Physical Exercise with or without Whole-Body Vibration in Breast Cancer Patients Suffering from Aromatase Inhibitor—Induced Musculoskeletal Symptoms: A Pilot Randomized Clinical Study
Abstract
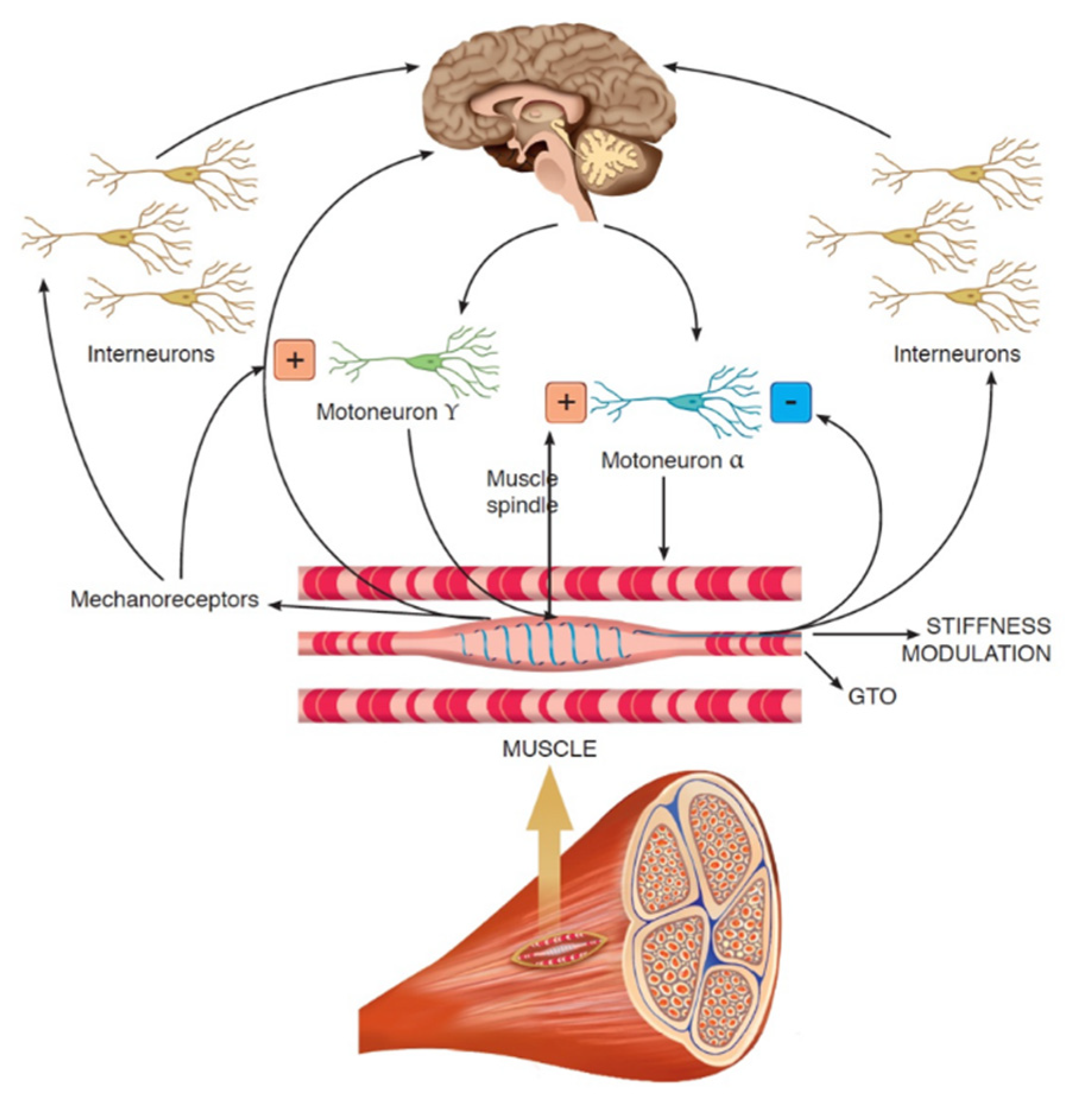
:1. Introduction
2. Materials and Methods
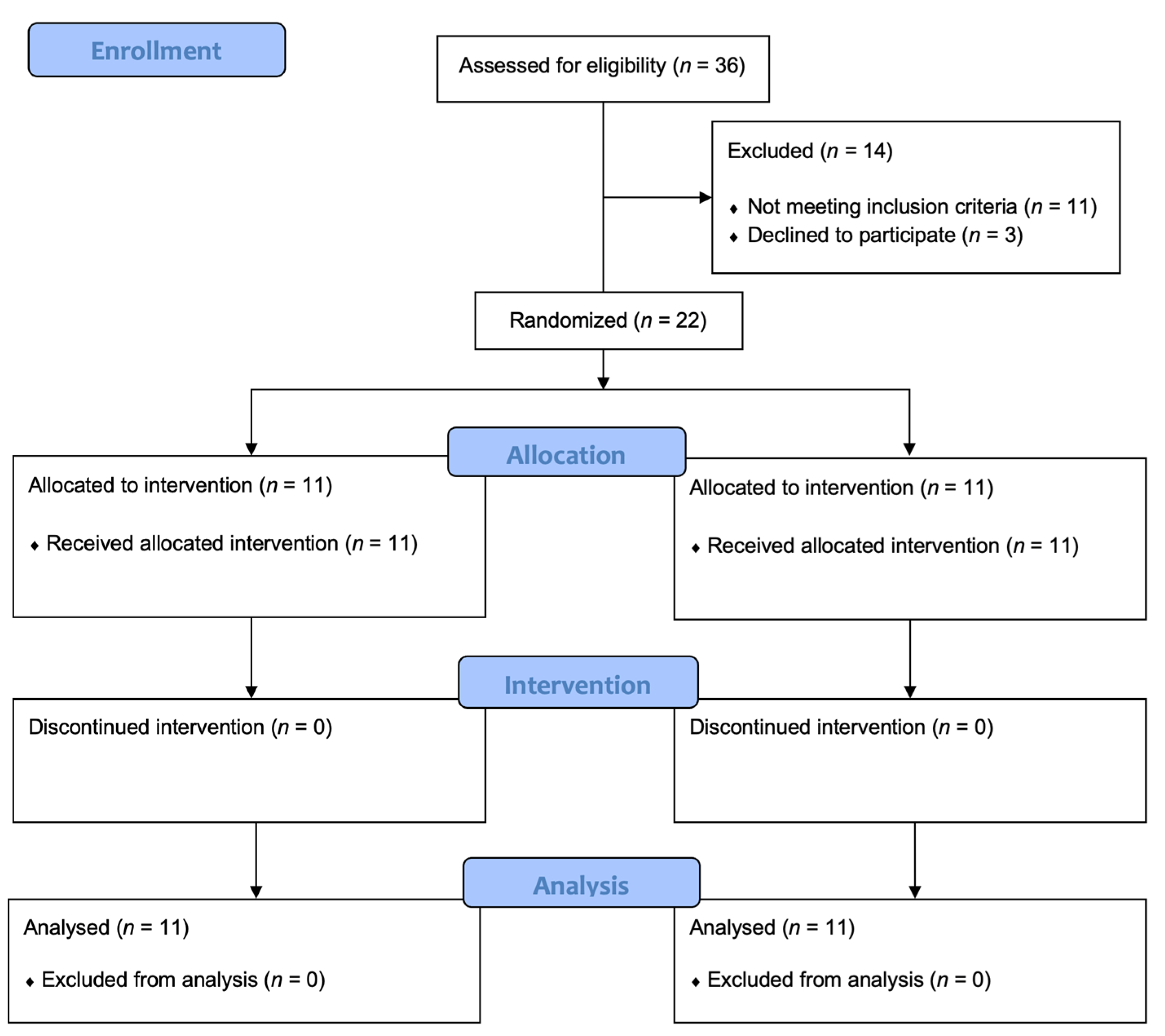
2.1. Study Design and Patients
2.2. Randomization and Procedures
- Phase I (common for both groups, lasting 10 min): a warm-up characterized by active and passive joint mobility exercises, performed to adapt the joints to the load and reduce the rate of musculoskeletal or other injuries.
- Phase II (common for both groups, lasting 10 min): aerobic training on treadmill (rapid walking at 3–5 km/h) with exercise intensity set according to 60–70% of maximal heart rate.
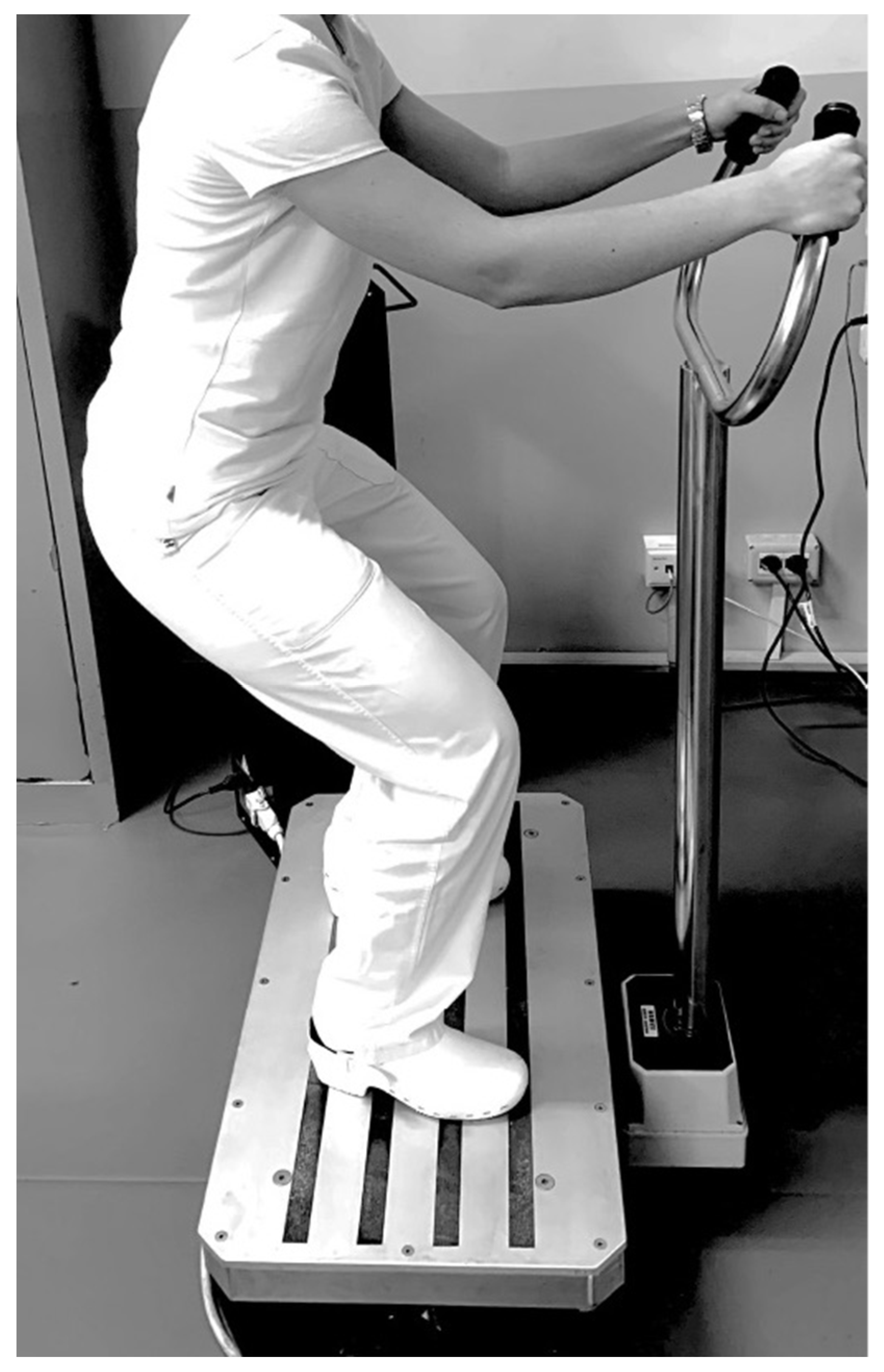
- Phase III (with differences between groups, lasting 10 min): a resistance exercise training protocol comprising both isotonic and isometric exercise, with intensity assessed by the Modified Borg Scale (MBS) [36]. The target of exercise intensity was set to moderate with an MBS score between 4 and 6. Moreover, all patients were encouraged to reach the target intensity during the training session. In this phase, the two groups performed the physical exercise with or without the WBV approach with the following differences: Group A (physical exercise plus WBV) performed 5 sets of 10 repetitions of squats without WBV alternating with 5 sets of 30 s on the side-alternating WBV platform (model NEMES-LB Bosco System® Rieti, Italy), with a frequency of 30 Hz, peak-to-peak amplitude of 1.15 mm (acceleration magnitude of 20.44 ms–2) in squatting position (110° knee flexion), as shown in Figure 2.
- Phase IV (common for both groups, lasting 10 min): equal to Phase II consisting of aerobic training on treadmill (rapid walking at 3–5 km/h) with exercise intensity set according to 60–70% of maximal heart rate.
- Phase V (common for both groups, lasting 10 min): cooling down with active and passive joint mobility exercises.
2.3. Outcome Measures
2.4. Statistical Analysis
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Sire, A.; Losco, L.; Cigna, E.; Lippi, L.; Gimigliano, F.; Gennari, A.; Cisari, C.; Chen, H.C.; Fusco, N.; Invernizzi, M. Three-dimensional laser scanning as a reliable and reproducible diagnostic tool in breast cancer related lymphedema rehabilitation: A proof-of-principle study. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4476–4485. [Google Scholar] [CrossRef]
- De Sire, A.; Losco, L.; Cisari, C.; Gennari, A.; Boldorini, R.; Fusco, N.; Cigna, E.; Invernizzi, M. Axillary web syndrome in women after breast cancer surgery referred to an Oncological Rehabilitation Unit: Which are the main risk factors? A retrospective case-control study. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 8028–8035. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, M.; Kim, J.; Fusco, N. Editorial: Quality of Life in Breast Cancer Patients and Survivors. Front. Oncol. 2020, 10, 620574. [Google Scholar] [CrossRef]
- Grizzi, G.; Ghidini, M.; Botticelli, A.; Tomasello, G.; Ghidini, A.; Grossi, F.; Fusco, N.; Cabiddu, M.; Savio, T.; Petrelli, F. Strategies for Increasing the Effectiveness of Aromatase Inhibitors in Locally Advanced Breast Cancer: An Evidence-Based Review on Current Options. Cancer Manag. Res. 2020, ume 12, 675–686. [Google Scholar] [CrossRef] [Green Version]
- Molehin, D.; Rasha, F.; Rahman, R.L.; Pruitt, K. Regulation of aromatase in cancer. Mol. Cell. Biochem. 2021, 476, 2449–2464. [Google Scholar] [CrossRef]
- de Sire, A.; Ferrillo, M.; Gennari, A.; Cisari, C.; Pasqua, S.; Bonda, P.L.F.; Invernizzi, M.; Migliario, M. Bone health, vitamin D status and oral hygiene screening in breast cancer women before starting osteoporosis treatment: A cross-sectional study. J. Biol. Regul. Homeost. Agents 2021, 35, 397–402. [Google Scholar]
- Scaturro, D.; de Sire, A.; Terrana, P.; Curci, C.; Vitagliani, F.; Falco, V.; Cuntrera, D.; Iolascon, G.; Mauro, G.L. Early Denosumab for the prevention of osteoporotic fractures in breast cancer women undergoing aromatase inhibitors: A case-control retrospective study. J. Back Musculoskelet. Rehabil. 2021, 1–6, Preprint. [Google Scholar] [CrossRef] [PubMed]
- Crew, K.D.; Capodice, J.L.; Greenlee, H.; Brafman, L.; Fuentes, D.; Awad, D.; Tsai, W.Y.; Hershman, D.L. Randomized, Blinded, Sham-Controlled Trial of Acupuncture for the Management of Aromatase Inhibitor–Associated Joint Symptoms in Women with Early-Stage Breast Cancer. J. Clin. Oncol. 2010, 28, 1154–1160. [Google Scholar] [CrossRef]
- Dos Santos, B.S.; Bordignon, C.; Rosa, D.D. Managing Common Estrogen Deprivation Side Effects in HR+ Breast Cancer: An Evidence-Based Review. Curr. Oncol. Rep. 2021, 23, 63. [Google Scholar] [CrossRef] [PubMed]
- Baum, M.; Budzar, A.U.; Cuzick, J.; Forbes, J.; Houghton, J.H.; Klijn, J.G.M.; Sahmoud, T. ATAC Trialists’ Group Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: First results of the ATAC randomised trial. Lancet 2002, 359, 2131–2139. [Google Scholar] [CrossRef]
- Coombes, R.C.; Hall, E.; Gibson, L.J.; Paridaens, R.; Jassem, J.; DeLozier, T.; Jones, S.E.; Alvarez, I.; Bertelli, G.; Ortmann, O.; et al. A Randomized Trial of Exemestane after Two to Three Years of Tamoxifen Therapy in Postmenopausal Women with Primary Breast Cancer. N. Engl. J. Med. 2004, 350, 1081–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crew, K.D.; Greenlee, H.; Capodice, J.; Raptis, G.; Brafman, L.; Fuentes, D.; Sierra, A.; Hershman, D.L. Prevalence of Joint Symptoms in Postmenopausal Women Taking Aromatase Inhibitors for Early-Stage Breast Cancer. J. Clin. Oncol. 2007, 25, 3877–3883. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.C.; Bartholomew, L.K.; Carpentier, M.Y.; Bluethmann, S.M.; Vernon, S.W. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: A systematic review. Breast Cancer Res. Treat. 2012, 134, 459–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Migliaccio, S.; de Sire, A.; Marocco, C.; Fornari, R.; Paoletta, M.; Greco, E.A.; Amar, I.D.; Moretti, A.; Ronzoni, S.; Gimigliano, F.; et al. Approach in aromatase inhibitors—Induced osteoporosis: Results from an Italian multicenter observational study. Clin. Cases Miner. Bone Metab. 2018, 15, 334–339. [Google Scholar]
- Sestak, I.; Cuzick, J.; Sapunar, F.; Eastell, R.; Forbes, J.F.; Bianco, A.R.; Buzdar, A.U. Risk factors for joint symptoms in patients enrolled in the ATAC trial: A retrospective, exploratory analysis. Lancet Oncol. 2008, 9, 866–872. [Google Scholar] [CrossRef] [Green Version]
- Nardin, S.; Mora, E.; Varughese, F.M.; D’Avanzo, F.; Vachanaram, A.R.; Rossi, V.; Saggia, C.; Rubinelli, S.; Gennari, A. Breast Cancer Survivorship, Quality of Life, and Late Toxicities. Front. Oncol. 2020, 10, 864. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.L.; Cartmel, B.; Gross, C.P.; Ercolano, E.; Li, F.; Yao, X.; Fiellin, M.; Capozza, S.; Rothbard, M.; Zhou, Y.; et al. Randomized Exercise Trial of Aromatase Inhibitor–Induced Arthralgia in Breast Cancer Survivors. J. Clin. Oncol. 2015, 33, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, H.; Crew, K.D.; Shao, T.; Kranwinkel, G.; Kalinsky, K.; Maurer, M.; Brafman, L.; Insel, B.; Tsai, W.Y.; Hershman, D.L. Phase II study of glucosamine with chondroitin on aromatase inhibitor-associated joint symptoms in women with breast cancer. Support. Care Cancer 2012, 21, 1077–1087. [Google Scholar] [CrossRef] [Green Version]
- Khan, Q.J.; Reddy, P.S.; Kimler, B.F.; Sharma, P.; Baxa, S.E.; O’Dea, A.P.; Klemp, J.R.; Fabian, C.J. Effect of vitamin D supplementation on serum 25-hydroxy vitamin D levels, joint pain, and fatigue in women starting adjuvant letrozole treatment for breast cancer. Breast Cancer Res. Treat. 2009, 119, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Oh, B.; Kimble, B.; Costa, D.S.J.; Davis, E.; McLean, A.; Orme, K.; Beith, J. Acupuncture for Treatment of Arthralgia Secondary to Aromatase Inhibitor Therapy in Women with Early Breast Cancer: Pilot Study. Acupunct. Med. 2013, 31, 264–271. [Google Scholar] [CrossRef]
- Arem, H.; Sorkin, M.; Cartmel, B.; Fiellin, M.; Capozza, S.; Harrigan, M.; Ercolano, E.; Zhou, Y.; Sanft, T.; Gross, C.; et al. Exercise adherence in a randomized trial of exercise on aromatase inhibitor arthralgias in breast cancer survivors: The Hormones and Physical Exercise (HOPE) study. J. Cancer Surviv. 2016, 10, 654–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Y.; Wang, H.; Zhu, Y.; Chen, B.; Zheng, Y.; Liu, X.; Qiao, J.; Wang, X. Effects of whole-body vibration exercise on lumbar-abdominal muscles activation for patients with chronic low back pain. BMC Sports Sci. Med. Rehabil. 2020, 12, 78. [Google Scholar] [CrossRef]
- Rabini, A.; de Sire, A.; Marzetti, E.; Gimigliano, R.; Ferriero, G.; Piazzini, D.B.; Iolascon, G.; Gimigliano, F. Effects of focal muscle vibration on physical functioning in patients with knee osteoarthritis: A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2015, 51, 513–520. [Google Scholar]
- Dong, Y.; Wang, W.; Zheng, J.; Chen, S.; Qiao, J.; Wang, X. Whole Body Vibration Exercise for Chronic Musculoskeletal Pain: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Arch. Phys. Med. Rehabil. 2019, 100, 2167–2178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, R.; Zheng, Y.; Xu, J.; Wu, Y.; Wang, X. Effect of Whole-Body Vibration Training on Muscle Activation for Individuals with Knee Osteoarthritis. BioMed. Res. Int. 2021, 2021, 6671390. [Google Scholar] [CrossRef]
- Lai, C.-C.; Tu, Y.-K.; Wang, T.-G.; Huang, Y.-T.; Chien, K.-L. Effects of resistance training, endurance training and whole-body vibration on lean body mass, muscle strength and physical performance in older people: A systematic review and network meta-analysis. Age Ageing 2018, 47, 367–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, M.M.; Khan, A.A.; Farooq, M. Effect of whole-body vibration on neuromuscular performance: A literature review. Work 2018, 59, 571–583. [Google Scholar] [CrossRef]
- Cardinale, M.B.C. The use of vibration as an exercise intervention. Exerc. Sport Sci. Rev. 2003, 31, 3–7. [Google Scholar] [CrossRef]
- Sitjà-Rabert, M.; Rigau, D.; Fort-Vanmeerhaeghe, A.; Romero-Rodríguez, D.; Subirana, M.B.; Bonfill, X. Efficacy of whole-body vibration exercise in older people: A systematic review. Disabil. Rehabil. 2012, 34, 883–893. [Google Scholar] [CrossRef]
- Sañudo, B.; De Hoyo, M.; Carrasco, L.; McVeigh, J.G.; Corral, J.; Cabeza, R.; Rodríguez, C.; Oliva, A. The effect of 6-week exercise programme and whole-body vibration on strength and quality of life in women with fibromyalgia: A randomised study. Clin. Exp. Rheumatol. 2010, 28, S40–S45. [Google Scholar] [PubMed]
- Micke, F.; Weissenfels, A.; Wirtz, N.; von Stengel, S.; Dörmann, U.; Kohl, M.; Kleinöder, H.; Donath, L.; Kemmler, W. Similar Pain Intensity Reductions and Trunk Strength Improvements Following Whole-Body Electromyostimulation vs. Whole-Body Vibration vs. Conventional Back-Strengthening Training in Chronic Non-specific Low Back Pain Patients: A Three-Armed Randomized Controlled Trial. Front. Physiol. 2021, 12, 664991. [Google Scholar]
- Streckmann, F.; Balke, M.; Lehmann, H.C.; Rustler, V.; Koliamitra, C.; Elter, T.; Hallek, M.; Leitzmann, M.; Steinmetz, T.; Heinen, P.; et al. The preventive effect of sensorimotor- and vibration exercises on the onset of Oxaliplatin- or vinca-alkaloid induced peripheral neuropathies—STOP. BMC Cancer 2018, 18, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, M.K.; Peddle-McIntyre, C.; Galvao, D.; Hunt, C.; Spry, N.; Newton, R.U. Whole Body Vibration Exposure on Markers of Bone Turnover, Body Composition, and Physical Functioning in Breast Cancer Patients Receiving Aromatase Inhibitor Therapy: A Randomized Controlled Trial. Integr. Cancer Ther. 2018, 17, 968–978. [Google Scholar] [CrossRef] [Green Version]
- Roberts, K.; Rickett, K.; Feng, S.; Vagenas, D.; Woodward, N. Exercise therapies for preventing or treating aromatase inhibitor-induced musculoskeletal symptoms in early breast cancer. Cochrane Database Syst. Rev. 2020, 1, CD012988. [Google Scholar] [CrossRef] [PubMed]
- Association, W.M. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar]
- Skinner, J.S.; Hutsler, E.; Bergsteinova, V.; Buskirk, E.R. The validity and reliability of a rating scale of perceived exertion. Med. Sci. Sports Exerc. 1973, 5, 94–96. [Google Scholar] [CrossRef]
- Jakobsen, L.H.; Rask, I.K.; Kondrup, J. Validation of handgrip strength and endurance as a measure of physical function and quality of life in healthy subjects and patients. Nutrition 2010, 26, 542–550. [Google Scholar] [CrossRef]
- Salaffi, F.; Leardini, G.; Canesi, B.; Mannoni, A.; Fioravanti, A.; Caporali, R.; Lapadula, G.; Punzi, L. Reliability and validity of the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index in Italian patients with osteoarthritis of the knee. Osteoarthr. Cartil. 2003, 11, 551–560. [Google Scholar] [CrossRef] [Green Version]
- Amatachaya, S.; Kwanmongkolthong, M.; Thongjumroon, A.; Boonpew, N.; Amatachaya, P.; Saensook, W.; Thaweewannakij, T.; Hunsawong, T. Influence of timing protocols and distance covered on the outcomes of the 10-meter walk test. Physiother. Theory Pract. 2020, 36, 1348–1353. [Google Scholar] [CrossRef]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; De Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Meisingset, I.; Stensdotter, A.K.; Woodhouse, A.; Vasseljen, O. Predictors for global perceived effect after physiotherapy in patients with neck pain: An observational study. Physiotherapy 2017, 104, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Hershman, D.L.; Shao, T.; Kushi, L.H.; Buono, D.; Tsai, W.Y.; Fehrenbacher, L.; Kwan, M.; Gomez, S.L.; Neugut, A.I. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res. Treat. 2010, 126, 529–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geirsdottir, O.G.; Arnarson, A.; Briem, K.; Ramel, A.; Jonsson, P.V.; Thorsdottir, I. Effect of 12-Week Resistance Exercise Program on Body Composition, Muscle Strength, Physical Function, and Glucose Metabolism in Healthy, Insulin-Resistant, and Diabetic Elderly Icelanders. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2012, 67, 1259–1265. [Google Scholar] [CrossRef] [Green Version]
- Hassan, B.H.; Hewitt, J.; Keogh, J.W.; Bermeo, S.; Duque, G.; Henwood, T.R. Impact of resistance training on sarcopenia in nursing care facilities: A pilot study. Geriatr. Nurs. 2015, 37, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Carroll, T.J. Cross education: Possible mechanisms for the contralateral effects of unilateral resistance training. Sports Med. 2007, 37, 1–14. [Google Scholar] [CrossRef]
- Zheng, Y.L.; Zhang, Z.J.; Peng, M.S.; Hu, H.Y.; Zhang, J.; Wang, X.Q. Whole-body vibration exercise for low back pain: A meta-analysis protocol of randomized controlled trial. Medicine 2018, 97, e12534. [Google Scholar] [CrossRef]
- Zafar, H.; Alghadir, A.; Anwer, S.; Al-Eisa, E. Therapeutic Effects of Whole-Body Vibration Training in Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2015, 96, 1525–1532. [Google Scholar] [CrossRef]
- Verhulst, A.L.; Savelberg, H.H.; Vreugdenhil, G.; Mischi, M.; Schep, G. Whole-body vibration as a modality for the rehabilitation of peripheral neuropathies: Implications for cancer survivors suffering from chemotherapy-induced peripheral neuropathy. Oncol. Rev. 2015, 9, 263. [Google Scholar] [CrossRef] [Green Version]
- Bidonde, J.; Busch, A.J.; van der Spuy, S.P.; Tupper, S.; Kim, S.Y.; Boden, C. Whole body vibration exercise training for fibromyalgia. Cochrane Database Syst. Rev. 2017, 9, CD011755. [Google Scholar] [CrossRef]
- Tonorezos, E.S.; Ford, J.S.; Wang, L.; Pt, K.K.N.; Yasui, Y.; Leisenring, W.; Sklar, C.A.; Robison, L.L.; Oeffinger, K.C.; Nathan, P.C.; et al. Impact of exercise on psychological burden in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer 2019, 125, 3059–3067. [Google Scholar] [CrossRef] [PubMed]
- Melzack, R.W.P. Pain mechanisms: A new theory. Science 1965, 150, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Longe, S.E.; Wise, R.; Bantick, S.; Lloyd, D.; Johansen-Berg, H.; McGlone, F.; Tracey, I. Counter-stimulatory effects on pain perception and processing are significantly altered by attention: An fMRI study. Neuroreport 2001, 12, 2021–2025. [Google Scholar] [CrossRef] [Green Version]
- Moggio, L.; de Sire, A.; Marotta, N.; Demeco, A.; Ammendolia, A. Vibration therapy role in neurological diseases rehabilitation: An umbrella review of systematic reviews. Disabil. Rehabil. 2021, 5, 1–9. [Google Scholar] [CrossRef]
- Jamal, A.; Ahmad, I.; Ahamed, N.; Azharuddin, M.; Alam, F.; Hussain, M.E. Whole body vibration showed beneficial effect on pain, balance measures and quality of life in painful diabetic peripheral neuropathy: A randomized controlled trial. J. Diabetes Metab. Disord. 2019, 19, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Coghill, R.C.T.J.; Evans, A.C.; Meyer, E.; Gjedde, A.; Bushnell, M.C.; Duncan, G.H. Distributed processing of pain and vibration by the human brain. J. Neurosci. 1994, 14, 4095–4108. [Google Scholar] [CrossRef] [Green Version]
| Sample Characteristics | Group A (n = 11) | Group B (n = 11) | p Value |
|---|---|---|---|
| Age (years) | 51.73 ± 10.73 | 58.55 ± 9.71 | 0.146 |
| Sex (female) | 11 (100.0) | 11 (100.0) | 0.999 |
| BMI (kg/m2) | 25.56 ± 5.17 | 27.31 ± 3.84 | 0.186 |
| Smokers (habitual smokers) | 2 (18.2) | 1 (9.1) | 0.999 |
| Breast surgery | |||
| Conservative | 6 (55.5) | 5 (44.5) | 0.999 |
| Mastectomy | 5 (44.5) | 6 (55.5) | 0.999 |
| Axillary surgery | |||
| Sentinel lymph node | 5 (36.4) | 5 (44.5) | 0.999 |
| En bloc dissection | 7 (63.6) | 6 (55.5) | 0.999 |
| Radiotherapy | 8 (72.7) | 6 (55.5) | 0.659 |
| Hormone therapy | 11 (100.0) | 11 (100.0) | 0.999 |
| Chemotherapy | 4 (36.4) | 3 (27.3) | 0.999 |
| Trastuzumab | 3 (27.3) | 4 (36.4) | 0.999 |
| Presence of Upper Limb Lymphedema | 0 (0.0) | 1 (9.1) | 0.999 |
| Outcome | Group A (n = 11) | Group B (n = 11) | Between-Group Analysis | |||||
|---|---|---|---|---|---|---|---|---|
| T0 | T1 | p-Value | T0 | T1 | p-Value | T0 p-Value | T1 p-Value | |
| NPRS | 6.82 ± 1.17 | 5.73 ± 1.01 | 0.031 | 6.91 ± 2.02 | 5.91 ± 2.51 | 0.070 | 0.430 | 0.434 |
| WOMAC | 52.69 ± 13.21 | 77.56 ± 9.85 | 0.001 | 59.12 ± 19.71 | 65.63 ± 14.27 | 0.023 | 0.508 | 0.044 |
| HGS | 15.81 ± 0.59 | 17.42 ± 1.06 | 0.001 | 13.24 ± 2.13 | 15.36 ± 3.37 | 0.016 | 0.077 | 0.185 |
| 6MWT | 465.51 ± 57.94 | 518.2 ± 44.79 | 0.017 | 423.7 ± 45.39 | 470.9 ± 57.52 | 0.001 | 0.063 | 0.186 |
| 10MWT | 1.46 ± 0.26 | 1.61 ± 0.17 | 0.001 | 1.42 ± 0.16 | 1.60 ± 0.16 | 0.001 | 0.885 | 0.884 |
| EORTC QLQ-C30 | ||||||||
| Functional score | 69.74 ± 12.42 | 80.61±10.14 | 0.027 | 70.71 ± 21.77 | 78.99 ± 16.28 | 0.004 | 0.736 | 0.783 |
| Symptom score | 31.72 ± 12.26 | 19.35 ± 7.46 | 0.008 | 28.44 ± 12.48 | 20.05 ± 11.46 | 0.001 | 0.548 | 0.505 |
| Global health score | 25.76 ± 13.67 | 60.61 ± 17.91 | 0.001 | 34.09 ± 9.47 | 68.18 ± 22.61 | 0.005 | 0.190 | 0.422 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Sire, A.; Lippi, L.; Ammendolia, A.; Cisari, C.; Venetis, K.; Sajjadi, E.; Fusco, N.; Invernizzi, M. Physical Exercise with or without Whole-Body Vibration in Breast Cancer Patients Suffering from Aromatase Inhibitor—Induced Musculoskeletal Symptoms: A Pilot Randomized Clinical Study. J. Pers. Med. 2021, 11, 1369. https://doi.org/10.3390/jpm11121369
de Sire A, Lippi L, Ammendolia A, Cisari C, Venetis K, Sajjadi E, Fusco N, Invernizzi M. Physical Exercise with or without Whole-Body Vibration in Breast Cancer Patients Suffering from Aromatase Inhibitor—Induced Musculoskeletal Symptoms: A Pilot Randomized Clinical Study. Journal of Personalized Medicine. 2021; 11(12):1369. https://doi.org/10.3390/jpm11121369
Chicago/Turabian Stylede Sire, Alessandro, Lorenzo Lippi, Antonio Ammendolia, Carlo Cisari, Konstantinos Venetis, Elham Sajjadi, Nicola Fusco, and Marco Invernizzi. 2021. "Physical Exercise with or without Whole-Body Vibration in Breast Cancer Patients Suffering from Aromatase Inhibitor—Induced Musculoskeletal Symptoms: A Pilot Randomized Clinical Study" Journal of Personalized Medicine 11, no. 12: 1369. https://doi.org/10.3390/jpm11121369
APA Stylede Sire, A., Lippi, L., Ammendolia, A., Cisari, C., Venetis, K., Sajjadi, E., Fusco, N., & Invernizzi, M. (2021). Physical Exercise with or without Whole-Body Vibration in Breast Cancer Patients Suffering from Aromatase Inhibitor—Induced Musculoskeletal Symptoms: A Pilot Randomized Clinical Study. Journal of Personalized Medicine, 11(12), 1369. https://doi.org/10.3390/jpm11121369













