Abstract
Rivaroxaban has become an alternative to vitamin K antagonists, which are considered to be at higher risk of drug-drug interactions (DDI) and more difficult to use. However, DDI do occur. We systematically reviewed studies that evaluated them and analysed DDI and subsequent adverse drug reactions (ADR) reported in spontaneous reports and VigiBase. We systematically searched articles that explored DDI with rivaroxaban up to 20 August 2018 via Medline, Embase and Google Scholar. Data from VigiBase came from spontaneous reports recovered up to 2 January 2018, where Omega was used to detect signals and identify potential interactions in terms of triplets with two drugs and one ADR. We identified 31 studies and 28 case reports. Studies showed significant variation in the pharmacokinetic for rivaroxaban, and an increased risk of haemorrhage or thromboembolic events due to DDI was highlighted in case reports. From VigiBase, a total of 21,261 triplets were analysed and the most reported was rivaroxaban–aspirin–gastrointestinal haemorrhage. In VigiBase, only 34.8% of the DDI reported were described or understood, and most were pharmacodynamic DDI. These data suggest that rivaroxaban should be considered to have significant potential for DDI, especially with CYP3A/P-gp modulators or with drugs that impair haemostasis.
1. Introduction
Unlike heparin or vitamin K antagonists (VKAs), direct oral anticoagulants (DOACs) act by direct inhibition of coagulation factor Xa or factor II (thrombin). Their pharmacological profile is deemed predictable, safe and suitable for long-term use [1]. While VKAs were the only available oral anticoagulants for more than 50 years, clinical requirements for a variety of indications in adults and the willingness to have new antithrombotic drugs on hand with an optimal balance between efficacy and risk of bleeding led to the emergence of DOACs [2]. Indeed, DOACs are considered easier to use because they have a wide therapeutic window, less interindividual variability, and higher oral bioavailability that is less impacted by food intake or bodyweight than warfarin, the reference treatment [3]. Therefore, they no longer needed to be individualised on a daily basis like VKAs, which require monitoring of the international normalised ratio (INR) [3]. However, although DOACs are less influenced by food or bodyweight, small dose adjustments are necessary for a high-dose of rivaroxaban and for low-weight patients < 60 kg taking apixaban respectively [3].
There are currently five DOACs approved for use worldwide: dabigatran, an oral direct thrombin (factor II) inhibitor [4]; rivaroxaban; apixaban; edoxaban, and; betrixaban [5], which are oral direct factor Xa inhibitors [5].
Rivaroxaban was the first oral direct factor Xa inhibitor approved, and it is used to prevent and/or treat venous thromboembolism (VTE) and prevent the occurrence of ischaemic stroke and embolism in individuals with nonvalvular atrial fibrillation (NVAF) [2]. In patients with NVAF and acute symptomatic VTE, studies have demonstrated that rivaroxaban is as effective as the standard therapy [6,7,8]. Moreover, rivaroxaban was superior to enoxaparin for the prevention of VTE in patients undergoing major orthopaedic surgery [9,10,11,12]. There is, therefore, no additional need for a priori monitoring of specific laboratory parameters, but anti-Xa factor could be used in specific cases where measurement is needed, for example, to confirm an overdose [13].
In addition to its ease of use and efficacy, rivaroxaban is considered to have a low risk of drug-drug interactions (DDIs), although two-thirds of rivaroxaban elimination takes place via conversion to inactive metabolites in the liver by CYP3A [3]. Rivaroxaban also carries an inherent risk of bleeding, and its coadministration with other drugs affecting haemostasis can lead to an increased risk of haemorrhage [14].
Like all DOACs, rivaroxaban has certain limitations in its use [15]. Rivaroxaban is contraindicated in women during pregnancy and lactation and in children because no data are available for these populations [16]. In addition, rivaroxaban should not be prescribed in patients with severe hepatic (Child Pugh C), renal impairment (creatinine clearance < 15 mL/min), antiphospholipid syndrome or mechanical heart valves [16]. No dose adjustments are recommended for rivaroxaban based on sex, age or bodyweight [17].
Regarding the safety profile, patients receiving rivaroxaban for any therapeutic indication have a lower risk of intracranial bleeding compared to patients receiving VKAs alone or in sequential treatment with low-molecular-weight heparins [18]. However, gastrointestinal bleeding seems to be more frequent [19,20]. Bleeding is not the only safety concern with rivaroxaban, as it has been associated with a risk of hepatotoxicity in a review that analysed data from case reports, case series and spontaneous reports [20,21,22]. However, in more than one-third of the drug-induced liver injuries (DILIs) observed, concomitant use of possible hepatotoxic and/or interacting drugs was also reported [21,22]. Based on these results, the authors suggested that there is a need to re-evaluate the risk of DILI associated with rivaroxaban and the importance of post-marketing pharmacovigilance to detect these potential adverse drug reactions (ADRs) [21,22].
The global aim of this study was to evaluate DDIs causing ADRs with rivaroxaban through a review of currently published data in the literature and a real-world evaluation of rivaroxaban’s interaction data from VigiBase, the WHO (World Health Organization) global database of individual case safety reports (https://www.who-umc.org) [23].
2. Materials and Methods
2.1. Literature Search in Biomedical Databases
As suggested by the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) Statement, the eligibility criteria were divided into two key categories [24]. The eligibility criteria were applied to select relevant publications and are described in Table 1 [25]. The literature search was done for articles published up to 20 August 2018 in Google Scholar and in two databases, specifically, Embase and PubMed via MEDLINE. The literature search was achieved for four DOACs (rivaroxaban, apixaban [25], edoxaban and dabigatran), and the search approach was developed independently for Google Scholar, Embase and PubMed, as previously described [25]. For Google Scholar, the keywords/strings were rivaroxaban OR apixaban OR dabigatran OR edoxaban AND interaction OR interactions AND “case report”. For Embase, the keywords/strings used were (rivaroxaban OR apixaban OR dabigatran OR edoxaban) OR (DOACs OR NOAC OR “direct oral anticoagulants” OR “new oral anticoagulants” OR “direct thrombin inhibitor” OR “direct factor Xa inhibitor”) AND drug interaction. Finally, for PubMed, the keywords/strings used were (rivaroxaban OR apixaban OR dabigatran OR edoxaban) OR (DOACs OR NOAC OR “direct oral anticoagulants” OR “new oral anticoagulants” OR “direct thrombin inhibitor” OR “direct factor Xa inhibitor”) AND (drug interaction OR interaction).

Table 1.
Eligibility criteria [25].
The reference managing software Zotero® (version 5.0.47) was used to remove duplicates, and the potential relevance of the remaining records was assessed by two reviewers who screened the title and abstract. If a single study was described in more than one article and each presented the same data, the most recent study was integrated. The included articles were divided into two groups, namely, interaction studies and case reports.
The mechanisms of interactions for interaction studies and case reports were checked by reviewing the Summary of Product Characteristics (SmPC) [14], UpToDate-Lexicomp [26] and the CYP450 substrates, inhibitor and inducers table (https://www.hug-ge.ch/sites/interhug/files/structures/pharmacologie_et_toxicologie_cliniques/a5_cytochromes_6_2.pdf (accessed on 20 August 2018)) [27]. Case reports that described DDIs that were not previously described or not understood from a pharmacological point of view were excluded.
As already done with apixaban in a previous article, the types of interactions assessed were PK interactions mediated by CYP3A, P-gp modulators and/or by gastric pH modifiers and PD interactions mediated by other antithrombotic agents and nonsteroidal anti-inflammatory drugs (NSAIDs) for interaction studies [25]. An additional category entitled “other drugs” pooled interactions not matching any of the previous categories. Each study was reviewed and described individually and categorised into in vitro/animal studies or phase I to phase IV human studies. Furthermore, a post hoc analysis was performed to allow us to assess if some DDIs were missing and if the SmPC included all the DDIs described in the literature.
Concerning case reports, the required information was patient characteristics, information on rivaroxaban (dosage, start and end of treatment, duration of treatment) and potential interacting drugs, adverse event descriptions and a list of additional medication when available.
2.2. Analysis of Data from Spontaneous Reports in VigiBase
Spontaneous reports from VigiBase were used to investigate DDIs between rivaroxaban and other drugs. The Uppsala Monitoring Centre (UMC) is the WHO Collaborating Centre for International Drug Monitoring and is responsible to maintain VigiBase. UMC receives reports of suspected ADRs from national centres in countries participating in the WHO Program for International Drug Monitoring (https://www.who-umc.org/vigibase/vigibase/ (accessed on 2 January 2018)). At the date of retrieval (accessed on 2 January 2018), there were a total of 16,329,758 individual case safety reports (ICSRs) in VigiBase that came from 131 countries. Drugs are coded according to WHODrug and ADRs are coded according to MedDRA (version 20.1) [28]. The information in VigiBase comes from multiple sources, and the probability that the suspected adverse effect is drug-related is not the same in all cases [29].
Each ICSR retrieved contained drug-drug-ADR (DDA) triplets that allowed the identification of potential DDIs. The analyses to detect potential signals of DDIs were performed using Omega (Ω), an observed-to expected three-way measure of disproportionate reporting developed by the UMC [30]. When Ω is positive and two drugs are used together, an increased risk of a specific ADR occurrence is emphasised over the sum of the individual risks when these same drugs are used separately. Thus, it is an indicator of the frequency of reporting of certain DDA triplets in the dataset compared to what is expected based on the relative reporting in the dataset. The Ω value is thereby dynamic because it can change as new reports are entered in VigiBase. Ω0.25 is used as a threshold in the screening of potential DDIs in data from ICSRs because it is the lower limit of a 95% credibility interval for Ω. Prior to analysis, the data set was thus cleaned by removing all DDAs with Ω0.25 less than or equal to 0. The next step to clean the data set was to exclude some non-relevant MedDRA preferred terms, such as “stent placement”, “vascular stent insertion” and “Dieulafoy’s vascular malformation”. Some non-relevant drug names were also excluded. Finally, all rows with drugs reported as “concomitant” were removed from the file; therefore, only drugs reported as “interacting” or “suspected” were kept. This cleaning procedure was the same as that already described in a previous publication [25].
The search and extraction of ICSRs related to rivaroxaban and DDIs from VigiBase were performed by the UMC on 24 April 2018 from a database freeze conducted on 2 January 2018 [25]. The number of DDA triplets for rivaroxaban related to each MedDRA system organ class (SOC), the number of DDA triplets for rivaroxaban and one specific ADR and the number of combinations for rivaroxaban and one specific suspected/interacting drug in the DDA triplet were studied.
According to SmPC, UpToDate and PubMed, DDIs were classified in PK and/or PD DDIs and in unknown DDIs. PK and PD DDIs were further classified into sub-classifications that included absorption (PKA), distribution (PKD), metabolism (PKM) or excretion (PKE) for PK mechanisms and direct effects on receptor function (PD1), interference with a biological physiological control process (PD2) or additive/opposed pharmacological effects (PD3) for PD mechanisms. DDIs were counted in when they were verified for the two mechanisms. All mechanisms were listed when more than one was found. This article focuses on rivaroxaban only, due to the large quantity of data extracted with the VigiBase analysis. As already mentioned, similar work was done for apixaban only [25].
3. Results
3.1. Literature
The literature search retrieved 31 interaction studies, some investigating several drugs, and 28 case reports. The selection process is illustrated in the following PRISMA flowchart (Figure 1).
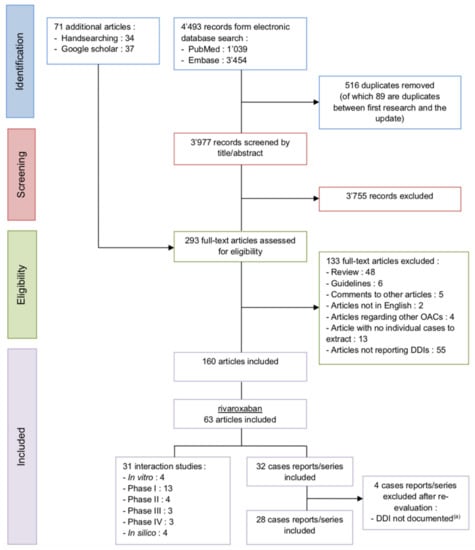
Figure 1.
PRISMA flowchart of the rivaroxaban studies selection process DDI (drug-drug interaction) and OAC (oral anticoagulant), (a) or not understood from a pharmacological point of view.
3.1.1. CYP3A and P-gp Inhibitors
In Vitro Studies
Rivaroxaban did not show any interaction with tacrolimus when both drugs were supplemented into citrated plasma in an in vitro study [31]. In vitro, type 5 phosphodiesterase inhibitors (PDE5is), such as sildenafil, tadalafil and vardenafil, inhibited the P-gp-mediated efflux of rivaroxaban [32]. According to the authors, this could have consequences on rivaroxaban’s safety, particularly in terms of bleeding risk [32].
Phase I Studies
In healthy volunteers, coadministration of ketoconazole increased the rivaroxaban AUC by 158% and the Cmax by 72% [33]. Similarly, ritonavir significantly increased the rivaroxaban AUC and Cmax by 153% and 55%, respectively [33]. Coadministration of clarithromycin, erythromycin and fluconazole with rivaroxaban significantly increased its AUC by 54%, 34% and 42%, respectively, but these moderate effects were not considered to be clinically relevant [33]. All of these coadministered drugs are CYP3A/P-gp inhibitors.
Another phase I study found a high impact of clarithromycin on rivaroxaban’s PK with an AUC increase of 94% and a Cmax increase of 92%, independent of the ABCB1 genotype [34]. The effect of erythromycin on rivaroxaban exposure was also assessed in another study but this time in subjects with normal and impaired renal function. In subjects with normal renal function, coadministration with erythromycin produced an increase in the rivaroxaban AUC and Cmax of 39% and 40%, respectively [35]. However, in subjects with mild renal impairment, the increase in the rivaroxaban AUC and Cmax when given erythromycin was 54% and 26%, respectively. Moderate renal impairment combined with erythromycin coadministration increased rivaroxaban’s AUC and Cmax by 71% and 21%, respectively [35]. A study conducted in healthy volunteers demonstrated that verapamil coadministration increased the AUC of rivaroxaban. Volunteers were separated into a normal renal function group and a mild renal impairment group. The increase in the AUC was of the same extent in both groups (ratio of geometric means: 1.39 vs. 1.43, respectively) [36].
Phase II Studies
Limited data from a small phase II study in liver transplant patients (n = 9) showed an increase in rivaroxaban plasma levels in the presence of cyclosporin A (n = 5) [37]. The rivaroxaban plasma levels were within therapeutic ranges in patients treated with tacrolimus instead of cyclosporin A [37]. In a study that compared patients taking rivaroxaban (controls) and patients taking rivaroxaban and diltiazem, there was no significant difference in the incidence of major and/or clinically relevant non-major bleeding events in either group [38]. The authors suggest that although diltiazem may increase rivaroxaban exposure because of its moderate inhibition of CYP3A/P-gp, there was no evidence of an increased risk of bleeding outcomes in patients taking both drugs [38].
Phase III Studies
A study that used data from the ROCKET study assessed the risk of coadministration of non-dihydropyridine calcium channel blockers (non-DHP CCBs) with rivaroxaban or warfarin. This coadministration was not associated with a significant increase in the risk of stroke or non-central nervous system systemic embolism (p = 0.11) or major or non-major clinically relevant bleeding (p = 0.087) [39]. However, major bleeding or intracranial haemorrhage occurred more frequently in the non-DHP CCB user group (p = 0.0091 and p = 0.001, respectively) [39]. Cardiovascular death, all-cause death, myocardial infarction and all-cause hospitalisation were not significantly different between the two groups [39]. Comparison between rivaroxaban and warfarin users showed no significant difference in safety outcomes such as major or non-major clinically relevant bleeding (p = 0.14) in non-DHP CCB users [39].
Phase IV Studies
A retrospective study concluded that coadministration of amiodarone and rivaroxaban is linked to an increased risk of bleeding [40]. The study compared the number of bleeding events in patients being treated simultaneously with both drugs with patients taking rivaroxaban only [40]. Another retrospective study assessed the bleeding risk of rivaroxaban and other DOACs when it was coadministered with verapamil, diltiazem, amiodarone, dronedarone, azoles (fluconazole, ketoconazole, itraconazole, voriconazole and posaconazole), cyclosporine, erythromycin or clarithromycin [41]. The combination of rivaroxaban with amiodarone and fluconazole was associated with a significantly increased risk of major bleeding [41]. In contrast, the coadministration of rivaroxaban and erythromycin or clarithromycin decreased the risk of major bleeding but it was not statistically significant [41]. Coadministration of rivaroxaban with cyclosporin, verapamil, diltiazem, ketoconazole and itraconazole, voriconazole, posaconazole and dronedarone did not significantly change the incidence rate of major bleeding [41]. Results for erythromycin, clarithromycin, cyclosporin, verapamil, ketoconazole and dronedarone are not in line with previously cited studies. This could be because this study has strong limitations of a retrospective design and of an analysis based on the Health Insurance database system, and thus, has a lack of detailed clinical information such as liver and kidney function [41]. Moreover, this study included an Asian population that has been recognised to have a different bleeding risk and anticoagulant therapy than the Western population [41]. Finally, rivaroxaban dosage and concomitant treatment were not considered in the model [41].
In Silico Studies
A study combined data from in vitro inhibition assays and static modelling to predict in vivo DDIs between rivaroxaban and amiodarone and dronedarone, two CYP3A/P-gp inhibitors. Thus, the study predicted an increased rivaroxaban exposure of 37% and 31%, respectively [42]. In addition, a nine percent increase in rivaroxaban exposure due to inhibition of P-gp-mediated efflux by either of the two inhibitors was estimated [42]. In a study that developed a physiologically based pharmacokinetic (PBPK) model, rivaroxaban exposure increased when DDIs with CYP3A/P-gp inhibitors (ketoconazole, ritonavir, clarithromycin) coexisted with mild or moderate hepatic dysfunction compared to hepatic dysfunction alone [43]. The simulation results revealed a synergistic effect of the addition of DDI and hepatic dysfunction, which was greatest when hepatic dysfunction was severe [43]. Another PBPK study showed that coadministration of verapamil and rivaroxaban increased rivaroxaban AUC by 1.48-fold and that coadministration of verapamil and renal insufficiency produced a synergistic increase in systemic exposure to rivaroxaban [44]. The authors suggested that subjects with mild to severe renal dysfunction who are taking verapamil should receive a reduced dose of rivaroxaban to minimise synergistic drug-drug disease interactions and prevent further bleeding risks [44]. In another PBPK model, systemic exposure to 20 mg of rivaroxaban once daily for 20 days increased when coadministered with a loading dose of amiodarone 200 mg three times a day during the last fifteen days in healthy subjects [45]. Simulations also indicated a significant 1.36-fold mean AUC increase [45]. Moreover, renal insufficiency had a synergistic effect, as the simulated mean AUC-fold change was 1.86- in patients with mild renal impairment and 1.61 in patients with moderate renal impairment where the rivaroxaban dosage was reduced to 15 mg [45].
3.1.2. CYP3A and P-gp Inducers
Phase IV Study
Coadministration of rivaroxaban with rifampicin and phenytoin was assessed and surprisingly showed an increased risk of major bleeding [41]. However, this effect was not statistically significant for rifampicin [41]. Phenytoin, as a CYP inducer, is expected to decrease rivaroxaban AUC and, therefore, the bleeding risk. The results of this phase IV study should be treated with caution due to the limitations mentioned above [41].
3.1.3. CYP3A and P-gp Substrates
Phase I Studies
No clinically relevant PK or PD interactions between rivaroxaban and the CYP3A substrate midazolam, the P-gp substrate digoxin and the CYP3A/P-gp substrate atorvastatin were observed in healthy volunteers [33,46].
Phase IV Study
The bleeding risk with rivaroxaban was assessed when coadministered with atorvastatin and digoxin and a significantly decreased risk of major bleeding was observed, while the effect of digoxin was not statistically significant [41]. In the phase I study, atorvastatin had no effect on rivaroxaban PK and this discrepancy in results can also be attributed to the limitations of the phase IV study [41].
3.1.4. Other Antithrombotic Agents and NSAIDs
In Vitro and Animal Studies
The combination of rivaroxaban with acetylsalicylic acid (ASA) and/or ticagrelor in vitro using human platelet-rich plasma and coadministration of low-dose rivaroxaban with ASA and clopidogrel in rat models of arterial thrombosis suggested that the combination of rivaroxaban with single or dual antiplatelet agents led to a synergistic increase in their antithrombotic activity compared with anticoagulant or antiplatelet therapy alone [47]. Furthermore, the authors considered that since the low dose of rivaroxaban tested was equivalent to the trough plasma concentration after a rivaroxaban 2.5 mg twice daily dose in humans, their results can be deemed of clinical relevance [47].
Phase I Studies
No clinically relevant PK interactions were observed between rivaroxaban and enoxaparin [48] or warfarin in phase I studies [49,50]. However, some rivaroxaban PD parameters were affected, and the anti-factor Xa activity of rivaroxaban increased by 50% when coadministered with enoxaparin [48]. Regarding warfarin, an additive effect on the prolongation of the PT/INR was observed during the initial transitioning period from warfarin to rivaroxaban, although pre-treatment with warfarin did not affect rivaroxaban anti-factor Xa activity [49]. Similar results arose during the co-treatment period when switching from rivaroxaban to warfarin (higher PT and greater than additive INR values than those measured when either drug was administered alone) [50]. The combination of rivaroxaban and the commonly used NSAID naproxen significantly increased the bleeding time compared with rivaroxaban alone. On the other hand, rivaroxaban exposure was only slightly affected by coadministration of both drugs (10% increase in the rivaroxaban AUC and Cmax). The authors of the study concluded that there was no clinically relevant interaction between rivaroxaban and naproxen [51]. Moreover, the same finding was found for rivaroxaban and ASA. Rivaroxaban’s bleeding time was prolonged when both drugs were coadministered as compared to rivaroxaban alone, while its PK characteristics/properties remained unchanged. Thus, the authors considered that the rivaroxaban-ASA interaction was not clinically relevant [52]. Coadministration of rivaroxaban and clopidogrel led to an additive effect on the bleeding time that was doubled when compared with the effect produced with clopidogrel alone, without affecting any other PK or PD parameters of rivaroxaban [53].
Phase II Studies
In acute coronary syndrome (ACS) patients, rivaroxaban increased the risk of bleeding events in a dose-dependent manner in both groups of patients (aspirin or aspirin and thienopyridine) compared to placebo [54]. Moreover, the absolute rate of clinically significant bleeding was higher in the group receiving dual antiplatelet therapy than in the group receiving ASA alone in addition to rivaroxaban [54]. In a study that compared the use of a low dose of rivaroxaban (2.5 mg twice daily) concomitant with either clopidogrel or ticagrelor to dual antiplatelet therapy (aspirin and either clopidogrel or ticagrelor) in patients who underwent percutaneous coronary intervention, there were no significant differences in the bleeding incidence [55]. However, in a post hoc analysis, the use of ticagrelor was associated with a significant increase in the bleeding rate (p = 0.0006) compared to clopidogrel [55]. As pointed out by the authors, a higher bleeding rate was found in regions where there was greater use of ticagrelor but was not associated with the randomised treatment assignment (rivaroxaban vs. aspirin) [55].
Phase III Studies
In a sub-analysis of pooled data from the RECORD programme, coadministration of NSAIDs (relative rate ratio = 1.22, CI95% = 0.99–1.50), platelet aggregation inhibitors (PAIs) and ASA (relative ratio = 1.32, CI95% = 0.85–2.05) together with rivaroxaban increased the risk of bleeding in hip or knee replacement surgery patients, although the effect was not considered significant [56]. However, the small proportion of patients using concomitant PAIs and ASA may not have been high enough to conclude on the risk of bleeding, which could explain the difference in results with other studies [56]. Regarding the increased risk of bleeding with concomitant use of NSAIDs, it was at the limit of statistical significance [56]. In the ROCKET-AF trial, more than one-third of patients were on ASA at baseline, and the concomitant use of rivaroxaban and ASA was associated with higher rates of all-cause death [57]. It is worth mentioning that the increase in all-cause death in the presence of aspirin was more pronounced for warfarin than for rivaroxaban, enhancing the difference between the two drugs regarding outcome [57].
Phase IV Studies
In a sub-analysis of the XAMOS study, coadministration of PAIs (including ASA) increased the incidence of symptomatic thromboembolic and bleeding events in patients taking rivaroxaban and in those who followed standard thrombophylaxis for VTE prophylaxis after major orthopaedic surgery [58]. However, this finding was largely attributable to a higher incidence of symptomatic arterial thromboembolic events [58]. This could be explained by the fact that PAIs users were older and had more comorbidities affecting cardiovascular risk [58]. Additionally, concomitant use of NSAIDs was also associated with an increased risk of bleeding, while it had no influence on the rate of symptomatic thromboembolic events [58].
3.1.5. Gastric pH Modifiers
Phase I Studies
Ranitidine, a H2 antagonist, has no significant impact on the PK/PD of rivaroxaban [59]. Similarly, the proton pump inhibitor (PPI) omeprazole showed no clinically relevant PK or PD interactions with rivaroxaban [60].
3.1.6. Other Drugs
In Vitro Studies
Irinotecan is metabolised by esterases to its active metabolite SN-38 (7-ethyl-10-hydroxycamptothecin), which is later detoxified via glucuronidation to form SN-38G. In human liver microsomes, rivaroxaban displayed dose-dependent inhibition of SN-38 glucuronidation, which may increase SN-38 toxicity [61]. These findings suggest a potential interaction between rivaroxaban and irinotecan [61]. The combination of rivaroxaban with drugs such as alendronate sodium, chondroitin sulfate, hydrocodone-acetaminophen, clonazepam, penicillin, tramadol and tranexamic acid did not exhibit any interactions [31].
Results are summarised in Table 2.

Table 2.
Summary of DDIs involving rivaroxaban.
3.2. Case Series or Reports
Twenty-eight case reports were found in the literature. Eleven cases were female, with an overall age range of 29–90 years. Among them, four patients died. The rivaroxaban indication was mainly AF (n = 16) but also VTE prevention after orthopaedic surgery (n = 7), recurrent VTE prevention (n = 2), VTE treatment (n = 1), transient ischaemic attack (n = 1) and unknown (n = 1). Renal impairment (n = 7) was the most relevant pathophysiological factor contributing to the development of ADRs. Concerning the mechanism of interaction, PK DDIs were involved in seventeen cases [63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79], PD DDIs in eight cases [80,81,82,83,84,85,86,87] and PK/PD DDIs in three cases [88,89,90]. Bleeding (n = 18) and thromboembolic events (n = 7) were the two main ADRs described in these case reports. In two other cases, the coagulation parameters were abnormal, and the anti-Factor Xa peak remained under the reference value, but this had no consequences [78,89]. In one case, rivaroxaban induced hepatic encephalopathy that led to death [90]. In the cases describing thromboembolic events or lack of efficacy measured with laboratory tests (coagulation parameters or anti-Factor Xa), the involved comedications were CYP3A and/or P-gp inducers, namely, rifampicin [68,73], nevirapine [72] and antiepileptic drugs, such as carbamazepine [64,66,77], oxcarbamazepine [65] or phenytoin [78]. PK DDIs with CYP3A and/or P-gp inhibitors led to bleeding events in all cases. The PD DDIs involved coadministration of alirocumab [80] and antiplatelet aggregation drugs such as clopidogrel [80,86] or aspirin [87], warfarin [81,85], NSAIDs [83,84] and cocaine [82].
3.3. VigiBase
A total of 21,261 DDAs with positive Ω0.25 values were extracted from VigiBase for the DDA combination of rivaroxaban with any suspected/interacting drug and any ADR. Those DDAs came from 18,928 ICSRs reported to VigiBase up to the database freeze in January 2018. After cleaning the datasets, 21,109 DDAs (corresponding to 862 unique DDA combinations of rivaroxaban with one specific suspected/interacting drug and one defined ADR, each observed in a certain number of ICSRs). In the dataset, the most represented MedDRA SOCs were GI disorders (n = 12,307, 58.3%), renal and urinary disorders (n = 1994, 9.4%) and vascular disorders (n = 1533, 7.3%). For the ADRs, the three most reported in combination with rivaroxaban and any other suspected/interacting drug were GI haemorrhage (n = 7182, 34.0%), upper GI haemorrhage (n = 1619, 7.7%) and rectal haemorrhage (n = 1355, 6.4%). Regardless of the ADR, acetylsalicylic acid (ASA) (n = 12,725, 60.3%), clopidogrel (n = 2464, 11.7%) and warfarin (n = 1110, 5.3%) were the three suspected/interacting drugs most co-reported with rivaroxaban. If the ADRs reported for each of those drug pairs were also considered, the most reported ADR was GI haemorrhage, with incidence rates of 38.0% (n = 4838), 40.9% (n = 1009) and 36.6% (n = 406), respectively.
The three most reported DDAs in the whole dataset were:
- rivaroxaban–ASA–GI haemorrhage (n = 4838, 22.9%)
- rivaroxaban–ASA–Upper GI haemorrhage (n = 1040, 4.9%)
- rivaroxaban–clopidogrel–GI haemorrhage (n = 1009, 4.8%)
Of the 862 DDAs reviewed, 559 DDIs were not verified in the literature. A total of 41 PK DDIs and 265 PD DDIs were verified in the literature. The most common PK DDI was inhibition of drug metabolism, and the most common PD DDI was additive pharmacological effects.
Concerning verified PK DDIs, inhibitors of CYP3A and P-gp were the most reported drugs, and bleeding was the most reported ADR (Table 3). Regarding verified PD DDIs, antithrombotic agents and NSAIDs were the most reported drugs, and bleeding was also the most reported ADR. Regarding bleeding, the most reported site was the gastrointestinal tract (Table 3). Table 3 shows the number of occurrences that represent the number of different ADRs that occurred after the interaction between rivaroxaban and drug B, and the number in parentheses is the number of the most frequently reported ADR.

Table 3.
Drug reported as interacting with rivaroxaban in VigiBase with interaction mechanism and most frequently reported adverse effect.
4. Discussion
Due to their ease of use and alleged favorable safety and efficacy profile, anticoagulation drug management experienced a major turning point with the arrival of DOACs, especially rivaroxaban, which was the first to be marketed in 2009 for cardiovascular indications [14,91]. As rivaroxaban has been on the market for several years, it has been increasingly possible to highlight DDIs in real-world situations. In line with this, we performed a systematic review of published studies and case reports, together with an analysis of data reported to VigiBase, as already done with apixaban in a previous article [25]. We showed that rivaroxaban is subject to a significant number of DDIs that need to be considered by clinicians and patients, especially DDIs with CYP3A/P-gp inhibitors and other antithrombotic agents/NSAIDs. The impact of inducers of CYP3A/P-gp on rivaroxaban is sparsely available in the literature. A post hoc comparison between collected interactions in the literature and interactions contained in rivaroxaban’s SmPC was performed to verify the accuracy of our review [14]. First, the DDI between rivaroxaban and rifampicin reported in the rivaroxaban SmPC was not detected by our literature search and not registered in clinicaltrials.gov, which means that this study is not publicly available in any form and seems not to have even been registered in any national or international registry so far, even though registries of clinical trials are an important data source in clinical research. Conversely, some interactions that were identified by our search are not included in the SmPC. This can be explained by the fact that not all information has to be disclosed in the SmPC. Concerning in vitro interaction studies, data are only integrated into the SmPC if they impact the use of the medicinal product [61,92,93]. A lack of interaction should only be mentioned in the SmPC if it is of major significance to the prescriber for data from in vivo studies. Moreover, phase I studies in healthy volunteers publication depends on the transparency policies of drug manufacturers because they are not subject to required data disclosure [94,95]. Compared to studies performed in patients, a recent study showed that phase I (conducted in healthy volunteers) studies had a significantly lower level of transparency [95]. Finally, data from post-marketing studies are only included if they result in a variation of the drug’s marketing authorisation [93,96].
Venous thromboembolism was identified in the case reports included in our literature search as one of the most frequently reported ADR of rivaroxaban, and it was not mentioned, per se, in rivaroxaban’s SmPC. This is likely due to the fact that interactions leading to this ADR are not recognised and are instead classified as treatment inefficacy [20]. Therefore, this is not a lack of coverage in our literature search.
Regarding data from VigiBase, the most co-reported suspected/interacting drug was ASA, the most co-reported ADR was GI haemorrhage, and consequently, rivaroxaban–ASA–GI haemorrhage was the most reported DDA triplet. These results are not surprising, as multiple studies have highlighted the increased risk of GI haemorrhage when DOACs were administered, including a thorough evaluation of their safety profile based on data from the same source, VigiBase [19,20]. More precisely, rivaroxaban showed a positive odds ratio of 1.38 (1.24–1.55) for GI haemorrhage compared to warfarin [20]. Several suspected/interacting drugs were not documented or understood from a pharmacological point of view to be associated with a DDI with rivaroxaban, so they were excluded from our analysis of the ICSRs. Moreover, with the dataset available, it was not possible to find a plausible explanation for some of the DDIs, and many DDA triplets did not seem to correlate, such as rivaroxaban with mesalazine and poor-quality sleep. The data stored in VigiBase come from regulatory and voluntary sources and may lack a proper causality assessment in some cases, since not all national pharmacovigilance centres contributing to VigiBase perform causality assessments of their ICSRs [97]. Additionally, some cases may lack completeness, and the data stored are heterogeneous. Rivaroxaban might be at higher risk of interacting with drugs with the same pharmacological profile because the proportion of DDIs involving the PD mechanism was higher than the proportion of DDIs involving the PK mechanism. This finding erroneously suggests that rivaroxaban might not interact with CYP3A/P-gp inhibitors or inducers. Indeed, this emphasises a bias in the data included in VigiBase, which depends on spontaneous reporting. As healthcare professionals and/or patients are the source of these spontaneous reports and as they are often less familiar with PK DDIs, these are underreported because they go undetected. These results are consistent with those of a study that used the same database, where PD and PK DDIs accounted for 41% and 25% of DDIs, respectively [98].
VigiBase has inherent limitations, as all ADR reporting databases [99]. Underreporting and selective reporting are the two first limitations worth mentioning. Another limitation of these databases is the unfeasibility of estimating risk, due to the absence of a denominator. Using certain reporting patterns as indicators of DDIs in addition to a positive Ω0.25 is one of the ideas that have been put forward for improving the database [100]. The existence of a plausible time course, a positive dechallenge and alternate causes of the reaction could help identify suspected adverse drug interactions from ICSRs more precisely [101]. For that, it should be useful to take into account information available in the free text of the original reports [101]. Nevertheless, the lack of completeness of each report is the root of the problem because not all fields are required to be completed for reports to be accepted in VigiBase, and a detailed case-by-case analysis of each ICSR is needed [102].
5. Conclusions
Contrary to what was mentioned at the time of marketing, rivaroxaban has significant DDI potential with other drugs. Data analysis of VigiBase and some articles in this review highlight that PD interactions, as well as drugs that may impair haemostasis such as ASA or antithrombotics, are widely known and reported. Indeed, they occur due to the known properties of the drug and are predictable. However, this literature review shows that rivaroxaban has particular DDI potential with CYP3A/P-gp inhibitors and CYP3A/P-gp inducers, but the analysis of VigiBase data shows that the detection and reporting of pharmacokinetic interactions are sparse because they are not well recognised. Moreover, SmPC does not contain all potentially described post-marketing DDIs. This should serve as a warning to healthcare professionals as to the likelihood of occurrence of ADRs due to DDIs, as they are avoidable.
Author Contributions
Conceptualization, S.F., V.R.; methodology, S.F., C.L., V.R.; investigation, S.F., C.L.; writing—original draft preparation, S.F., C.L.; writing—review and editing, C.F.S., V.R.; supervision, C.F.S. and V.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The information in this article does not represent the opinion of the Uppsala Monitoring Centre (UMC) or the World Health Organization. The authors are very grateful to Camilla Westerberg, research pharmacist, and Lucie Gattepaille, data scientist, for extracting and providing data from VigiBase. We thank the UMC (Uppsala, Sweden) for its valuable support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Weitz, J.I. Blood Coagulation and Anticoagulant, Fibrinolytic, and Antiplatelet Drugs. In Goodman & Gilman’s: The Pharmacological Basis of Therapeutics; Brunton, L.L., Chabner, B.A., Knollmann, B.C., Eds.; McGraw-Hill Education: New York, NY, USA, 2015. [Google Scholar]
- Kvasnicka, T.; Malikova, I.; Zenahlikova, Z.; Kettnerova, K.; Brzezkova, R.; Zima, T.; Ulrych, J.; Briza, J.; Netuka, I.; Kvasnicka, J. Rivaroxaban—Metabolism, Pharmacologic Properties and Drug Interactions. Curr. Drug Metab. 2017, 18, 636–642. [Google Scholar] [CrossRef]
- Bauer, K.A. Pros and Cons of New Oral Anticoagulants. Hematol. Am. Soc. Hematol. Educ. Program 2013, 2013, 464–470. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency—Europa EU Pradaxa, INN-Dabigatran—Summary of Product Characteristics. Available online: https://www.ema.europa.eu/documents/product-information/pradaxa-epar-product-information_en.pdf (accessed on 25 October 2018).
- Lippi, G.; Gosselin, R.; Favaloro, E.J. Current and Emerging Direct Oral Anticoagulants: State-of-the-Art. Semin. Thromb. Hemost. 2019, 45, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef]
- EINSTEIN Investigators; Bauersachs, R.; Berkowitz, S.D.; Brenner, B.; Buller, H.R.; Decousus, H.; Gallus, A.S.; Lensing, A.W.; Misselwitz, F.; Prins, M.H.; et al. Oral Rivaroxaban for Symptomatic Venous Thromboembolism. N. Engl. J. Med. 2010, 363, 2499–2510. [Google Scholar] [CrossRef] [PubMed]
- EINSTEIN–PE Investigators; Büller, H.R.; Prins, M.H.; Lensin, A.W.A.; Decousus, H.; Jacobson, B.F.; Minar, E.; Chlumsky, J.; Verhamme, P.; Wells, P.; et al. Oral Rivaroxaban for the Treatment of Symptomatic Pulmonary Embolism. N. Engl. J. Med. 2012, 366, 1287–1297. [Google Scholar] [CrossRef]
- Eriksson, B.I.; Borris, L.C.; Friedman, R.J.; Haas, S.; Huisman, M.V.; Kakkar, A.K.; Bandel, T.J.; Beckmann, H.; Muehlhofer, E.; Misselwitz, F.; et al. Rivaroxaban versus Enoxaparin for Thromboprophylaxis after Hip Arthroplasty. N. Engl. J. Med. 2008, 358, 2765–2775. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, A.K.; Brenner, B.; Dahl, O.E.; Eriksson, B.I.; Mouret, P.; Muntz, J.; Soglian, A.G.; Pap, A.F.; Misselwitz, F.; Haas, S.; et al. Extended Duration Rivaroxaban versus Short-Term Enoxaparin for the Prevention of Venous Thromboembolism after Total Hip Arthroplasty: A Double-Blind, Randomised Controlled Trial. Lancet 2008, 372, 31–39. [Google Scholar] [CrossRef]
- Lassen, M.R.; Ageno, W.; Borris, L.C.; Lieberman, J.R.; Rosencher, N.; Bandel, T.J.; Misselwitz, F.; Turpie, A.G.G.; RECORD3 Investigators. Rivaroxaban versus Enoxaparin for Thromboprophylaxis after Total Knee Arthroplasty. N. Engl. J. Med. 2008, 358, 2776–2786. [Google Scholar] [CrossRef]
- Turpie, A.G.G.; Lassen, M.R.; Davidson, B.L.; Bauer, K.A.; Gent, M.; Kwong, L.M.; Cushner, F.D.; Lotke, P.A.; Berkowitz, S.D.; Bandel, T.J.; et al. Rivaroxaban versus Enoxaparin for Thromboprophylaxis after Total Knee Arthroplasty (RECORD4): A Randomised Trial. Lancet 2009, 373, 1673–1680. [Google Scholar] [CrossRef]
- Harter, K.; Levine, M.; Henderson, S.O. Anticoagulation Drug Therapy: A Review. West. J. Emerg. Med. 2015, 16, 11–17. [Google Scholar] [CrossRef]
- European Medicines Agency—Europa EU Xarelto, INN-Rivaroxaban—Summary of Product Characteristics. Available online: https://www.ema.europa.eu/documents/product-information/xarelto-epar-product-information_en.pdf (accessed on 25 October 2018).
- Lee, L.H. DOACs—Advances and Limitations in Real World. Thromb. J. 2016, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- Fontana, P.; Robert-Ebadi, H.; Bounameaux, H.; Boehlen, F.; Righini, M. Direct Oral Anticoagulants: A Guide for Daily Practice. Swiss Med. Wkly. 2016, 146, w14286. [Google Scholar] [CrossRef] [PubMed]
- Mueck, W.; Stampfuss, J.; Kubitza, D.; Becka, M. Clinical Pharmacokinetic and Pharmacodynamic Profile of Rivaroxaban. Clin. Pharmacokinet. 2014, 53, 1–16. [Google Scholar] [CrossRef]
- Caldeira, D.; Barra, M.; Pinto, F.J.; Ferreira, J.J.; Costa, J. Intracranial Hemorrhage Risk with the New Oral Anticoagulants: A Systematic Review and Meta-Analysis. J. Neurol. 2015, 262, 516–522. [Google Scholar] [CrossRef]
- Holster, I.L.; Valkhoff, V.E.; Kuipers, E.J.; Tjwa, E.T.T.L. New Oral Anticoagulants Increase Risk for Gastrointestinal Bleeding: A Systematic Review and Meta-Analysis. Gastroenterology 2013, 145, 105–112.e15. [Google Scholar] [CrossRef] [PubMed]
- Monaco, L.; Biagi, C.; Conti, V.; Melis, M.; Donati, M.; Venegoni, M.; Vaccheri, A.; Motola, D. Safety Profile of the Direct Oral Anticoagulants: An Analysis of the WHO Database of Adverse Drug Reactions. Br. J. Clin. Pharmacol. 2017, 83, 1532–1543. [Google Scholar] [CrossRef]
- Liakoni, E.; Rätz Bravo, A.E.; Krähenbühl, S. Hepatotoxicity of New Oral Anticoagulants (NOACs). Drug Saf. 2015, 38, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Raschi, E.; Poluzzi, E.; Koci, A.; Salvo, F.; Pariente, A.; Biselli, M.; Moretti, U.; Moore, N.; De Ponti, F. Liver Injury with Novel Oral Anticoagulants: Assessing Post-Marketing Reports in the US Food and Drug Administration Adverse Event Reporting System. Br. J. Clin. Pharm. 2015, 80, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Hugman, B. From the Uppsala Monitoring Centre: A Review of Viewpoint Part 1 and Part 2. Drug Saf. 2005, 28, 645–646. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, S.; Lenoir, C.; Samer, C.; Rollason, V. Drug Interactions with Apixaban: A Systematic Review of the Literature and an Analysis of VigiBase, the World Health Organization Database of Spontaneous Safety Reports. Pharm. Res. Perspect. 2020, 8, e00647. [Google Scholar] [CrossRef] [PubMed]
- Wolters Kluwer Health. UpToDate, the Evidence-Based Clinical Decision Support Resource. Available online: https://www.uptodate.com/home/linking-policy (accessed on 25 October 2018).
- Samer, C.F.; Lorenzini, K.I.; Rollason, V.; Daali, Y.; Desmeules, J.A. Applications of CYP450 Testing in the Clinical Setting. Mol. Diagn. Ther. 2013, 17, 165–184. [Google Scholar] [CrossRef] [PubMed]
- Lagerlund, O.; Strese, S.; Fladvad, M.; Lindquist, M. WHODrug: A Global, Validated and Updated Dictionary for Medicinal Information. Ther. Innov. Regul. Sci. 2020, 54, 1116–1122. [Google Scholar] [CrossRef]
- Uppsala Monitoring Center; WHO Collaborating Centre for International Drug Monitoring Caveat Document. Statement of Reservations, Limitations and Conditions Relating to Data Released from VigiBase, the WHO Global Database of Individual Case Safety Reports (ICSRs); Uppsala Monitoring Center: Uppsala, Sweden, 2018. [Google Scholar]
- Norén, G.N.; Sundberg, R.; Bate, A.; Edwards, I.R. A Statistical Methodology for Drug-Drug Interaction Surveillance. Stat. Med. 2008, 27, 3057–3070. [Google Scholar] [CrossRef]
- Sayani, S.; Iqbal, O.; Hoppensteadt, D.; Fareed, J. Drug Interactions of Newer Oral Anticoagulants Dabigatran, Rivaroxaban, and Apixaban with Routinely Used Nonanticoagulant/Antiplatelet Drugs. Blood 2014, 124. [Google Scholar] [CrossRef]
- Margelidon-Cozzolino, V.; Hodin, S.; Jacqueroux, E.; Delezay, O.; Bertoletti, L.; Delavenne, X. In Vitro Assessment of Pharmacokinetic Drug-Drug Interactions of Direct Oral Anticoagulants: Type 5-Phosphodiesterase Inhibitors Are Inhibitors of Rivaroxaban and Apixaban Efflux by P-Glycoprotein. J. Pharmacol. Exp. Ther. 2018, 365, 519–525. [Google Scholar] [CrossRef]
- Mueck, W.; Kubitza, D.; Becka, M. Co-Administration of Rivaroxaban with Drugs That Share Its Elimination Pathways: Pharmacokinetic Effects in Healthy Subjects. Br. J. Clin. Pharmacol. 2013, 76, 455–466. [Google Scholar] [CrossRef]
- Gouin-Thibault, I.; Delavenne, X.; Blanchard, A.; Siguret, V.; Salem, J.E.; Narjoz, C.; Gaussem, P.; Beaune, P.; Funck-Brentano, C.; Azizi, M.; et al. Interindividual Variability in Dabigatran and Rivaroxaban Exposure: Contribution of ABCB1 Genetic Polymorphisms and Interaction with Clarithromycin. J. Thromb. Haemost. 2017, 15, 273–283. [Google Scholar] [CrossRef]
- Moore, K.T.; Vaidyanathan, S.; Natarajan, J.; Ariyawansa, J.; Haskell, L.; Turner, K.C. An Open-Label Study to Estimate the Effect of Steady-State Erythromycin on the Pharmacokinetics, Pharmacodynamics, and Safety of a Single Dose of Rivaroxaban in Subjects with Renal Impairment and Normal Renal Function. J. Clin. Pharmacol. 2014, 54, 1407–1420. [Google Scholar] [CrossRef]
- Greenblatt, D.J.; Patel, M.; Harmatz, J.S.; Nicholson, W.T.; Rubino, C.M.; Chow, C.R. Impaired Rivaroxaban Clearance in Mild Renal Insufficiency With Verapamil Coadministration: Potential Implications for Bleeding Risk and Dose Selection. J. Clin. Pharmacol. 2018, 58, 533–540. [Google Scholar] [CrossRef]
- Wannhoff, A.; Weiss, K.; Schemmer, P.; Stremmel, W.; Gotthardt, D. Increased Anti-Xa Activity of Rivaroxaban in Patients after Liver Transplantation Treated with Cyclosporine a. Transplantation 2014, 14, 712. [Google Scholar] [CrossRef]
- Bartlett, J.W.; Renner, E.; Mouland, E.; Barnes, G.D.; Kuo, L.; Ha, N.B. Clinical Safety Outcomes in Patients with Nonvalvular Atrial Fibrillation on Rivaroxaban and Diltiazem. Ann. Pharmacol. 2018, 53, 21–27. [Google Scholar] [CrossRef]
- Washam, J.B.; Hellkamp, A.S.; Lokhnygina, Y.; Piccini, J.P.; Berkowitz, S.D.; Nessel, C.C.; Becker, R.C.; Breithardt, G.; Fox, K.A.A.; Halperin, J.L.; et al. Efficacy and Safety of Rivaroxaban Versus Warfarin in Patients Taking Nondihydropyridine Calcium Channel Blockers for Atrial Fibrillation (from the ROCKET AF Trial). Am. J. Cardiol. 2017, 120, 588–594. [Google Scholar] [CrossRef]
- Howell, D.; Hoch, E.; Shulman, E.H.; Di Biase, L.; Ferrick, K.J.; Fisher, J.D.; Krumerman, A. Interaction between Amiodarone and Rivaroxaban and the Risk of Major Bleeding. Heart Rhythm 2016, 13, S512. [Google Scholar]
- Chang, S.-H.; Chou, I.-J.; Yeh, Y.-H.; Chiou, M.-J.; Wen, M.-S.; Kuo, C.-T.; See, L.-C.; Kuo, C.-F. Association Between Use of Non-Vitamin K Oral Anticoagulants With and Without Concurrent Medications and Risk of Major Bleeding in Nonvalvular Atrial Fibrillation. JAMA 2017, 318, 1250–1259. [Google Scholar] [CrossRef]
- Cheong, E.J.Y.; Goh, J.J.N.; Hong, Y.; Venkatesan, G.; Liu, Y.; Chiu, G.N.C.; Kojodjojo, P.; Chan, E.C.Y. Application of Static Modeling in the Prediction of in Vivo Drug-Drug Interactions between Rivaroxaban and Antiarrhythmic Agents Based on in Vitro Inhibition Studies. Drug Metab. Dispos. 2017, 45, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Ge, W.; Jiang, Q. Application of Physiologically Based Pharmacokinetic Modeling to the Prediction of Drug-Drug and Drug-Disease Interactions for Rivaroxaban. Eur. J. Clin. Pharmacol. 2018, 74, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Lee, V.H.; Chow, C.R.; Rubino, C.M. Minimal Physiologically Based Pharmacokinetic and Drug-Drug-Disease Interaction Model of Rivaroxaban and Verapamil in Healthy and Renally Impaired Subjects. J. Clin. Pharmacol. 2018, 58, 541–548. [Google Scholar] [CrossRef]
- Cheong, E.J.Y.; Goh, J.J.N.; Hong, Y.; Kojodjojo, P.; Chan, E.C.Y. Rivaroxaban With and Without Amiodarone in Renal Impairment. J. Am. Coll. Cardiol. 2018, 71, 1395–1397. [Google Scholar] [CrossRef] [PubMed]
- Kubitza, D.; Becka, M.; Roth, A.; Mueck, W. Absence of Clinically Relevant Interactions between Rivaroxaban–an Oral, Direct Factor Xa Inhibitor–and Digoxin or Atorvastatin in Healthy Subjects. J. Int. Med. Res. 2012, 40, 1688–1707. [Google Scholar] [CrossRef] [PubMed]
- Perzborn, E.; Heitmeier, S.; Laux, V. Effects of Rivaroxaban on Platelet Activation and Platelet-Coagulation Pathway Interaction: In Vitro and In Vivo Studies. J. Cardiovasc. Pharmacol. Ther. 2015, 20, 554–562. [Google Scholar] [CrossRef]
- Kubitza, D.; Becka, M.; Schwers, S.; Voith, B. Investigation of Pharmacodynamic and Pharmacokinetic Interactions between Rivaroxaban and Enoxaparin in Healthy Male Subjects. Clin. Pharm. Drug Dev. 2013, 2, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Kubitza, D.; Becka, M.; Mück, W.; Krätzschmar, J. Pharmacodynamics and Pharmacokinetics during the Transition from Warfarin to Rivaroxaban: A Randomized Study in Healthy Subjects: Pharmacodynamics during Transition from Warfarin to Rivaroxaban. Br. J. Clin. Pharmacol. 2014, 78, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.T.; Byra, W.; Vaidyanathan, S.; Natarajan, J.; Ariyawansa, J.; Salih, H.; Turner, K.C. Switching from Rivaroxaban to Warfarin: An Open Label Pharmacodynamic Study in Healthy Subjects. Br. J. Clin. Pharmacol. 2015, 79, 907–917. [Google Scholar] [CrossRef]
- Kubitza, D.; Becka, M.; Mueck, W.; Zuehlsdorf, M. Rivaroxaban (BAY 59-7939)—An Oral, Direct Factor Xa Inhibitor—Has No Clinically Relevant Interaction with Naproxen. Br. J. Clin. Pharmacol. 2007, 63, 469–476. [Google Scholar] [CrossRef]
- Kubitza, D.; Becka, M.; Mueck, W.; Zuehlsdorf, M. Safety, Tolerability, Pharmacodynamics, and Pharmacokinetics of Rivaroxaban—An Oral, Direct Factor Xa Inhibitor—Are Not Affected by Aspirin. J. Clin. Pharmacol. 2006, 46, 981–990. [Google Scholar] [CrossRef]
- Kubitza, D.; Becka, M.; Mück, W.; Schwers, S. Effect of Co-Administration of Rivaroxaban and Clopidogrel on Bleeding Time, Pharmacodynamics and Pharmacokinetics: A Phase I Study. Pharmaceuticals 2012, 5, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Mega, J.L.; Braunwald, E.; Mohanavelu, S.; Burton, P.; Poulter, R.; Misselwitz, F.; Hricak, V.; Barnathan, E.S.; Bordes, P.; Witkowski, A.; et al. Rivaroxaban versus Placebo in Patients with Acute Coronary Syndromes (ATLAS ACS-TIMI 46): A Randomised, Double-Blind, Phase II Trial. Lancet 2009, 374, 29–38. [Google Scholar] [CrossRef]
- Ohman, E.M.; Roe, M.T.; Steg, P.G.; James, S.K.; Povsic, T.J.; White, J.; Rockhold, F.; Plotnikov, A.; Mundl, H.; Strony, J.; et al. Clinically Significant Bleeding with Low-Dose Rivaroxaban versus Aspirin, in Addition to P2Y12 Inhibition, in Acute Coronary Syndromes (GEMINI-ACS-1): A Double-Blind, Multicentre, Randomised Trial. Lancet 2017, 389, 1799–1808. [Google Scholar] [CrossRef]
- Eriksson, B.I.; Rosencher, N.; Friedman, R.J.; Homering, M.; Dahl, O.E. Concomitant Use of Medication with Antiplatelet Effects in Patients Receiving Either Rivaroxaban or Enoxaparin after Total Hip or Knee Arthroplasty. Thromb. Res. 2012, 130, 147–151. [Google Scholar] [CrossRef]
- Shah, R.; Hellkamp, A.; Lokhnygina, Y.; Becker, R.C.; Berkowitz, S.D.; Breithardt, G.; Hacke, W.; Halperin, J.L.; Hankey, G.J.; Fox, K.A.; et al. Use of Concomitant Aspirin in Patients with Atrial Fibrillation: Findings from the ROCKET AF Trial. Am. Heart J. 2016, 179, 77–86. [Google Scholar] [CrossRef]
- Kreutz, R.; Haas, S.; Holberg, G.; Lassen, M.R.; Mantovani, L.G.; Schmidt, A.; Turpie, A.G.G. Rivaroxaban Compared with Standard Thromboprophylaxis after Major Orthopaedic Surgery: Co-Medication Interactions. Br. J. Clin. Pharmacol. 2016, 81, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Kubitza, D.; Becka, M.; Zuehlsdorf, M.; Mueck, W. Effect of Food, an Antacid, and the H2 Antagonist Ranitidine on the Absorption of BAY 59-7939 (Rivaroxaban), an Oral, Direct Factor Xa Inhibitor, in Healthy Subjects. J. Clin. Pharmacol. 2006, 46, 549–558. [Google Scholar] [CrossRef]
- Moore, K.T.; Plotnikov, A.N.; Thyssen, A.; Vaccaro, N.; Ariyawansa, J.; Burton, P.B. Effect of Multiple Doses of Omeprazole on the Pharmacokinetics, Pharmacodynamics, and Safety of a Single Dose of Rivaroxaban. J. Cardiovasc. Pharmacol. 2011, 58, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, H.; Xu, F.; Zhang, B. The Utilization Risk of Rivaroxaban in the Anti-Glioma Therapy Regimen. Lat. Am. J. Pharm. 2015, 34, 616–618. [Google Scholar]
- Abstracts of the XXV Congress of the International Society on Thrombosis and Haemostasis, June 20–25, 2015. J. Thromb. Haemost. 2015, 13, 1–997. [CrossRef]
- Stöllberger, C.; Bastovansky, A.; Finsterer, J. Fatal Intracerebral Bleeding under Rivaroxaban. Int. J. Cardiol. 2015, 201, 110–112. [Google Scholar] [CrossRef]
- Stollberger, C.; Finsterer, J. Recurrent Venous Thrombosis under Rivaroxaban and Carbamazepine for Symptomatic Epilepsy. Neurol. Neurochir. Pol. 2017, 51, 194–196. [Google Scholar] [CrossRef]
- Serra, W.; Li Calzi, M.; Coruzzi, P. Left Atrial Appendage Thrombosis during Therapy with Rivaroxaban in Elective Cardioversion for Permanent Atrial Fibrillation. Clin. Pract. 2015, 5, 788. [Google Scholar] [CrossRef] [PubMed]
- Risselada, A.J.; Visser, M.J.; Van Roon, E.N. Pulmonary Embolism Due to Interaction between Rivaroxaban and Carbamazepine. Nederlands Tijdschrift voor Geneeskunde 2013, 157, A6568. [Google Scholar]
- Lakatos, B.; Stoeckle, M.; Elzi, L.; Battegay, M.; Marzolini, C. Gastrointestinal Bleeding Associated with Rivaroxaban Administration in a Treated Patient Infected with Human Immunodeficiency Virus. Swiss Med. Wkly. 2014, 22, 13906. [Google Scholar] [CrossRef]
- Kaur, J.; Rizvi, S.; Tewari, P.; Tamer, S.; Nafsi, T. Rivaroxaban Treatment Failure from Possible Drug Interaction: A Case Report. Chest 2016, 149, A501. [Google Scholar] [CrossRef]
- Ing Lorenzini, K.; Daali, Y.; Fontana, P.; Desmeules, J.; Samer, C. Rivaroxaban-Induced Hemorrhage Associated with ABCB1 Genetic Defect. Front. Pharmacol. 2016, 7. [Google Scholar] [CrossRef]
- Fralick, M.; Juurlink, D.N.; Marras, T. Bleeding Associated with Coadministration of Rivaroxaban and Clarithromycin. CMAJ 2016, 188, 669–672. [Google Scholar] [CrossRef][Green Version]
- Corallo, C.E.; Grannell, L.; Tran, H. Postoperative Bleeding After Administration of a Single Dose of Rivaroxaban to a Patient Receiving Antiretroviral Therapy. Drug Saf. Case Rep. 2015, 2, 11. [Google Scholar] [CrossRef][Green Version]
- Bates, D.; Dalton, B.; Gilmour, J.; Kapler, J. Venous Thromboembolism Due to Suspected Interaction between Rivaroxaban and Nevirapine. Can. J. Hosp. Pharm. 2013, 66, 125–129. [Google Scholar] [CrossRef]
- Altena, R.; van Roon, E.; Folkeringa, R.; de Wit, H.; Hoogendoorn, M. Clinical Challenges Related to Novel Oral Anticoagulants: Drug-Drug Interactions and Monitoring. Haematology 2014, 99, e26–e27. [Google Scholar] [CrossRef][Green Version]
- Stöllberger, C.; Valentin, A.; Finsterer, J. Severe Bleeding after Jugular Central Venous Line Insertion in a Patient under Rivaroxaban. Anaesth. Intensive Care 2014, 42, 419–420. [Google Scholar]
- Menendez, D.; Michel, J. Hemopericardium with Tamponade Following Rivaroxaban Administration and Its Attenuation by CYP3A4 Inhibitors. Proceedings (Bayl. Univ. Med. Cent.) 2016, 29, 414–415. [Google Scholar] [CrossRef]
- Yoong, D.; Naccarato, M.; Gough, K. Extensive Bruising and Elevated Rivaroxaban Plasma Concentration in a Patient Receiving Cobicistat-Boosted Elvitegravir. Ann. Pharmacol. 2017, 51, 713–714. [Google Scholar] [CrossRef] [PubMed]
- Burden, T.; Thompson, C.; Bonanos, E.; Medford, A.R. Lesson of the Month 2: Pulmonary Embolism in a Patient on Rivaroxaban and Concurrent Carbamazepine. Clin. Med. 2018, 18, 103–105. [Google Scholar] [CrossRef]
- Becerra, A.F.; Amuchastegui, T.; Tabares, A.H. Decreased Rivaroxaban Levels in a Patient with Cerebral Vein Thrombosis Receiving Phenytoin. Case Rep. Hematol. 2017, 2017, 4760612. [Google Scholar] [CrossRef] [PubMed]
- Oladiran, O.; Segal, J.; Nwosu, I.; Nazir, S. A Rare Case of Spontaneous Cardiac Tamponade Induced by Concomitant Use of Rivaroxaban and Amiodarone. Case Rep. Cardiol. 2018, 2018. [Google Scholar] [CrossRef]
- Shah, P.; Glueck, J.; Jetty, V. Bilateral Upper Extremity Ecchymotic, Bruising, and Bleeding in a Patient on Alirocumab, Rivaroxaban, and Anti-Platelet Therapy. J. Investig. Med. 2016, 64, 923–924. [Google Scholar] [CrossRef]
- Egger, F.; Targa, F.; Unterholzner, I.; Grant, R.P.; Herrmann, M.; Wiedermann, C.J. Medication Error When Switching from Warfarin to Rivaroxaban Leading to Spontaneous Large Ecchymosis of the Abdominal and Chest Wall. Clin. Pract. 2016, 6, 873. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.; Auraha, N.; Dyal, H.; Patel, R.; DiGiovine, B. Reversal of Cocaine-Induced Diffuse Alveolar Hemorrhage in a Patient on Rivaroxaban with Activated Prothrombin Complex Concentrate. Am. J. Respir. Crit. Care Med. 2015, 191, A4612. [Google Scholar]
- Stöllberger, C.; Zuntner, G.; Bastovansky, A.; Finsterer, J. Cerebral Hemorrhage under Rivaroxaban. Int. J. Cardiol. 2013, 167, e179–e181. [Google Scholar] [CrossRef]
- Jaeger, M.; Jeanneret, B.; Schaeren, S. Spontaneous Spinal Epidural Haematoma during Factor Xa Inhibitor Treatment (Rivaroxaban). Eur. Spine J. 2012, 21, 433–435. [Google Scholar] [CrossRef][Green Version]
- Sen, P.; Casaus, L.; Majumdar, U.; Saeed, A. Fatal Consequences of Synergistic Anticoagulation. Chest 2017, 152, A298. [Google Scholar] [CrossRef]
- Patel, J.S.; Rahbar, A.J.; Patel, K.; Sigal, T.W. Rivaroxaban-Associated Intraparenchymal Hemorrhage Managed with 4-Factor Prothrombin Complex Concentrate. Curr. Emerg. Hosp. Med. Rep. 2018, 6, 1–7. [Google Scholar] [CrossRef]
- Arioli, D.; Donelli, D.; Morini, L.; Leone, M.C.; Negri, E.A. Drug Plasma Level Measurement in Management of Severe Bleeding during Direct Oral Anticoagulant Treatment: Case Report and Perspective. Intern. Emerg. Med. 2018, 13, 1–4. [Google Scholar] [CrossRef]
- Gruenebaum, D.D.; Alsarah, A.; Alsara, O.; Laird-Fick, H. Bleeding Complication of Triple Therapy of Rivaroxaban, Prasugrel, and Aspirin: A Case Report and General Discussion. Case Rep. Cardiol. 2014, 2014, 1–4. [Google Scholar] [CrossRef]
- Stöllberger, C.; Finsterer, J. Prolonged Anticoagulant Activity of Rivaroxaban in a Polymorbid Elderly Female with Non-Convulsive Epileptic State. Heart Lung J. Acute Crit. Care 2014, 43, 262–263. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.; Wool, K.; Halanych, J.; Sarmad, R. Acute Liver Failure after Initiation of Rivaroxaban: A Case Report and Review of the Literature. N. Am. J. Med. Sci. 2015, 7, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Almarshad, F.; Alaklabi, A.; Bakhsh, E.; Pathan, A.; Almegren, M. Use of Direct Oral Anticoagulants in Daily Practice. Am. J. Blood Res. 2018, 8, 57–72. [Google Scholar]
- European Commission—Enterprise and Industry Directorate-General a Guideline on Summary of Product Characteristics. Available online: http://www.kardio.hr/wp-content/uploads/2012/12/spcguidrev1-oct2005_en.pdf (accessed on 25 October 2018).
- European Medicines Agency—Europa EU Section 4.5 Interaction with Other Medicinal Products and Other Forms of Interaction. Available online: https://www.ema.europa.eu/en/documents/presentation/presentation-section-45-interaction-other-medicinal-products-other-forms-interaction_en.pdf (accessed on 13 January 2020).
- Home—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 25 October 2018).
- Miller, J.E.; Wilenzick, M.; Ritcey, N.; Ross, J.S.; Mello, M.M. Measuring Clinical Trial Transparency: An Empirical Analysis of Newly Approved Drugs and Large Pharmaceutical Companies. BMJ Open 2017, 7, e017917. [Google Scholar] [CrossRef]
- European Medicines Agency—Europa EU Guideline on Good Pharmacovigilance Practices (GVP)—Module VIII—Post-Authorisation Safety Studies (Rev 3). Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-gvp-module-viii-post-authorisation-safety-studies-rev-3_en.pdf (accessed on 13 January 2020).
- Uppsala Monitoring Centre UMC. Know More about VigiBase. Available online: https://www.who-umc.org/vigibase/vigibase/know-more-about-vigibase/ (accessed on 25 October 2018).
- Strandell, J.; Wahlin, S. Pharmacodynamic and Pharmacokinetic Drug Interactions Reported to VigiBase, the WHO Global Individual Case Safety Report Database. Eur. J. Clin. Pharmacol. 2011, 67, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Hult, S.; Sartori, D.; Bergvall, T.; Hedfors Vidlin, S.; Grundmark, B.; Ellenius, J.; Norén, G.N. A Feasibility Study of Drug-Drug Interaction Signal Detection in Regular Pharmacovigilance. Drug Saf. 2020, 43, 775–785. [Google Scholar] [CrossRef]
- Strandell, J.; Caster, O.; Bate, A.; Norén, N.; Edwards, I.R. Reporting Patterns Indicative of Adverse Drug Interactions: A Systematic Evaluation in VigiBase. Drug Saf. 2011, 34, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Strandell, J.; Norén, G.N.; Hägg, S. Key Elements in Adverse Drug Interaction Safety Signals: An Assessment of Individual Case Safety Reports. Drug Saf. 2013, 36, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, M. VigiBase, the WHO Global ICSR Database System: Basic Facts. Drug Inf. J. 2008, 42, 409–419. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).