Relationship between the miRNA Profiles and Oncogene Mutations in Non-Smoker Lung Cancer. Relevance for Lung Cancer Personalized Screenings and Treatments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Recruitment and Sampling
2.2. DNA Extraction
2.3. Somatic Mutation Identification
2.4. Total RNA Extraction
2.5. MiRNA Microarray Analysis
2.6. Statistical Analysis
3. Results
3.1. Mutations in Oncogenes
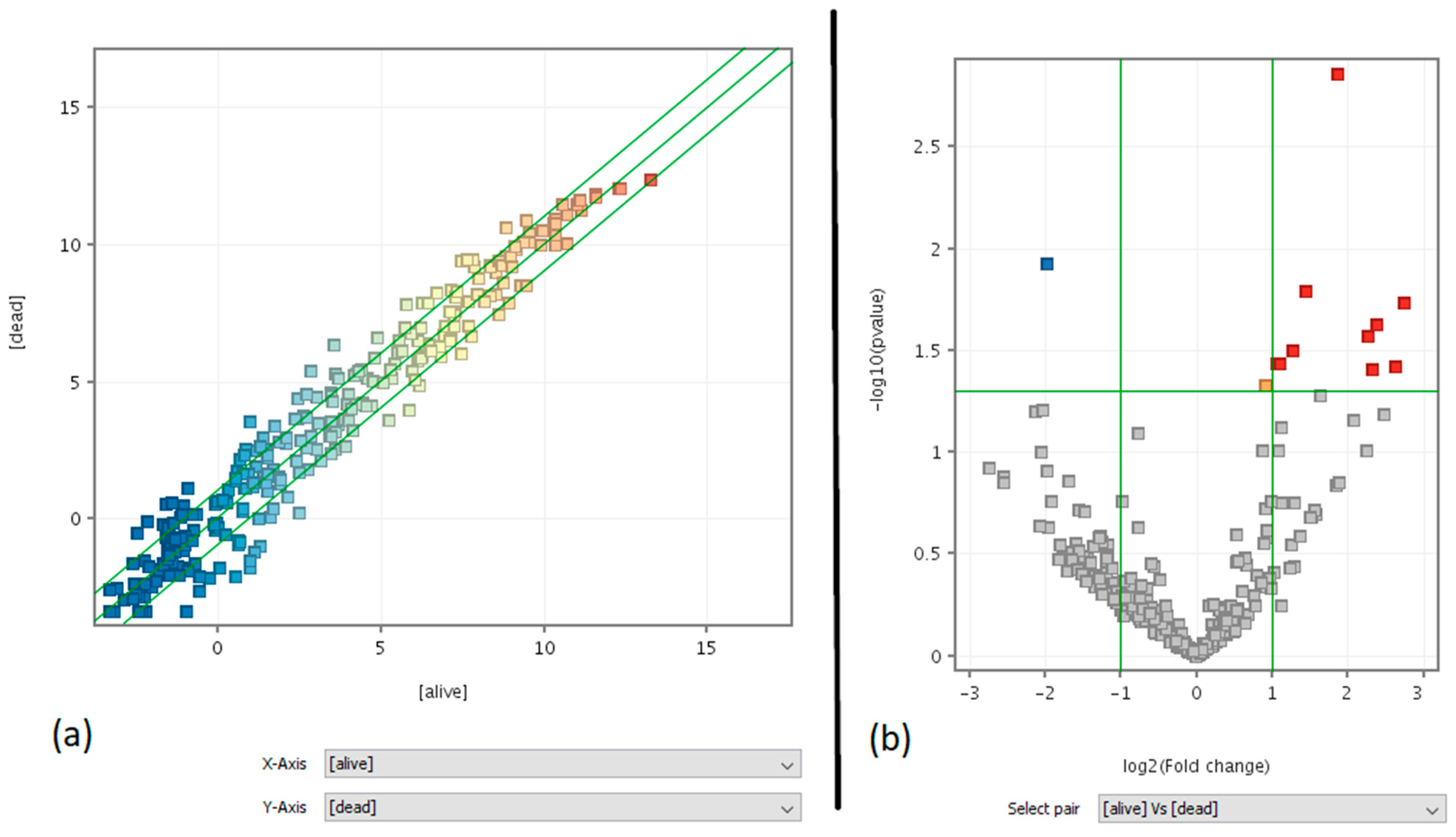
3.2. Relationship between miRNA Profiles and Mutations in Oncogenes
3.3. Clinical Predictivity of miRNA Profiling
3.4. Liquid Biopsy: Lung Versus Blood
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rupaimoole, R.; Slack, F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liang, A.-L.; Liu, Y.-J. Research progress on the relationship between lung cancer drug-resistance and microRNAs. J. Cancer 2019, 10, 6865–6875. [Google Scholar] [CrossRef]
- Ren, P.; Gong, F.; Zhang, Y.; Jiang, J.; Zhang, H. MicroRNA-92a promotes growth, metastasis, and chemoresistance in non-small cell lung cancer cells by targeting PTEN. Tumor Biol. 2015, 37, 3215–3225. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Kim, T.; Fassan, M.; Meng, W.; Sun, H.-L.; Jeon, Y.-J. MicroRNA-224 is implicated in lung cancer pathogenesis through targeting caspase-3 and caspase-7. Oncotarget 2015, 6, 21802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Han, M.; Xiong, Y.; Wang, L.; Fei, Y.; Shen, X.; Zhu, Y.; Liang, Z.Q. A miRNA-200c/cathepsin L feedback loop determines paclitaxel resistance in human lung cancer A549 cells in vitro through regulating epithelial–mesenchymal transition. Acta Pharmacol. Sin. 2018, 39, 1034. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhou, C.; Zhao, X.; He, J.; Tian, H.; Shen, W.; Han, Y.; Chen, J.; Fang, S.; Meng, X.; et al. A two-miRNA signature (miR-33a-5p and miR-128-3p) in whole blood as potential biomarker for early diagnosis of lung cancer. Sci. Rep. 2018, 8, 16699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izzotti, A.; Carozzo, S.; Pulliero, A.; Zhabayeva, D.; Ravetti, J.L.; Bersimbaev, R. Extracellular MicroRNA in liquid biopsy: Applicability in cancer diagnosis and prevention. Am. J. Cancer Res. 2016, 6, 1461–1493. [Google Scholar] [PubMed]
- Izzotti, A.; Longobardi, M.G.; La Maestra, S.; Micale, R.T.; Pulliero, A.; Camoirano, A.; Geretto, M.; D’Agostini, F.; Balansky, R.; Miller, M.S.; et al. Release of microRNAs into body fluids form ten organs in mice exposed to cigarette smoke. Theranostic 2018, 8, 2147–2160. [Google Scholar] [CrossRef]
- Izzotti, A.; Pulliero, A. Molecular damage and lung tumors in cigarette smoke exposed mice. Ann. N. Y. Acad. Sci. 2015, 1340, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Heyn, H.; Engelmann, M.; Schreek, S.; Ahrens, P.; Lehmann, U.; Kreipe, H.; Schlegelberger, B.; Beger, C. MicroRNA miR-335 is crucial for the BRCA1 regulatory cascade in breast cancer development. Int. J. Cancer 2011, 129, 2797–2806. [Google Scholar] [CrossRef] [PubMed]
- Izzotti, A.; Calin, G.A.; Arrigo, P.; Steele, V.E.; Croce, C.M.; De Flora, S. Downregulation of microRNA expression in the lungs of rats exposed to cigarette smoke. FASEB J. 2009, 23, 806–812. [Google Scholar] [CrossRef]
- Vanni, I.; Coco, S.; Truini, A.; Rusmini, M.; Dal Bello, M.G.; Alama, A.; Banelli, B.; Mora, M.; Rijavec, E.; Barletta, G.; et al. Next-Generation Sequencing Workflow for NSCLC Critical Samples Using a Targeted Sequencing Approach by Ion Torrent PGM™ Platform. Int. J. Mol. Sci. 2015, 16, 28765–28782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stracquadanio, M.; Dinelli, E.; Trombini, C. Role of volcanic dust in the atmospheric transport and deposition of polycyclic aromatic hydrocarbons and mercury. J. Environ. Monit. 2003, 5, 984. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, K.; Rojas, M.; Geneste, O.; Castegnaro, M.; Camus, A.M.; Petruzzelli, S.; Giuntini, C.; Bartsch, H. An improved fluorometric assay for dosimetry of benzo(a)pyrene diol-epoxide-DNA adducts in smokers’ lung: Comparisons with total bulky adducts and aryl hydrocarbon hydroxylase activity. Cancer Res. 1992, 52, 6248–6253. [Google Scholar] [PubMed]
- Zaporozhchenko, I.A.; Morozkin, E.S.; Ponomaryova, A.A.; Rykova, E.Y.; Cherdyntseva, N.V.; Zheravin, A.A. Profiling of 179 miRNA Expression in Blood Plasma of Lung Cancer Patients and Cancer-Free Individuals. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sharawat, S.K.; Ali, A.; Gaur, V.; Malik, P.S.; Kumar, S.; Mohan, A.; Guleria, R. Identification of differentially expressed circulating serum microRNA for the diagnosis and prognosis of Indian non–small cell lung cancer patients. Curr. Probl. Cancer 2020, 100540. [Google Scholar] [CrossRef]
- Bianchi, F.; Nicassio, F.; Marzi, M.; Belloni, E.; Dall’Olio, V.; Bernard, L.; Pelosi, G.; Maisonneuve, P.; Veronesi, G.; Di Fiore, P.P. A serum circulating miRNA diagnostic test to identify asymptomatic high-risk individuals with early stage lung cancer. Embo Mol. Med. 2011, 3, 495–503. [Google Scholar] [CrossRef]
- Boeri, M.; Verri, C.; Conte, D.; Roz, L.; Modena, P.; Facchinetti, F.; Calabrò, E.; Croce, C.M.; Pastorino, U.; Sozzi, G. MicroRNA signatures in tissues and plasma predict development and prognosis of computed tomography detected lung cancer. Proc. Natl. Acad. Sci. USA 2011, 108, 3713–3718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.; Chen, X.; Zhao, Y.; Tian, T.; Jin, G.; Shu, Y.; Chen, Y.; Xu, L.; Zen, K.; Zhang, C.; et al. Serum MicroRNA Signatures Identified in a Genome-Wide Serum MicroRNA Expression Profiling Predict Survival of Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2010, 28, 1721–1726. [Google Scholar] [CrossRef]
- Mao, M.; Wu, Z.; Chen, J. MicroRNA-187-5p suppresses cancer cell progression in non-small cell lung cancer (NSCLC) through down-regulation of CYP1B1. Biochem. Biophys. Res. Commun. 2016, 478, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.-Y.; Liu, C.-H.; Xue, Y.; Chen, Y.-Y.; Wang, Y.-L.; Wu, X.-Z. MicroRNA-147b promotes lung adenocarcinoma cell aggressiveness through negatively regulating microfibril-associated glycoprotein 4 (MFAP4) and affects prognosis of lung adenocarcinoma patients. Gene 2020, 730, 144316. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, L.; Zhou, J.; Cui, X.; Tian, Q.; Jin, Y.; Zhu, Y. MiR-2861 Behaves as a Biomarker of Lung Cancer Stem Cells and Regulates the HDAC5-ERK System Genes. Cell. Reprogram. 2018, 20, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Asakura, K.; Kadota, T.; Matsuzaki, J.; Yoshida, Y.; Yamamoto, Y.; Nakagawa, K.A. miRNA-based diagnostic model predicts resectable lung cancer in humans with high accuracy. Commun. Biol. 2020, 3, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]


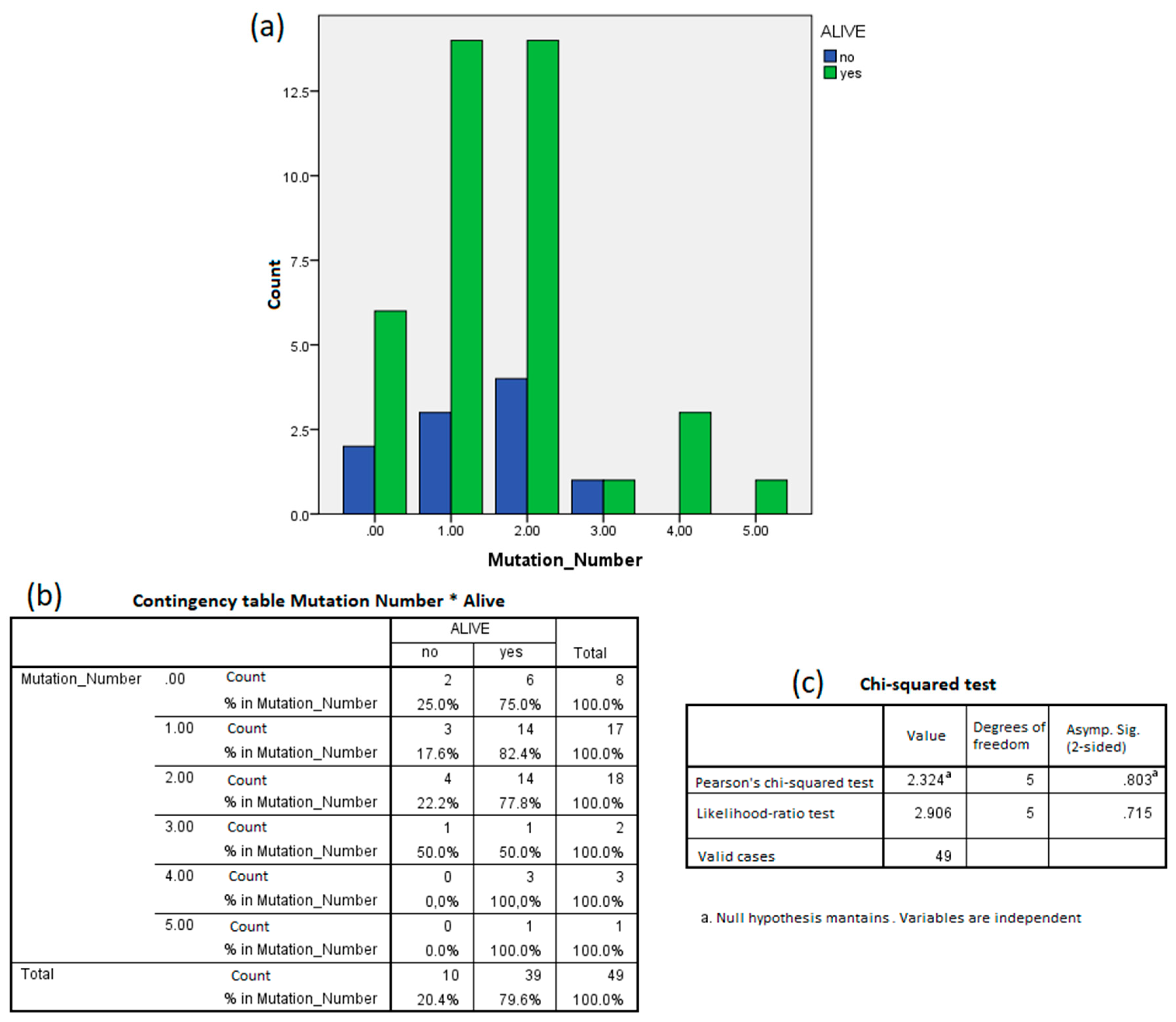
| Number of Mutations | Frequency | Percentage |
|---|---|---|
| 0 | 10 | 19.2 |
| 1 | 17 | 32.7 |
| 2 | 19 | 36.5 |
| 3 | 2 | 3.8 |
| 4 | 3 | 5.8 |
| 5 | 1 | 1.9 |
| Total | 52 | 100.0 |
| Gene | Mutated/Total Patients | Number of Entities (Altered miRNAs) | Up | Down | miRNAs Targeting Mutated Oncogene |
|---|---|---|---|---|---|
| BRAF | 2/33 | 7 | 1 | 6 | 0 |
| EGFR | 9/33 | 1 | 0 | 1 | 0 |
| ERBB2 | 1/33 | - | - | - | - |
| ERBB4 | 1/33 | - | - | - | - |
| FGFR3 | 1/33 | - | - | - | - |
| KRAS | 10/33 | 13 | 13 | 0 | 2 (hsa-miR-15b-3p, hsa-miR-21-3p) |
| NOTCH1 | 3/33 | 1 | 1 | 0 | 0 |
| PIK3CA | 3/33 | 0 | 0 | 0 | 0 |
| PTEN | 2/33 | 0 | 0 | 0 | 0 |
| STK11 | 2/33 | 31 | 30 | 1 | 1 (hsa-miR-548aa) |
| TP53 | 10/33 | 4 | 4 | 0 | 1 (hsa-miR-205-3p) |
| Tumoral miRNAs in Blood | Average Signal in Blood Plasma Microarray (Fluorescence Units) | Reference in Literature |
|---|---|---|
| hsa-let-7b-5p | 1556.03 | [16,17] |
| hsa-let-7e-5p | 2570.68 | [15] |
| hsa-let-7g-5p | 1591.89 | ND |
| hsa-miR-103a-3p | 1966.77 | ND |
| hsa-miR-107 | 2307.96 | ND |
| hsa-miR-1247-5p | 1619.35 | ND |
| hsa-miR-143-3p | 1517.48 | ND |
| hsa-miR-147b | 2867.22 | ND |
| hsa-miR-150-5p | 1728.39 | [15] |
| hsa-miR-151a-5p | 2375.45 | ND |
| hsa-miR-151b | 2375.45 | ND |
| hsa-miR-181a-2-3p | 1714.68 | ND |
| hsa-miR-183-3p | 4515.51 | ND |
| hsa-miR-184 | 3097.26 | ND |
| hsa-miR-185-5p | 2809.76 | ND |
| hsa-miR-193a-5p | 1482.98 | ND |
| hsa-miR-22-5p | 1889.39 | [15,17] |
| hsa-miR-224-3p | 1805.07 | ND |
| hsa-miR-23a-5p | 2880.99 | ND |
| hsa-miR-26a-5p | 1497.29 | [17] |
| hsa-miR-2861 | 3086.05 | ND |
| hsa-miR-29b-2-5p | 1481.13 | ND |
| hsa-miR-30b-5p | 2117.35 | [16] |
| hsa-miR-30c-2-3p | 2919.97 | ND |
| hsa-miR-30c-5p | 3299.46 | [17,18] |
| hsa-miR-3149 | 2367.91 | ND |
| hsa-miR-361-3p | 1454.65 | ND |
| hsa-miR-371b-5p | 12,583.51 | ND |
| hsa-miR-424-5p | 1660.83 | ND |
| hsa-miR-4252 | 1558.12 | ND |
| hsa-miR-4290 | 1875.54 | ND |
| hsa-miR-4306 | 7145.12 | ND |
| hsa-miR-4324 | 1530.87 | ND |
| hsa-miR-4440 | 1537.86 | ND |
| hsa-miR-4443 | 3859.37 | ND |
| hsa-miR-4481 | 1605.44 | ND |
| hsa-miR-450a-5p | 4646.58 | ND |
| hsa-miR-4516 | 11,603.31 | ND |
| hsa-miR-452-5p | 1629.61 | ND |
| hsa-miR-4532 | 13,231.17 | ND |
| hsa-miR-4634 | 1884.47 | ND |
| hsa-miR-483-3p | 3177.46 | ND |
| hsa-miR-486-3p | 2337.52 | [14,18] |
| hsa-miR-490-3p | 1882.31 | ND |
| hsa-miR-505-5p | 3775.67 | ND |
| hsa-miR-516b-5p | 5121.31 | ND |
| hsa-miR-548aa | 3480.4 | ND |
| hsa-miR-548q | 1453.76 | ND |
| hsa-miR-642b-5p | 2070.84 | ND |
| hsa-miR-664b-3p | 1539.91 | ND |
| hsa-miR-744-5p | 3112.18 | ND |
| hsa-miR-99a-3p | 1503.5 | ND |
| hsa-miR-99b-5p | 1575.63 | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izzotti, A.; Coronel Vargas, G.; Pulliero, A.; Coco, S.; Vanni, I.; Colarossi, C.; Blanco, G.; Agodi, A.; Barchitta, M.; Maugeri, A.; et al. Relationship between the miRNA Profiles and Oncogene Mutations in Non-Smoker Lung Cancer. Relevance for Lung Cancer Personalized Screenings and Treatments. J. Pers. Med. 2021, 11, 182. https://doi.org/10.3390/jpm11030182
Izzotti A, Coronel Vargas G, Pulliero A, Coco S, Vanni I, Colarossi C, Blanco G, Agodi A, Barchitta M, Maugeri A, et al. Relationship between the miRNA Profiles and Oncogene Mutations in Non-Smoker Lung Cancer. Relevance for Lung Cancer Personalized Screenings and Treatments. Journal of Personalized Medicine. 2021; 11(3):182. https://doi.org/10.3390/jpm11030182
Chicago/Turabian StyleIzzotti, Alberto, Gabriela Coronel Vargas, Alessandra Pulliero, Simona Coco, Irene Vanni, Cristina Colarossi, Giuseppina Blanco, Antonella Agodi, Martina Barchitta, Andrea Maugeri, and et al. 2021. "Relationship between the miRNA Profiles and Oncogene Mutations in Non-Smoker Lung Cancer. Relevance for Lung Cancer Personalized Screenings and Treatments" Journal of Personalized Medicine 11, no. 3: 182. https://doi.org/10.3390/jpm11030182
APA StyleIzzotti, A., Coronel Vargas, G., Pulliero, A., Coco, S., Vanni, I., Colarossi, C., Blanco, G., Agodi, A., Barchitta, M., Maugeri, A., CT-ME-EN Cancer Registry Workers, Oliveri Conti, G., Ferrante, M., & Sciacca, S. (2021). Relationship between the miRNA Profiles and Oncogene Mutations in Non-Smoker Lung Cancer. Relevance for Lung Cancer Personalized Screenings and Treatments. Journal of Personalized Medicine, 11(3), 182. https://doi.org/10.3390/jpm11030182













