Variations of Serum Oxidative Stress Biomarkers under First-Line Antituberculosis Treatment: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Biochemical Analysis
2.2.1. Determination of SOD Activity
2.2.2. Determination of GPx Activity
2.2.3. Determination of CAT Activity
2.2.4. Determination of GSH Concentration
2.2.5. Total Antioxidant Capacity (TAC) Assay
2.2.6. Thiobarbituric Acid Reactive Substances Assay
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Europe—Tuberculosis News. Available online: http://www.euro.who.int/en/health-topics/communicable-diseases/tuberculosis/news/news/2019/3/drug-resistant-strains-could-become-the-dominant-form-of-tb-in-europe-its-time-to-end-tb (accessed on 15 October 2020).
- Uberti, F.; Morsanuto, V.; Molinari, C. Vitamin D in Oxidative Stress and Diseases. Crit. Eval. Vitam. D Basic Overv. 2017, 47–73. [Google Scholar] [CrossRef]
- Brighenti, S.; Bergman, P.; Martineau, A.R. Vitamin D and tuberculosis: Where next? J. Intern. Med. 2018, 284, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Balcells, M.E.; Yokobori, N.; Hong, B.-Y.; Corbett, J.; Cervantes, J.L. The lung microbiome, vitamin D, and the tuberculous granuloma: A balance triangle. Microb. Pathog. 2019, 131, 158–163. [Google Scholar] [CrossRef]
- Shastri, M.D.; Shukla, S.D.; Chong, W.C.; Dua, K.; Peterson, G.M.; Patel, R.P.; Hansbro, P.M.; Eri, R.; O’Toole, R.F. Role of Oxidative Stress in the Pathology and Management of Human Tuberculosis. Oxidative Med. Cell. Longev. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- De Almeida, A.J.P.O.; Rezende, M.S.D.A.; Dantas, S.H.; Silva, S.D.L.; De Oliveira, J.C.P.L.; Azevedo, F.D.L.A.A.D.; Alves, R.M.F.R.; De Menezes, G.M.S.; Dos Santos, P.F.; Gonçalves, T.A.F.; et al. Unveiling the Role of Inflammation and Oxidative Stress on Age-Related Cardiovascular Diseases. Oxidative Med. Cell. Longev. 2020, 2020, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Vemula, M.H.; Ganji, R.; Sivangala, R.; Jakkala, K.; Gaddam, S.; Penmetsa, S.; Banerjee, S. Mycobacterium tuberculosis Zinc Metalloprotease-1 Elicits Tuberculosis-Specific Humoral Immune Response Independent of Mycobacterial Load in Pulmonary and Extra-Pulmonary Tuberculosis Patients. Front. Microbiol. 2016, 7, 418. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Molin, M.D.; Ganguli, G.; Padhi, A.; Jena, P.; Selchow, P.; Sengupta, S.; Meuli, M.; Sander, P.; Sonawane, A.; et al. Mycobacterium tuberculosis EsxO (Rv2346c) promotes bacillary survival by inducing oxidative stress mediated genomic instability in macrophages. Tuberculosis 2016, 96, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Shen, M. Associations between vitamin D receptor genetic variants and tuberculosis: A meta-analysis. Innate Immun. 2019, 25, 305–313. [Google Scholar] [CrossRef]
- Vidhya, R.; Rathnakumar, K.; Balu, V.; Pugalendi, K.V. Oxidative stress, antioxidant status and lipid profile in pulmonary tuberculosis patients before and after anti-tubercular therapy. Indian J. Tuberc. 2018, 66, 375–381. [Google Scholar] [CrossRef]
- Gough, M.E.; Graviss, E.A.; Chen, T.-A.; Obasi, E.M.; May, E.E. Compounding effect of vitamin D3 diet, supplementation, and alcohol exposure on macrophage response to mycobacterium infection. Tuberculosis 2019, 116, S42–S58. [Google Scholar] [CrossRef]
- Rajopadhye, S.H. Oxidative Stress Markers in Tuberculosis and HIV/TB Co-Infection. J. Clin. Diagn. Res. 2017, 11, BC24–BC28. [Google Scholar] [CrossRef]
- Abrahams, S.; Haylett, W.L.; Johnson, G.; Carr, J.A.; Bardien, S. Antioxidant effects of curcumin in models of neurodegeneration, aging, oxidative and nitrosative stress: A review. Neuroscience 2019, 406, 1–21. [Google Scholar] [CrossRef]
- Kruk, J.; Aboul-Enein, H.Y.; Kładna, A.; Bowser, J.E. Oxidative stress in biological systems and its relation with pathophysiological functions: The effect of physical activity on cellular redox homeostasis. Free Radic. Res. 2019, 53, 497–521. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P.; Rahman, H.S. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef]
- Amaral, E.P.; Vinhaes, C.L.; Oliveira-De-Souza, D.; Nogueira, B.; Akrami, K.M.; Andrade, B.B. The Interplay Between Systemic Inflammation, Oxidative Stress, and Tissue Remodeling in Tuberculosis. Antioxidants Redox Signal. 2021, 34, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Teskey, G.; Abrahem, R.; Cao, R.; Gyurjian, K.; Islamoglu, H.; Lucero, M.; Martinez, A.; Paredes, E.; Salaiz, O.; Robinson, B.; et al. Glutathione as a Marker for Human Disease. In Advances in Applied Microbiology; Elsevier BV: London, UK, 2018; Volume 87, pp. 141–159. [Google Scholar]
- Farazi, A.; Didgar, F.; Sarafraz, A. The effect of vitamin D on clinical outcomes in tuberculosis. Egypt. J. Chest Dis. Tuberc. 2017, 66, 419–423. [Google Scholar] [CrossRef]
- Wahyunitisari, M.R.; Mertaniasih, N.M.; Amin, M.; Artama, W.T.; Koendhori, E.B. Vitamin D, cell death pathways, and tuberculosis. Int. J. Mycobacteriology 2017, 6, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Gois, P.H.F.; Ferreira, D.; Olenski, S.; Seguro, A.C. Vitamin D and Infectious Diseases: Simple Bystander or Contributing Factor? Nutrients 2017, 9, 651. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yeo, H.C.; Doniger, S.J.; Ames, B.N. Assay of Aldehydes from Lipid Peroxidation: Gas Chromatography–Mass Spectrometry Compared to Thiobarbituric Acid. Anal. Biochem. 1997, 245, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Spanidis, Y.; Mpesios, A.; Stagos, D.; Goutzourelas, N.; Bar-Or, D.; Karapetsa, M.; Zakynthinos, E.; Spandidos, D.A.; Tsatsakis, A.; Leon, G.; et al. Assessment of the redox status in patients with metabolic syndrome and type 2 diabetes reveals great variations. Exp. Ther. Med. 2016, 11, 895–903. [Google Scholar] [CrossRef]
- Suresh, D.R.; Annam, V. Lipid peroxidation and total antioxidant capacity in health and disease—Pathophysiology and markers: An overview. Int. J. Med. Sci. Public Health 2013, 2, 478–479. [Google Scholar] [CrossRef]
- Keles, M.; Taysi, S.; Sen, N.; Aksoy, H.; Akçay, F. Effect of Corticosteroid Therapy on Serum and CSF Malondialdehyde and Antioxidant Proteins in Multiple Sclerosis. Can. J. Neurol. Sci./J. Can. des Sci. Neurol. 2001, 28, 141–143. [Google Scholar] [CrossRef]
- Janaszewska, A.; Bartosz, G. Assay of total antioxidant capacity: Comparison of four methods as applied to human blood plasma. Scand. J. Clin. Lab. Investig. 2002, 62, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Pădureanu, R.; Albu, C.V.; Mititelu, R.R.; Bacanoiu, M.V.; Docea, A.O.; Calina, D.; Pădureanu, V.; Olaru, G.; Sandu, R.E.; Malin, R.D.; et al. Oxidative Stress and Inflammation Interdependence in Multiple Sclerosis. J. Clin. Med. 2019, 8, 1815. [Google Scholar] [CrossRef] [PubMed]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione perox-idase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Packer, L., Ed.; Academic Press: Orlando, FL, USA, 1984; pp. 121–126. [Google Scholar]
- Sicińska, P.; Kik, K.; Bukowska, B. Human Erythrocytes Exposed to Phthalates and Their Metabolites Alter Antioxidant Enzyme Activity and Hemoglobin Oxidation. Int. J. Mol. Sci. 2020, 21, 4480. [Google Scholar] [CrossRef]
- Jarosiewicz, M.; Krokosz, A.; Marczak, A.; Bukowska, B. Changes in the activities of antioxidant enzymes and reduced glutathione level in human erythrocytes exposed to selected brominated flame retardants. Chemosphere 2019, 227, 93–99. [Google Scholar] [CrossRef]
- Hassanein, E.G.; Mohamed, E.E.; Baess, A.I.; El-Sayed, E.T.; Yossef, A.M.; Mohammad, E.E.S. The role of supplementary vitamin D in treatment course of pulmonary tuberculosis. Egypt. J. Chest Dis. Tuberc. 2016, 65, 629–635. [Google Scholar] [CrossRef]
- Sun, D.; Luo, F.; Xing, J.-C.; Zhang, F.; Xu, J.; Zhang, Z.-H. 1,25(OH)2 D3 inhibited Th17 cells differentiation via regulating the NF-κB activity and expression of IL-17. Cell Prolif. 2018, 51, e12461. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Battu, P.; Singla, M.; Goyal, N.; Sharma, V.L. Expression profile of markers of oxidative stress, injury and apoptosis in anti-tuberculosis drugs induced nephrotoxicity. Nephrology 2019, 24, 689–695. [Google Scholar] [CrossRef]
- Hassan, H.M.; Guo, H.; Yousef, B.A.; Guerram, M.; Hamdi, A.M.; Zhang, L.; Jiang, Z. Role of Inflammatory and Oxidative Stress, Cytochrome P450 2E1, and Bile Acid Disturbance in Rat Liver Injury Induced by Isoniazid and Lipopolysaccharide Cotreatment. Antimicrob. Agents Chemother. 2016, 60, 5285–5293. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodpoor, A.; Hamishehkar, H.; Shadvar, K.; Ostadi, Z.; Sanaie, S.; Saghaleini, S.H.; Nader, N.D. The Effect of Intravenous Selenium on Oxidative Stress in Critically Ill Patients with Acute Respiratory Distress Syndrome. Immunol. Investig. 2019, 48, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Khoubnasabjafari, M.; Soleymani, J.; Jouyban, A. Avoid using spectrophotometric determination of malondialdehyde as a biomarker of oxidative stress. Biomark. Med. 2018, 12, 551–554. [Google Scholar] [CrossRef] [PubMed]
| Demographic Data | Values: Mean (±SD) or n (%) |
|---|---|
| Age | 48.80 (±9.283) |
| Weight | 54.27 (±8.189) |
| Gender | |
| Female | 4 (26.7%) |
| Male | 11 (73.3%) |
| Environment | |
| Urban | 4 (26.7%) |
| Rural | 11 (73.3%) |
| Biomarkers | T0 Mean ± Standard Deviation (range) | T2 Mean ± Standard Deviation (range) | p-Value |
|---|---|---|---|
| TBARS (μmol/L) | 0.73 ± 0.29 (0.50–1.36) | 0.68 ± 0.29 (0.39–1.23) | 0.551 |
| TAC (mmol DPPH/L) | 49.49 ± 4.94 (38.15–56.23) | 49.14 ± 9.80 (29.33–63.51) | 0.691 |
| SOD (U/mL) | 283.99 ± 16.05 (231.00–297.15) | 291.62 ± 8.03 (275.62–305.66) | 0.074 |
| GPx (U/L) | 1617.35 ± 750.40 (656.14–3768.58) | 1441.82 ± 345.52 (1093.56–2187.12) | 0.333 |
| CAT (U/mgHb) | 1.07 ± 0.63 (0.41–2.38) | 1.41 ± 0.77 (0.62–3.54) | 0.008 * |
| GSH (mg/dL) | 7.63 ± 1.60 (4.80–10.60) | 8.16 ± 3.01 (4.00–12.60) | 0.490 |
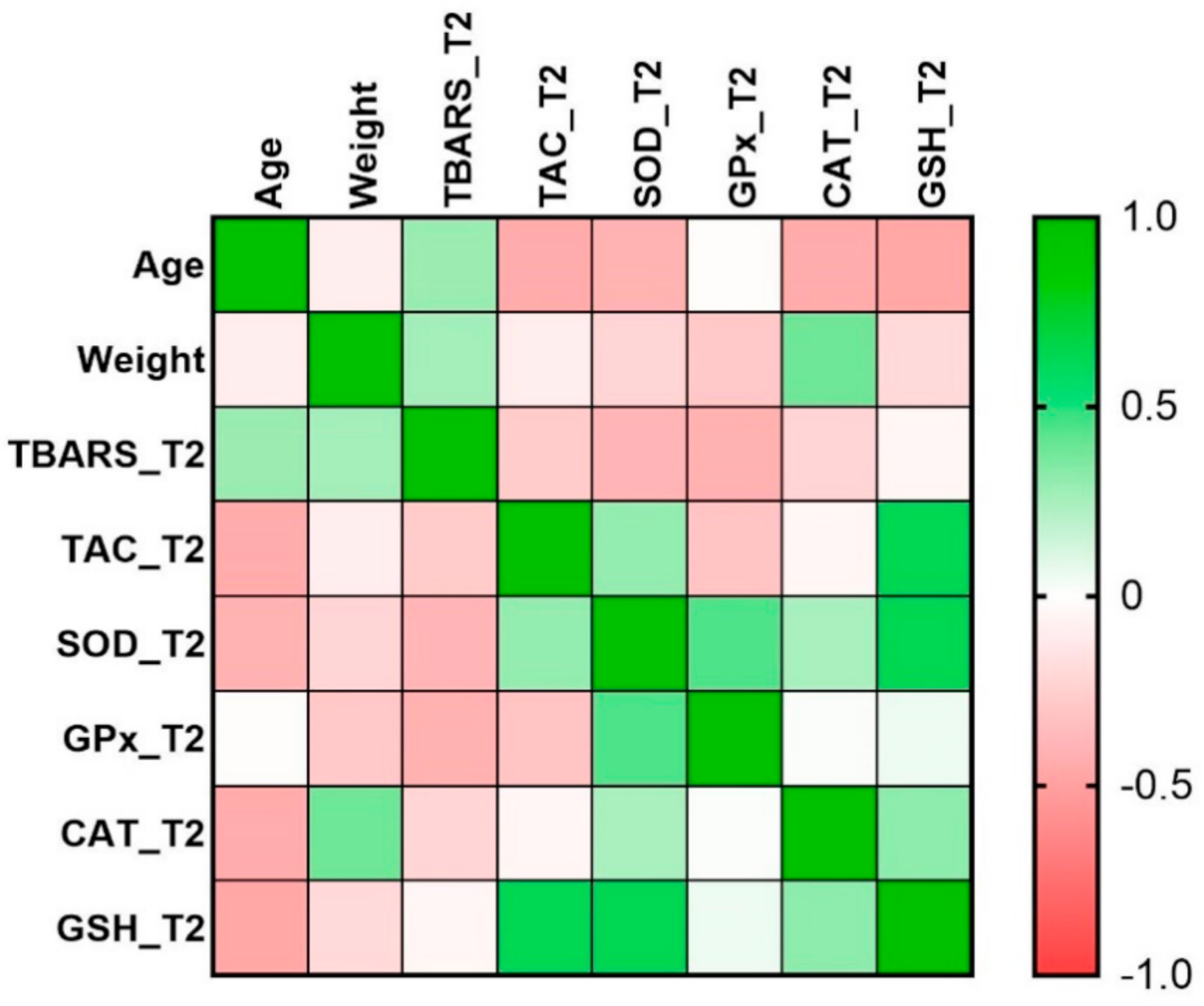
| Age | Weight | TBARS_T2 | TAC _T2 | SOD _T2 | GPx _T2 | CAT _T2 | GSH_T2 | |
|---|---|---|---|---|---|---|---|---|
| Age | 1.00 | |||||||
| Weight | −0.10 | 1.00 | ||||||
| TBARS_T2 | 0.29 | 0.26 | 1.00 | |||||
| TAC_T2 | −0.45 | −0.10 | −0.28 | 1.00 | ||||
| SOD_T2 | −0.40 | −0.22 | −0.40 | 0.29 | 1.00 | |||
| GPx_T2 | −0.02 | −0.29 | −0.42 | −0.32 | 0.45 | 1.00 | ||
| CAT_T2 | −0.45 | 0.39 | −0.23 | −0.05 | 0.24 | 0.02 | 1.00 | |
| GSH_T2 | −0.47 | −0.20 | −0.05 | 0.63 * | 0.64 * | 0.05 | 0.32 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meca, A.-D.; Turcu-Stiolica, A.; Stanciulescu, E.C.; Andrei, A.M.; Nitu, F.M.; Banita, I.M.; Matei, M.; Pisoschi, C.-G. Variations of Serum Oxidative Stress Biomarkers under First-Line Antituberculosis Treatment: A Pilot Study. J. Pers. Med. 2021, 11, 112. https://doi.org/10.3390/jpm11020112
Meca A-D, Turcu-Stiolica A, Stanciulescu EC, Andrei AM, Nitu FM, Banita IM, Matei M, Pisoschi C-G. Variations of Serum Oxidative Stress Biomarkers under First-Line Antituberculosis Treatment: A Pilot Study. Journal of Personalized Medicine. 2021; 11(2):112. https://doi.org/10.3390/jpm11020112
Chicago/Turabian StyleMeca, Andreea-Daniela, Adina Turcu-Stiolica, Elena Camelia Stanciulescu, Ana Marina Andrei, Floarea Mimi Nitu, Ileana Monica Banita, Marius Matei, and Catalina-Gabriela Pisoschi. 2021. "Variations of Serum Oxidative Stress Biomarkers under First-Line Antituberculosis Treatment: A Pilot Study" Journal of Personalized Medicine 11, no. 2: 112. https://doi.org/10.3390/jpm11020112
APA StyleMeca, A.-D., Turcu-Stiolica, A., Stanciulescu, E. C., Andrei, A. M., Nitu, F. M., Banita, I. M., Matei, M., & Pisoschi, C.-G. (2021). Variations of Serum Oxidative Stress Biomarkers under First-Line Antituberculosis Treatment: A Pilot Study. Journal of Personalized Medicine, 11(2), 112. https://doi.org/10.3390/jpm11020112








