Real Life Use of Bendamustine in Elderly Patients with Lymphoid Neoplasia
Abstract
1. Background
2. Aims
3. Materials and Methods
4. Results
4.1. Patients and Treatments
4.2. Efficacy Outcomes
4.2.1. Overall Response Rate
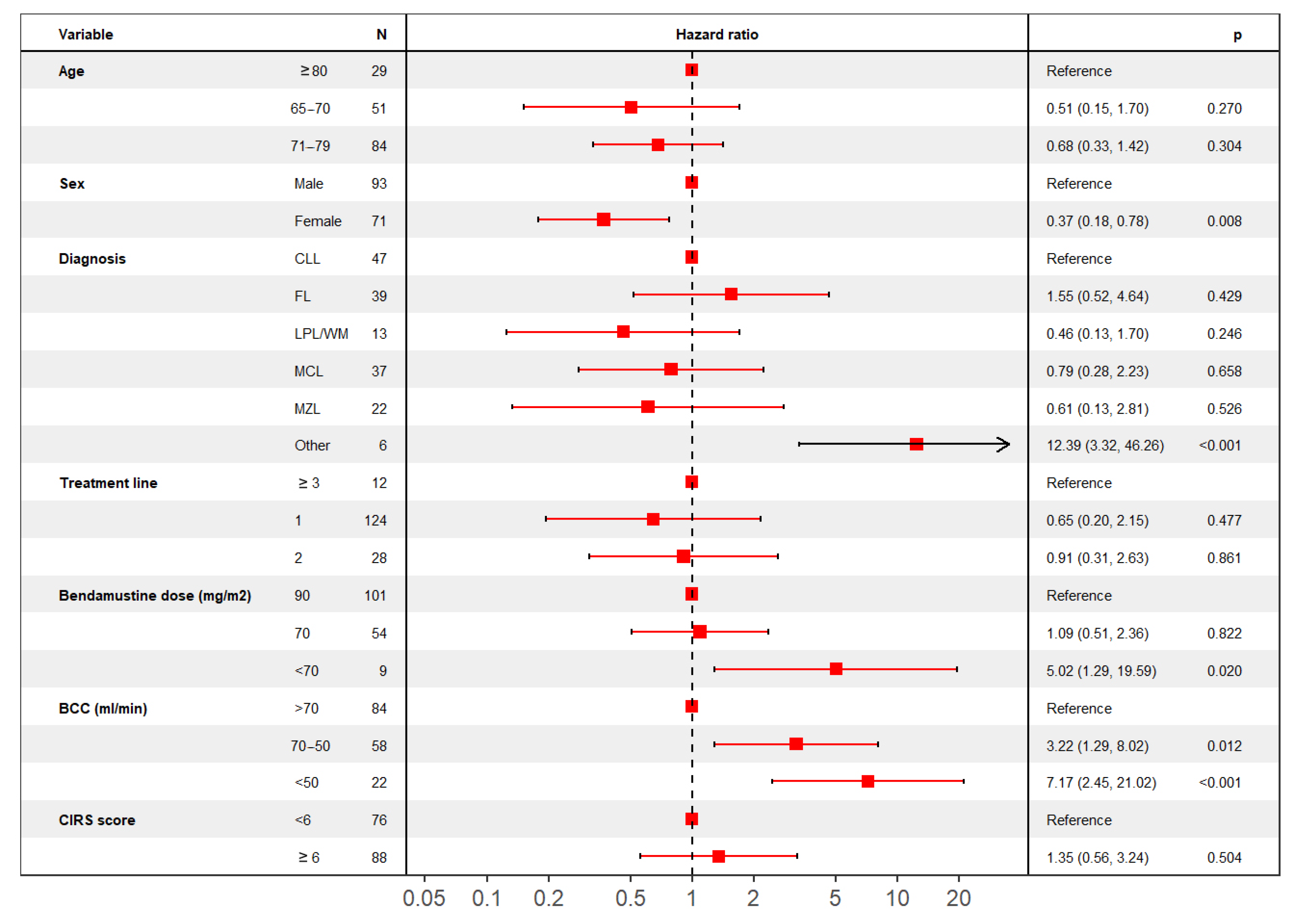
4.2.2. Overall Survival
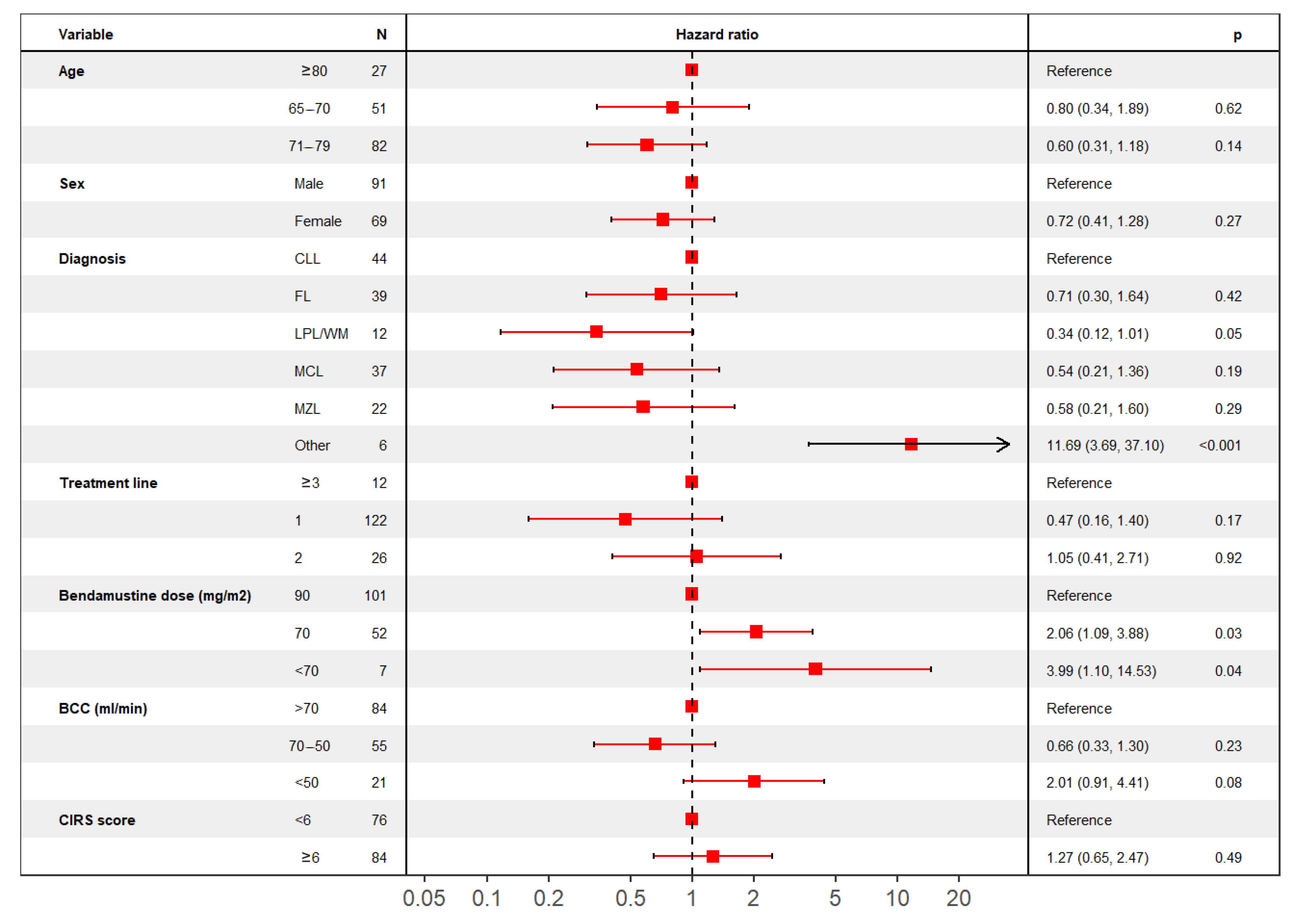
4.2.3. Time to Progression
4.3. Safety
5. Discussion
Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Darwish, M.; Bond, M.; Hellriegel, E.; Robertson, P.J.; Chovan, J.P. Pharmacokinetic and pharmacodynamic profile of bendamustine and its metabolites. Cancer Chemother. Pharmacol. 2015, 75, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Cheson, B.D.; Brugger, W.; Damaj, G.; Dreyling, M.; Kahl, B.; Kimby, E.; Ogura, M.; Weidmann, E.; Wendtner, C.-M.; Zinzani, P.L. Optimal use of bendamustine in hematologic disorders: Treatment recommendations from an international consensus panel—An update. Leuk. Lymphoma 2016, 57, 766–782. [Google Scholar] [CrossRef] [PubMed]
- Eichhorst, B.; Ghia, P. EHA Endorsement of ESMO Clinical Practice Guidelines for Diagnosis, Treatment, and Follow-up of Chronic Lymphocytic Leukemia. HemaSphere 2021, 5, e520. [Google Scholar] [CrossRef]
- Kater, A.P.; Wu, J.Q.; Kipps, T.; Eichhorst, B.; Hillmen, P.; D’Rozario, J.; Assouline, S.; Owen, C.; Robak, T.; de la Serna, J.; et al. Venetoclax Plus Rituximab in Relapsed Chronic Lymphocytic Leukemia: 4-Year Results and Evaluation of Impact of Genomic Complexity and Gene Mutations From the MURANO Phase III Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 4042–4054. [Google Scholar] [CrossRef] [PubMed]
- Woyach, J.A.; Ruppert, A.S.; Heerema, N.A.; Zhao, W.; Booth, A.M.; Ding, W.; Bartlett, N.L.; Brander, D.M.; Barr, P.M.; Rogers, K.A.; et al. Ibrutinib Regimens versus Chemoimmunotherapy in Older Patients with Untreated CLL. N. Engl. J. Med. 2018, 379, 2517–2528. [Google Scholar] [CrossRef]
- Cuneo, A.; Follows, G.; Rigolin, G.M.; Piciocchi, A.; Tedeschi, A.; Trentin, L.; Perez, A.M.; Coscia, M.; Laurenti, L.; Musuraca, G.; et al. Efficacy of bendamustine and rituximab as first salvage treatment in chronic lymphocytic leukemia and indirect comparison with ibrutinib: A GIMEMA, ERIC and UK CLL FORUM study. Haematologica 2018, 103, 1209–1217. [Google Scholar] [CrossRef]
- Cuneo, A.; Mato, A.R.; Rigolin, G.M.; Piciocchi, A.; Gentile, M.; Laurenti, L.; Allan, J.N.; Pagel, J.M.; Brander, D.M.; Hill, B.T.; et al. Efficacy of bendamustine and rituximab in unfit patients with previously untreated chronic lymphocytic leukemia. Indirect comparison with ibrutinib in a real-world setting. A GIMEMA-ERIC and US study. Cancer Med. 2020, 9, 8468–8479. [Google Scholar] [CrossRef]
- Rummel, M.J.; Niederle, N.; Maschmeyer, G.; Banat, G.A.; von Grünhagen, U.; Losem, C.; Kofahl-Krause, D.; Heil, G.; Welslau, M.; Balser, C.; et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: An open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 2013, 381, 1203–1210. [Google Scholar] [CrossRef]
- Rummel, M.; Kaiser, U.; Balser, C.; Stauch, M.; Brugger, W.; Welslau, M.; Niederle, N.; Losem, C.; Boeck, H.-P.; Weidmann, E.; et al. Bendamustine plus rituximab versus fludarabine plus rituximab for patients with relapsed indolent and mantle-cell lymphomas: A multicentre, randomised, open-label, non-inferiority phase 3 trial. Lancet Oncol. 2016, 17, 57–66. [Google Scholar] [CrossRef]
- Flinn, I.W.; van der Jagt, R.; Kahl, B.S.; Wood, P.; Hawkins, T.E.; Macdonald, D.; Hertzberg, M.; Kwan, Y.-L.; Simpson, D.; Craig, M.; et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: The BRIGHT study. Blood 2014, 123, 2944–2952. [Google Scholar] [CrossRef]
- Flinn, I.; van der Jagt, R.; Chang, J.E.; Wood, P.; Hawkins, T.E.; MacDonald, D.; Trotman, J.; Simpson, D.; Kolibaba, K.S.; Issa, S.; et al. First-line treatment of iNHL or MCL patients with BR or R-CHOP/R-CVP: Results of the BRIGHT 5-year follow-up study. J. Clin. Oncol. 2017, 35, 7500. [Google Scholar] [CrossRef]
- Michallet, A.-S.; Aktan, M.; Hiddemann, W.; Ilhan, O.; Johansson, P.; Laribi, K.; Meddeb, B.; Moreno, C.; Raposo, J.; Schuh, A.; et al. Rituximab plus bendamustine or chlorambucil for chronic lymphocytic leukemia: Primary analysis of the randomized, open-label MABLE study. Haematologica 2018, 103, 698–706. [Google Scholar] [CrossRef]
- Vidal, L.; Gafter-Gvili, A.; Gurion, R.; Raanani, P.; Dreyling, M.; Shpilberg, O. Bendamustine for patients with indolent B cell lymphoid malignancies including chronic lymphocytic leukaemia. Cochrane Database Syst. Rev. 2012, CD009045. [Google Scholar] [CrossRef] [PubMed]
- Gafter-Gvili, A.; Gurion, R.; Raanani, P.; Shpilberg, O.; Vidal, L. Bendamustine-associated infections-systematic review and meta-analysis of randomized controlled trials. Hematol. Oncol. 2017, 35, 424–431. [Google Scholar] [CrossRef]
- Fung, M.; Jacobsen, E.; Freedman, A.; Prestes, D.; Farmakiotis, D.; Gu, X.; Nguyen, P.L.; Koo, S. Increased Risk of Infectious Complications in Older Patients With Indolent Non-Hodgkin Lymphoma Exposed to Bendamustine. Clin. Infect. Dis. 2018, 68, 247–255. [Google Scholar] [CrossRef]
- Kath, R.; Blumenstengel, K.; Fricke, H.J.; Höffken, K. Bendamustine monotherapy in advanced and refractory chronic lymphocytic leukemia. J. Cancer Res. Clin. Oncol. 2001, 127, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Danilov, A.V.; Lewis, L.D.; Lansigan, F.; Roudaia, L.; Findley, D.L.; Jones, S.Y.; Highhouse, B.; Beaulieu, B.B.; Brown, J.R. A phase I dose-ranging study of bendamustine and rituximab in chronic lymphocytic leukemia patients with comorbidities. Br. J. Haematol. 2017, 178, 820–823. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.J.; Lewis, L.D.; Brown, J.R.; Danilov, A. V Bendamustine hydrochloride in patients with B-cell malignancies who have comorbidities—Is there an optimal dose? Expert Rev. Hematol. 2017, 10, 707–718. [Google Scholar] [CrossRef]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 3059–3068. [Google Scholar] [CrossRef] [PubMed]
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Döhner, H.; Hillmen, P.; Keating, M.; Montserrat, E.; Chiorazzi, N.; et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood 2018, 131, 2745–2760. [Google Scholar] [CrossRef]
- Assembly, W.G. WMA Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar]
- Cockcroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604. [Google Scholar] [CrossRef]
- Boccomini, C.; Ladetto, M.; Rigacci, L.; Puccini, B.; Rattotti, S.; Volpetti, S.; Ferrero, S.; Chiarenza, A.; Freilone, R.; Novo, M.; et al. A brief rituximab, bendamustine, mitoxantrone (R-BM) induction followed by rituximab consolidation in elderly patients with advanced follicular lymphoma: A phase II study by the Fondazione Italiana Linfomi (FIL). Br. J. Haematol. 2021. [Google Scholar] [CrossRef]
- Eichhorst, B.; Fink, A.-M.; Bahlo, J.; Busch, R.; Kovacs, G.; Maurer, C.; Lange, E.; Köppler, H.; Kiehl, M.; Sökler, M.; et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): An international, open-label, randomised, phase 3, non-inferiority trial. Lancet. Oncol. 2016, 17, 928–942. [Google Scholar] [CrossRef]
- Ghia, P.; Pluta, A.; Wach, M.; Lysak, D.; Kozak, T.; Simkovic, M.; Kaplan, P.; Kraychok, I.; Illes, A.; de la Serna, J.; et al. ASCEND: Phase III, Randomized Trial of Acalabrutinib Versus Idelalisib Plus Rituximab or Bendamustine Plus Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 2849–2861. [Google Scholar] [CrossRef]
- Seymour, J.F.; Kipps, T.J.; Eichhorst, B.; Hillmen, P.; D’Rozario, J.; Assouline, S.; Owen, C.; Gerecitano, J.; Robak, T.; De la Serna, J.; et al. Venetoclax–Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2018, 378, 1107–1120. [Google Scholar] [CrossRef]
- Zelenetz, A.D.; Barrientos, J.C.; Brown, J.R.; Coiffier, B.; Delgado, J.; Egyed, M.; Ghia, P.; Illés, Á.; Jurczak, W.; Marlton, P.; et al. Idelalisib or placebo in combination with bendamustine and rituximab in patients with relapsed or refractory chronic lymphocytic leukaemia: Interim results from a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. Oncol. 2017, 18, 297–311. [Google Scholar] [CrossRef]
- Mattsson, A.; Sylvan, S.E.; Asklid, A.; Wiggh, J.; Winqvist, M.; Lundin, J.; Mansouri, L.; Rosenquist, R.; Johansson, H.; Österborg, A.; et al. Risk-adapted bendamustine + rituximab is a tolerable treatment alternative for elderly patients with chronic lymphocytic leukaemia: A regional real-world report on 141 consecutive Swedish patients. Br. J. Haematol. 2020, 191, 426–432. [Google Scholar] [CrossRef]
- Kleeberg, U.R.; Linde, H.; Gunther, G.; Tessen, H.-W.; Kersting, M. Bendamustin-Rituximab Combination Is a Safe and Effective, Ambulatory Treatment for Elderly Patients with Chronic Lymphocytic Leukemia: Retrospective Real-world Analysis by Age from a German Registry and Review of the Literature. Anticancer Res. 2016, 36, 2827–2838. [Google Scholar]
- Panovská, A.; Němcová, L.; Nekvindová, L.; Špaček, M.; Šimkovič, M.; Papajík, T.; Brejcha, M.; Lysák, D.; Zuchnická, J.; Novák, J.; et al. Real-world data on efficacy and safety of obinutuzumab plus chlorambucil, rituximab plus chlorambucil, and rituximab plus bendamustine in the frontline treatment of chronic lymphocytic leukemia: The GO-CLLEAR Study by the Czech CLL Study Group. Hematol. Oncol. 2020, 38, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Hiddemann, W.; Barbui, A.M.; Canales, M.A.; Cannell, P.K.; Collins, G.P.; Dürig, J.; Forstpointner, R.; Herold, M.; Hertzberg, M.; Klanova, M.; et al. Immunochemotherapy With Obinutuzumab or Rituximab for Previously Untreated Follicular Lymphoma in the GALLIUM Study: Influence of Chemotherapy on Efficacy and Safety. J. Clin. Oncol. 2018, 36, 2395–2404. [Google Scholar] [CrossRef] [PubMed]
- Flinn, I.W.; van der Jagt, R.; Kahl, B.; Wood, P.; Hawkins, T.; MacDonald, D.; Simpson, D.; Kolibaba, K.; Issa, S.; Chang, J.; et al. First-Line Treatment of Patients With Indolent Non-Hodgkin Lymphoma or Mantle-Cell Lymphoma With Bendamustine Plus Rituximab Versus R-CHOP or R-CVP: Results of the BRIGHT 5-Year Follow-Up Study. J. Clin. Oncol. 2019, 37, 984–991. [Google Scholar] [CrossRef]
- Saito, H.; Maruyama, D.; Maeshima, A.M.; Makita, S.; Kitahara, H.; Miyamoto, K.; Fukuhara, S.; Munakata, W.; Suzuki, T.; Kobayashi, Y.; et al. Prolonged lymphocytopenia after bendamustine therapy in patients with relapsed or refractory indolent B-cell and mantle cell lymphoma. Blood Cancer J. 2015, 5, e362. [Google Scholar] [CrossRef] [PubMed]



| Characteristic (n. of Analyzed Patients) * | Median (IQR) or n. (%) |
|---|---|
| All patients | 179 |
| Age (median [IQR]) | 74.00 (70.00, 78.00) |
| Age distribution (%) | |
| 65–70 | 54 (30.2) |
| 71–79 | 94 (52.5) |
| ≥80 | 31 (17.3) |
| Sex (%) | |
| Male | 98 (54.7) |
| Female | 81 (45.3) |
| Diagnosis (%) | |
| FL | 43 (24.0) |
| CLL | 51 (28.5) |
| MCL | 41 (22.9) |
| MZL | 22 (12.3) |
| LPL/WM | 16 (8.9) |
| HD | 3 (1.7) |
| DLBCL | 1 (0.6) |
| other | 2 (1.1) |
| Stage (%) (151) | |
| 0 | 12 (7.9) |
| 1 | 13 (8.6) |
| 2 | 12 (7.9) |
| 3 | 17 (11.3) |
| 4 | 97 (64.2) |
| Symptoms (%) (158) | |
| A | 130 (82.3) |
| B | 28 (17.7) |
| Total lines (%) (178) | |
| 1 | 97 (54.5) |
| 2 | 31 (17.4) |
| ≥3 | 50 (28.1) |
| Previous ASCT (%) (178) | |
| No | 175 (98.3) |
| Yes | 3 (1.7) |
| Bendamustine treatment line (%) | |
| 1 | 131 (73.2) |
| 2 | 32 (17.9) |
| ≥3 | 16 (8.9) |
| BCC at bendamustine treatment start (%) (166) | |
| >70 mL/min | 85 (51.2) |
| 70–50 mL/min | 58 (34.9) |
| <50 mL/min | 23 (13.9) |
| CIRS at bendamustine treatment start (%) (177) | |
| <6 | 81 (45.8) |
| ≥6 | 96 (54.2) |
| Severe comorbidity § (%) (175) | |
| Yes | 159 (90.9) |
| No | 16 (9.1) |
| Characteristic (n. of Analyzed Patients) * | n. (%) |
|---|---|
| Bendamustine containing regimen (%) | |
| Bendamustine ± antiCD20 | 146 (81.6) |
| BEGEV | 6 (3.4) |
| BAC | 13 (7.3) |
| Other | 14 (7.8) |
| Bendamustine dose (%) (173) | |
| 90 mg/m2 | 106 (61.3) |
| 70 mg/m2 | 58 (33.5) |
| <70 mg/m2 | 9 (5.2) |
| Anti-CD20 monoclonal antibody (%) | |
| No | 17 (9.5) |
| Rituximab | 160 (89.4) |
| Obinutuzumab | 2 (1.1) |
| N. of completed cycles (%) | |
| <4 | 28 (15.7) |
| At least 4 | 150 (84.3) |
| Up to 6 | 120 (67.4) |
| Therapy interruption (%) | |
| No | 142 (79.3) |
| Yes | 37 (20.7) |
| Dose delay (%) (177) | |
| No | 112 (63.3) |
| Yes (at least 1 week delay) | 65 (36.7) |
| Dose reduction (%) (178) | |
| No | 155 (87.1) |
| Yes | 23 (12.9) |
| G-CSF prophylaxis (%) | |
| No | 102 (57.0) |
| Yes | 77 (43.0) |
| Erythropoietin prophylaxis (%) (177) | |
| No | 148 (83.6) |
| Yes | 29 (16.4) |
| Trimethoprim-sulfamethoxazole prophylaxis (%) (177) | |
| No | 31 (17.5) |
| Yes | 146 (82.5) |
| Acyclovir prophylaxis (%) (177) | |
| No | 105 (59.3) |
| Yes | 72 (40.7) |
| Adverse Event (n. of Analyzed Patients) * | n. (%) |
|---|---|
| Hematologic | |
| Neutropenia (%) | |
| No | 78 (43.6) |
| Yes (any grade) | 101 (56.4) |
| Grade < 3 | 22 (12.3) |
| Grade ≥ 3 | 79 (43.5) |
| Thrombocytopenia (%) | |
| No | 100 (55.9) |
| Yes (any grade) | 79 (44.1) |
| Grade < 3 | 58 (32.4) |
| Grade ≥ 3 | 21 (11.7) |
| Anemia (%) | |
| No | 74 (41.3) |
| Yes (any grade) | 105 (58.7) |
| Grade < 3 | 84 (46.9) |
| Grade ≥ 3 | 21 (11.7) |
| Extra-hematologic | |
| Patients who had infections (%) | |
| No | 115 (64.2) |
| Yes | 64 (35.7) |
| Infectious events (%) | |
| Respiratory infections | 25 (14) |
| Fever of unknown origin (FUO) | 13 (7.3) |
| Gastrointestinal infections | 7 (3.9) |
| VZV cutaneous reactivation | 6 (3.3) |
| UTI | 5 (2.8) |
| Oral infections | 5 (2.8) |
| Sepsis | 5 (2.8) |
| CMV reactivation | 2 (1.1) |
| Febrile neutropenia | 2 (1.1) |
| Other | 11 (6.1) |
| Infectious etiology (%) | |
| Bacterial | 27 (15.1) |
| Viral | 14 (7.8) |
| Fungal | 6 (3.3) |
| Unknown | 34 (19) |
| Other toxicities (%) (177) | |
| No | 126 (71.2) |
| Autoimmune disorder (e.g., AIHA) | 4 (2.3) |
| Skin toxicity | 15 (8.5) |
| Other | 32 (18.1) |
| Admission-requiring AEs (%) | |
| No | 148 (82.7) |
| Yes | 31 (17.3) |
| Length of stay (days [median]) | 10.00 [6.00, 20.00] |
| Cause of admission (%) | |
| Sepsis | 9 (5) |
| Fever of unknown origin (FUO) | 5 (2.8) |
| Pneumonia | 4 (2.2) |
| Gastrointestinal complications | 3 (1.7) |
| Cardiovascular complications | 3 (1.7) |
| Acute kidney injury | 2 (1.1) |
| CMV reactivation | 1 (0.6) |
| Steven-Johnson syndrome | 1 (0.6) |
| Other complications | 3 (1.7) |
| Second cancer (%) | |
| No | 139 (77.7) |
| Yes | 40 (22.3) |
| Second cancer type (%) | |
| Hematologic cancer | 10 (5.6) |
| Urogenital cancer | 8 (4.5) |
| Gastrointestinal cancer | 8 (4.5) |
| Skin cancer | 7 (3.9) |
| Lung cancer | 3 (1.7) |
| Breast cancer | 1 (0.6) |
| Other | 3 (1.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dogliotti, I.; Ragaini, S.; Vassallo, F.; Boccellato, E.; De Luca, G.; Perutelli, F.; Boccomini, C.; Clerico, M.; Botto, B.; Grimaldi, D.; et al. Real Life Use of Bendamustine in Elderly Patients with Lymphoid Neoplasia. J. Pers. Med. 2021, 11, 249. https://doi.org/10.3390/jpm11040249
Dogliotti I, Ragaini S, Vassallo F, Boccellato E, De Luca G, Perutelli F, Boccomini C, Clerico M, Botto B, Grimaldi D, et al. Real Life Use of Bendamustine in Elderly Patients with Lymphoid Neoplasia. Journal of Personalized Medicine. 2021; 11(4):249. https://doi.org/10.3390/jpm11040249
Chicago/Turabian StyleDogliotti, Irene, Simone Ragaini, Francesco Vassallo, Elia Boccellato, Gabriele De Luca, Francesca Perutelli, Carola Boccomini, Michele Clerico, Barbara Botto, Daniele Grimaldi, and et al. 2021. "Real Life Use of Bendamustine in Elderly Patients with Lymphoid Neoplasia" Journal of Personalized Medicine 11, no. 4: 249. https://doi.org/10.3390/jpm11040249
APA StyleDogliotti, I., Ragaini, S., Vassallo, F., Boccellato, E., De Luca, G., Perutelli, F., Boccomini, C., Clerico, M., Botto, B., Grimaldi, D., Orsucci, L., Ferrero, S., Vitale, C., Ferrero, D., Coscia, M., & Cavallo, F. (2021). Real Life Use of Bendamustine in Elderly Patients with Lymphoid Neoplasia. Journal of Personalized Medicine, 11(4), 249. https://doi.org/10.3390/jpm11040249







