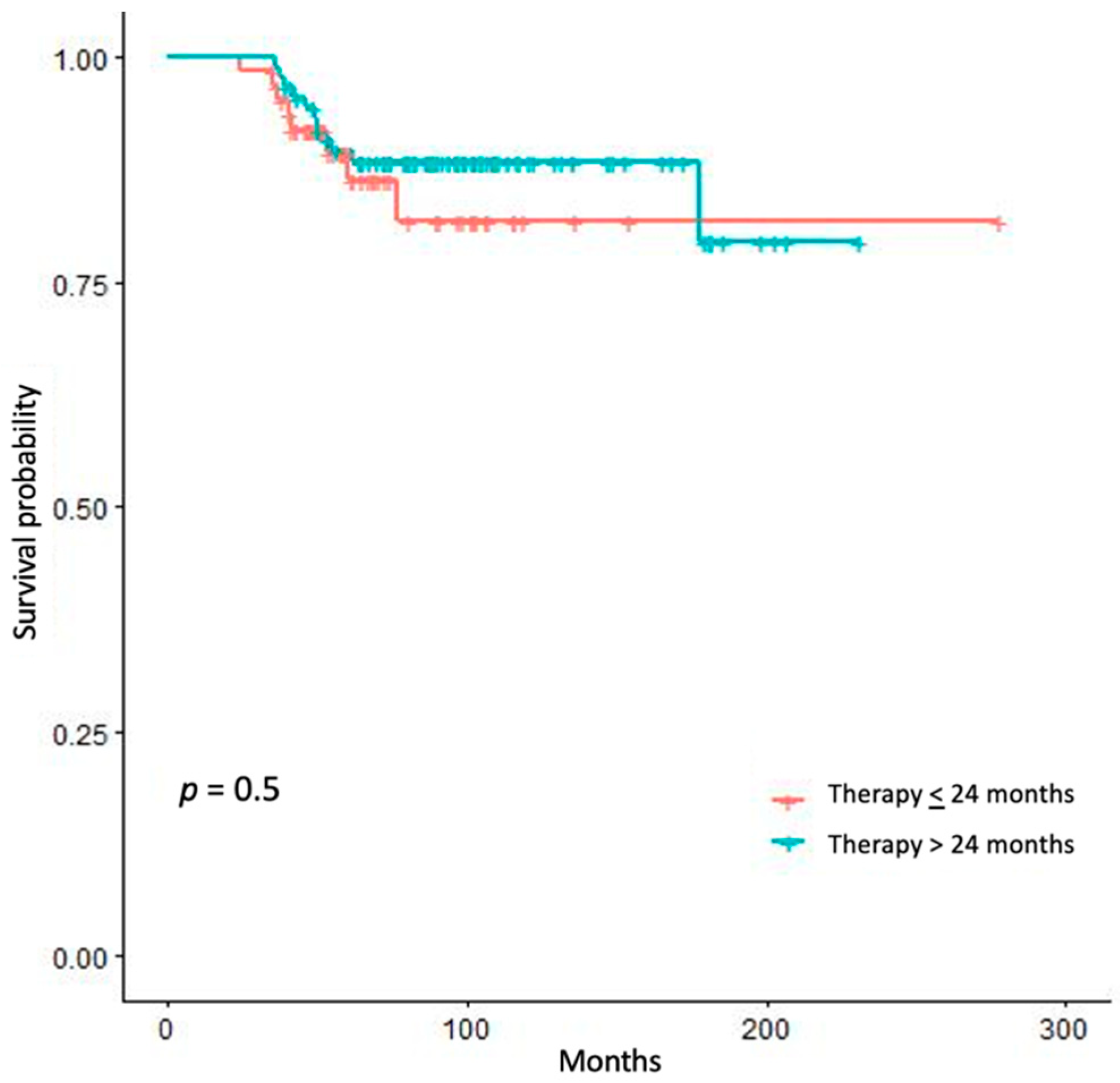
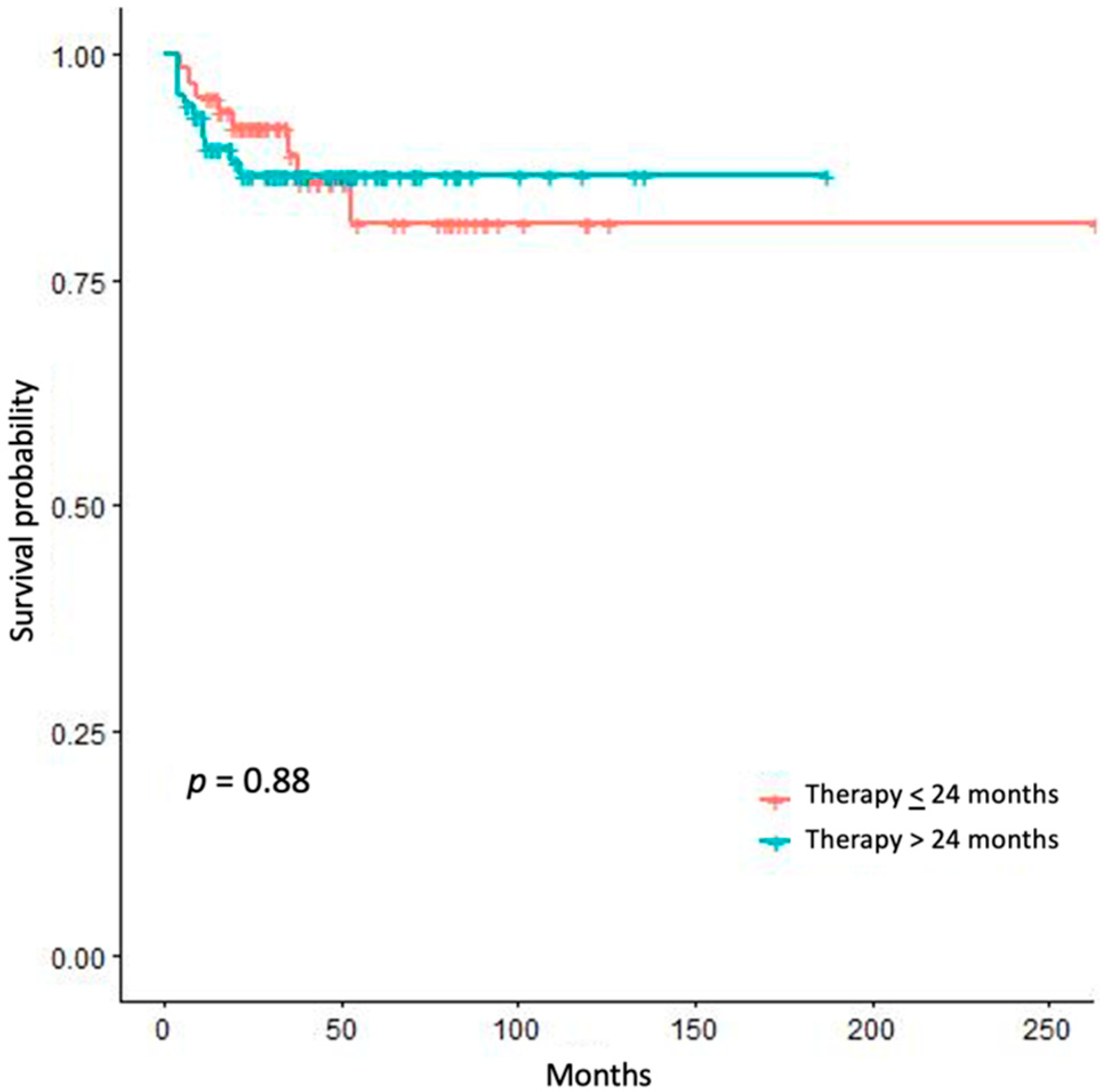
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Else, T.; Kim, A.C.; Sabolch, A.; Raymond, V.M.; Kandathil, A.; Caoili, E.M.; Jolly, S.; Miller, B.S.; Giordano, T.J.; Hammer, G.D. Adrenocortical carcinoma. Endocr. Rev. 2014, 35, 282–326. [Google Scholar] [CrossRef]
- Tierney, J.F.; Chivukula, S.V.; Poirier, J.; Pappas, S.G.; Schadde, E.; Hertl, M.; Kebebew, E.; Keutgen, X. National Treatment Practice for Adrenocortical Carcinoma: Have They Changed and Have We Made Any Progress? J. Clin. Endocrinol. Metab. 2019, 104, 5948–5956. [Google Scholar] [CrossRef] [PubMed]
- Souteiro, P.; Donato, S.; Costa, C.; Pereira, C.A.; Simoes-Pereira, J.; Oliveira, J.; Belo, S.; Santos, A.P.; Cardoso, H.; Leite, V.; et al. Diagnosis, treatment, and survival analysis of adrenocortical carcinomas: A multicentric study. Hormones 2020, 19, 197–203. [Google Scholar] [CrossRef]
- Amini, N.; Margonis, G.A.; Kim, Y.; Tran, T.B.; Postlewait, L.M.; Maithel, S.K.; Wang, T.S.; Evans, D.B.; Hatzaras, I.; Shenoy, R.; et al. Curative Resection of Adrenocortical Carcinoma: Rates and Patterns of Postoperative Recurrence. Ann. Surg. Oncol. 2016, 23, 126–133. [Google Scholar] [CrossRef]
- Bedrose, S.; Daher, M.; Altameemi, L.; Habra, M.A. Adjuvant Therapy in Adrenocortical Carcinoma: Reflections and Future Directions. Cancers 2020, 12, 508. [Google Scholar] [CrossRef]
- Lo, W.M.; Kariya, C.M.; Hernandez, J.M. Operative Management of Recurrent and Metastatic Adrenocortical Carcinoma: A Systematic Review. Am. Surg. 2019, 85, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Glenn, J.A.; Else, T.; Hughes, D.T.; Cohen, M.S.; Jolly, S.; Giordano, T.J.; Worden, F.P.; Gauger, P.G.; Hammer, G.D.; Miller, B.S. Longitudinal patterns of recurrence in patients with adrenocortical carcinoma. Surgery 2019, 165, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Gaujoux, S.; Mihai, R.; Carnaille, B.; Dousset, B.; Fiori, C.; Porpiglia, F.; Hellman, P.; Iacobone, M.; Kraimps, J.L.; Donatini, G.; et al. European Society of Endocrine Surgeons (ESES) and European Network for the Study of Adrenal Tumours (ENSAT) recommendations for the surgical management of adrenocortical carcinoma. Br. J. Surg. 2017, 104, 358–376. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, A.; Basile, V.; Puglisi, S.; Perotti, P.; Pia, A.; Saba, L.; Berchialla, P.; Porpiglia, F.; Veltri, A.; Volante, M.; et al. Adjuvant mitotane therapy is beneficial in non-metastatic adrenocortical carcinoma at high risk of recurrence. Eur. J. Endocrinol. 2019, 180, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Dekkers, O.M.; Else, T.; Baudin, E.; Berruti, A.; de Krijger, R.; Haak, H.R.; Mihai, R.; Assie, G.; Terzolo, M. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2018, 179, G1–G46. [Google Scholar] [CrossRef]
- Paragliola, R.M.; Corsello, A.; Locantore, P.; Papi, G.; Pontecorvi, A.; Corsello, S.M. Medical Approaches in Adrenocortical Carcinoma. Biomedicines 2020, 8, 551. [Google Scholar] [CrossRef]
- Terzolo, M.; Angeli, A.; Fassnacht, M.; Daffara, F.; Tauchmanova, L.; Conton, P.A.; Rossetto, R.; Buci, L.; Sperone, P.; Grossrubatscher, E.; et al. Adjuvant mitotane treatment for adrenocortical carcinoma. N. Engl. J. Med. 2007, 356, 2372–2380. [Google Scholar] [CrossRef]
- Huang, H.; Fojo, T. Adjuvant mitotane for adrenocortical cancer—A recurring controversy. J. Clin. Endocrinol. Metab. 2008, 93, 3730–3732. [Google Scholar] [CrossRef]
- Bertherat, J.; Coste, J.; Bertagna, X. Adjuvant mitotane in adrenocortical carcinoma. N. Engl. J. Med. 2007, 357, 1256–1257, author reply 1259. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, S.; Calabrese, A.; Basile, V.; Pia, A.; Reimondo, G.; Perotti, P.; Terzolo, M. New perspectives for mitotane treatment of adrenocortical carcinoma. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 101415. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, Z.; Zou, Z.; Liang, J.; Lu, Y.; Zhu, Y. Benefits of Adjuvant Mitotane after Resection of Adrenocortical Carcinoma: A Systematic Review and Meta-Analysis. Biomed. Res. Int. 2018, 2018, 9362108. [Google Scholar] [CrossRef]
- Fassnacht, M.; Assie, G.; Baudin, E.; Eisenhofer, G.; de la Fouchardiere, C.; Haak, H.R.; de Krijger, R.; Porpiglia, F.; Terzolo, M.; Berruti, A.; et al. Adrenocortical carcinomas and malignant phaeochromocytomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1476–1490. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, S.; Perotti, P.; Cosentini, D.; Roca, E.; Basile, V.; Berruti, A.; Terzolo, M. Decision-making for adrenocortical carcinoma: Surgical, systemic, and endocrine management options. Expert Rev. Anticancer Ther. 2018, 18, 1125–1133. [Google Scholar] [CrossRef]
- Weiss, L.M.; Medeiros, L.J.; Vickery, A.L. Pathologic features of prognostic significance in adrenocortical carcinoma. Am. J. Surg. Pathol. 1989, 13, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Johanssen, S.; Quinkler, M.; Bucsky, P.; Willenberg, H.S.; Beuschlein, F.; Terzolo, M.; Mueller, H.H.; Hahner, S.; Allolio, B.; et al. Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: Proposal for a Revised TNM Classification. Cancer 2009, 115, 243–250. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference; Springer: New York, NY, USA, 2002. [Google Scholar]
- Heinze, G.; Schemper, M. A solution to the problem of monotone likelihood in Cox regression. Biometrics 2001, 57, 114–119. [Google Scholar] [CrossRef]
- Dekkers, O.M.; Groenwold, R.H.H. When observational studies can give wrong answers: The potential of immortal time bias. Eur. J. Endocrinol. 2021, 184, E1–E4. [Google Scholar] [CrossRef]
- Grubbs, E.G.; Callender, G.G.; Xing, Y.; Perrier, N.D.; Evans, D.B.; Phan, A.T.; Lee, J.E. Recurrence of adrenal cortical carcinoma following resection: Surgery alone can achieve results equal to surgery plus mitotane. Ann. Surg. Oncol. 2010, 17, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Else, T.; Williams, A.R.; Sabolch, A.; Jolly, S.; Miller, B.S.; Hammer, G.D. Adjuvant therapies and patient and tumor characteristics associated with survival of adult patients with adrenocortical carcinoma. J. Clin. Endocrinol. Metab. 2014, 99, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Beuschlein, F.; Weigel, J.; Saeger, W.; Kroiss, M.; Wild, V.; Daffara, F.; Libé, R.; Ardito, A.; Al Ghuzlan, A.; Quinkler, M.; et al. Major prognostic role of Ki67 in localized adrenocortical carcinoma after complete resection. J. Clin. Endocrinol. Metab. 2015, 100, 841–849. [Google Scholar] [CrossRef]
- Postlewait, L.M.; Ethun, C.G.; Tran, T.B.; Prescott, J.D.; Pawlik, T.M.; Wang, T.S.; Glenn, J.; Hatzaras, I.; Shenoy, R.; Phay, J.E.; et al. Outcomes of Adjuvant Mitotane after Resection of Adrenocortical Carcinoma: A 13-Institution Study by the US Adrenocortical Carcinoma Group. J. Am. Coll. Surg. 2016, 222, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Berruti, A.; Grisanti, S.; Pulzer, A.; Claps, M.; Daffara, F.; Loli, P.; Mannelli, M.; Boscaro, M.; Arvat, E.; Tiberio, G.; et al. Long-Term Outcomes of Adjuvant Mitotane Therapy in Patients With Radically Resected Adrenocortical Carcinoma. J. Clin. Endocrinol. Metab. 2017, 102, 1358–1365. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Terzolo, M.; Allolio, B.; Baudin, E.; Haak, H.; Berruti, A.; Welin, S.; Schade-Brittinger, C.; Lacroix, A.; Jarzab, B.; et al. Combination chemotherapy in advanced adrenocortical carcinoma. N. Engl. J. Med. 2012, 366, 2189–2197. [Google Scholar] [CrossRef]
- Fassnacht, M.; Berruti, A.; Baudin, E.; Demeure, M.J.; Gilbert, J.; Haak, H.; Kroiss, M.; Quinn, D.I.; Hesseltine, E.; Ronchi, C.L.; et al. Linsitinib (OSI-906) versus placebo for patients with locally advanced or metastatic adrenocortical carcinoma: A double-blind, randomised, phase 3 study. Lancet Oncol. 2015, 16, 426–435. [Google Scholar] [CrossRef]

| Characteristics | Valid Cases (N) | Values |
|---|---|---|
| Sex, N (%) | 154 | |
| Male | 51 (33%) | |
| Female | 103 (67%) | |
| Age at diagnosis, years | 154 | |
| Median (IQR) | 45 (34–54) | |
| Tumor stage at diagnosis, N (%) | 154 | |
| Stage I | 14 (9%) | |
| Stage II | 110 (71%) | |
| Stage III | 30 (19%) | |
| Hormone secretion at diagnosis, N (%) | 113 | |
| No | 43 (38%) | |
| Yes | 70 (62%) | |
| Glucocorticoid | 41 (59%) | |
| Androgen | 21 (30%) | |
| Aldosterone | 5 (7%) | |
| Other | 3 (4%) | |
| Tumor size, cm | 148 | |
| Median (IQR) | 10 (7–15) | |
| Ki67 at diagnosis | 125 | |
| Median (IQR) | 10 (5–20) | |
| ≤10% | 75 (60%) | |
| >10% | 50 (40%) | |
| Weiss at diagnosis | 132 | |
| Median (IQR) | 6 (4–6) | |
| Duration of mitotane therapy, months | 154 | |
| Median (IQR) | 33 (24–59) |
| Characteristics | Group 1 n. 52 Patients (Treated for 13–25 Months) | Group 2 n. 51 Patients (Treated for 26–48 Months) | Group 3 n. 51 Patients (Treated for 49–143 Months) | p-Value |
|---|---|---|---|---|
| Sex, N (%) | 0.15 | |||
| Male | 16 (31%) | 13 (25%) | 22 (43%) | |
| Female | 36 (69%) | 38 (75%) | 29 (57%) | |
| Age at diagnosis, years | 0.15 | |||
| Median (IQR) | 47.5 (38.5–58) | 45 (32.5–53) | 43 (34–51.5) | |
| Tumor stage at diagnosis, N (%) | 0.44 | |||
| Stage I | 4 (8%) | 5 (10%) | 5 (10%) | |
| Stage II | 41 (79%) | 37 (72%) | 32 (63%) | |
| Stage III | 7 (13%) | 9 (18%) | 14 (27%) | |
| Hormone secretion at diagnosis, N (%) | 0.41 | |||
| No | 31 (60%) | 24 (47%) | 29 (57%) | |
| Yes | 21 (40%) | 27 (53%) | 22 (43%) | |
| Size tumor at diagnosis, cm | 0.80 | |||
| Median (IQR) | 9.5 (7.2–14.5) | 10.5 (7.6–14) | 10 (6.7–15.5) | |
| Ki67 at diagnosis | 0.014 | |||
| Median (IQR) | 10 (5–10) | 10 (5–19) | 15 (6–23) | |
| Weiss at diagnosis | 0.39 | |||
| Median (range) | 5 (4–6) | 6 (4–7) | 6 (5–7) | |
| Recurrence, N (%) | 0.001 | |||
| No | 47 (90%) | 38 (75%) | 50 (98%) | |
| Yes | 5 (10%) | 13 (25%) | 1 (2%) | |
| RFS, months | <0.001 | |||
| Median (IQR) | 61 (49–97) | 59 (48–85) | 108 (90–151) | |
| RFSAM, months | 0.002 | |||
| Median (IQR) | 38 (26–78) | 22 (11–47) | 35 (24–62) | |
| OSAM, months | 0.19 | |||
| Median (IQR) | 44 (26–78) | 37 (22–53) | 35 (27–62) |
| Causes of Mitotane Discontinuation | Group 1 n. 52 Patients (Treated for 13–25 Months) | Group 2 n. 51 Patients (Treated for 26–48 Months) | Group 3 n. 51 Patients (Treated for 49–143 Months) |
|---|---|---|---|
| End of schedule | 30 (57.7%) | 38 (74.5%) | 47 (92.2%) |
| Adverse effects | 20 (38.5%) | 8 (15.7%) | 2 (3.9%) |
| Unattainable target level | 0 | 2 (3.9%) | 0 |
| Severe concomitant disease | 1 (1.9%) | 1 (1.9%) | 0 |
| Other * | 1 (1.9%) | 2 (3.9%) | 2 (3.9%) |
| Univariate Analysis | Diff | HR | 95% CI | p | |
|---|---|---|---|---|---|
| Duration of mitotane therapy + | 1.302 | 0.509 | 3.334 | 0.58 | |
| § R status | 0.722 | 0.208 | 2.503 | 0.61 | |
| ‡ * Hormone secretion | 1.441 | 0.571 | 3.640 | 0.44 | |
| * ° Stage | 0.917 | 0.272 | 2.526 | 0.87 | |
| * Tumor size | 7.925 | 0.942 | 0.514 | 1.727 | 0.85 |
| * Weiss | 2.000 | 1.589 | 0.861 | 2.932 | 0.14 |
| * Ki67% | 15.000 | 0.805 | 0.426 | 1.521 | 0.50 |
| Univariate Analysis | Diff | HR | 95% CI | p | |
|---|---|---|---|---|---|
| Duration of mitotane therapy + | 0.894 | 0.354 | 2.257 | 0.812 | |
| § R status | 0.843 | 0.243 | 2.924 | 0.788 | |
| ‡ * Hormone secretion | 1.357 | 0.543 | 3.391 | 0.513 | |
| * ° Stage | 1.118 | 0.342 | 2.993 | 0.838 | |
| * Tumor size | 7.925 | 0.977 | 0.532 | 1.792 | 0.939 |
| * Weiss | 2.000 | 1.766 | 0.957 | 3.260 | 0.069 |
| * Ki67% | 15.000 | 0.820 | 0.442 | 1.521 | 0.529 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basile, V.; Puglisi, S.; Altieri, B.; Canu, L.; Libè, R.; Ceccato, F.; Beuschlein, F.; Quinkler, M.; Calabrese, A.; Perotti, P.; et al. What Is the Optimal Duration of Adjuvant Mitotane Therapy in Adrenocortical Carcinoma? An Unanswered Question. J. Pers. Med. 2021, 11, 269. https://doi.org/10.3390/jpm11040269
Basile V, Puglisi S, Altieri B, Canu L, Libè R, Ceccato F, Beuschlein F, Quinkler M, Calabrese A, Perotti P, et al. What Is the Optimal Duration of Adjuvant Mitotane Therapy in Adrenocortical Carcinoma? An Unanswered Question. Journal of Personalized Medicine. 2021; 11(4):269. https://doi.org/10.3390/jpm11040269
Chicago/Turabian StyleBasile, Vittoria, Soraya Puglisi, Barbara Altieri, Letizia Canu, Rossella Libè, Filippo Ceccato, Felix Beuschlein, Marcus Quinkler, Anna Calabrese, Paola Perotti, and et al. 2021. "What Is the Optimal Duration of Adjuvant Mitotane Therapy in Adrenocortical Carcinoma? An Unanswered Question" Journal of Personalized Medicine 11, no. 4: 269. https://doi.org/10.3390/jpm11040269
APA StyleBasile, V., Puglisi, S., Altieri, B., Canu, L., Libè, R., Ceccato, F., Beuschlein, F., Quinkler, M., Calabrese, A., Perotti, P., Berchialla, P., Dischinger, U., Megerle, F., Baudin, E., Bourdeau, I., Lacroix, A., Loli, P., Berruti, A., Kastelan, D., ... Terzolo, M. (2021). What Is the Optimal Duration of Adjuvant Mitotane Therapy in Adrenocortical Carcinoma? An Unanswered Question. Journal of Personalized Medicine, 11(4), 269. https://doi.org/10.3390/jpm11040269











