A Prospective Study on the Association between Oxidative Stress and Duration of Symptoms in Allergic Rhinitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. TAS and TOS Measurements
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Study Participants
3.2. Distribution of Number and Duration of Symptoms
3.3. Comparison of TAS and TOS
3.4. Associations between Symptom Duration and Mean TAS and TOS Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz-Guilloty, F.; Perez, V.L. Molecular medicine: Defense against oxidative damage. Nature 2011, 478, 42–43. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [Green Version]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [Green Version]
- Bishopp, A.; Sathyamurthy, R.; Manney, S.; Webbster, C.; Krishna, M.T.; Mansur, A.H. Biomarkers of oxidative stress and antioxidants in severe asthma: A Prospective Case-Control Study. Ann. Allergy Asthma Immunol. 2017, 118, 445–451. [Google Scholar] [CrossRef]
- Kirkham, P.A.; Barnes, P.J. Oxidative stress in COPD. Chest 2013, 144, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Sackesen, C.; Ercan, H.; Dizdar, E.; Soyer, O.; Gumus, P.; Tosun, B.N.; Büyüktuncer, Z.; Karabulut, E.; Besler, T.; Kalayci, O. A comprehensive evaluation of the enzymatic and nonenzymatic antioxidant systems in childhood asthma. J. Allergy Clin. Immunol. 2008, 122, 78–85. [Google Scholar] [CrossRef]
- Henricks, P.A.; Nijkamp, F.P. Reactive oxygen species as mediators in asthma. Pulm. Pharmacol. Ther. 2001, 14, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.-H.; Kwon, S.-O.; Lee, S.-Y.; Kim, H.Y.; Kwon, J.-W.; Kim, B.-J.; Yu, J.; Kim, H.-B.; Kim, W.K.; Jang, G.C.; et al. Association of Antioxidants with Allergic Rhinitis in Children from Seoul. Allergy Asthma Immunol. Res. 2013, 5, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Emin, O.; Hasan, A.; Aysegul, D.; Rusen, D. Total antioxidant status and oxidative stress and their relationship to total IgE levels and eosinophil counts in children with allergic rhinitis. J. Investig. Allergol. Clin. Immunol. 2012, 22, 188–192. [Google Scholar] [PubMed]
- Celik, M.; Tuncer, A.; Soyer, O.U.; Saçkesen, C.; Besler, H.T.; Kalayci, O. Oxidative stress in the airways of children with asthma and allergic rhinitis. Pediatr. Allergy Immunol. 2012, 23, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Bakkeheim, E.; Mowinckel, P.; Carlsen, K.H.; Burney, P.; Carlsen, K.C.L. Altered oxidative state in schoolchildren with asthma and allergic rhinitis. Pediatr. Allergy Immunol. 2011, 22, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Sim, C.S.; Lee, J.H.; Kim, S.H.; Han, M.W.; Kim, Y.; Oh, I.; Yun, S.-C.; Lee, J.C. Oxidative stress in schoolchildren with allergic rhinitis: Propensity score matching case-control study. Ann. Allergy Asthma Immunol. 2015, 115, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-J.; Kim, S.-W.; Oh, J.-W.; Rah, Y.-H.; Ahn, Y.-M.; Kim, K.-E.; Koh, Y.Y.; Lee, S.I. The validity of the ISAAC written questionnaire and the ISAAC video questionnaire (AVQ 3.0) for predicting asthma associated with bronchial hyperreactivity in a group of 13–14 year old Korean schoolchildren. J. Korean Med. Sci. 2003, 18, 48–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Björkstén, B.; Clayton, T.; Ellwood, P.; Stewart, A.; Strachan, D.; The ISAAC Phase III Study Group. Worldwide time trends for symptoms of rhinitis and conjunctivitis: Phase III of the International Study of Asthma and Allergies in Childhood. Pediatr. Allergy Immunol. 2008, 19, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Erel, O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin. Biochem. 2004, 37, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Brożek, J.L.; Bousquet, J.; Agache, I.; Agarwal, A.; Bachert, C.; Bosnic-Anticevich, S.; Brignardello-Petersen, R.; Canonica, G.W.; Casale, T.; Chavannes, N.H.; et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J. Allergy Clin. Immunol. 2017, 140, 950–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montoro, J.; Del Cuvillo, A.; Mullol, J.; Molina, X.; Bartra, J.; Dávila, I.; Ferrer, M.; Jáuregui, I.; Sastre, J.; Valero, A. Validation of the modified allergic rhinitis and its impact on asthma (ARIA) severity classification in allergic rhinitis children: The PEDRIAL study. Allergy 2012, 67, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, P.J.; Combescure, C.; Neukirch, F.; Klossek, J.M.; Mechin, H.; Daures, J.P.; Bousquet, J. Visual analog scales can assess the severity of rhinitis graded according to ARIA guidelines. Allergy 2007, 62, 367–372. [Google Scholar] [CrossRef]
- Chen, J.; Peng, L.; He, S.; Li, Y.; Mu, Z. Association between environmental factors and hospital visits among allergic patients: A retrospective study. Asian Pac. J. Allergy Immunol. 2016, 34, 21–29. [Google Scholar]
- Morgenstern, V.; Zutavern, A.; Cyrys, J.; Brockow, I.; Gehring, U.; Koletzko, S.; Bauer, C.P.; Reinhardt, D.; Wichmann, H.-E.; Heinrich, J. Respiratory health and individual estimated exposure to traffic-related air pollutants in a cohort of young children. Occup. Environ. Med. 2007, 64, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Bowatte, G.; Lodge, C.; Lowe, A.J.; Erbas, B.; Perret, J.; Abramson, M.J.; Matheson, M.; Dharmage, S.C. The influence of childhood traffic-related air pollution exposure on asthma, allergy and sensitization: A systematic review and a meta-analysis of birth cohort studies. Allergy 2015, 70, 245–256. [Google Scholar] [CrossRef]
- Murray, A.B.; Milner, R.A. The accuracy of features in the clinical history for predicting atopic sensitization to airborne allergens in children. J. Allergy Clin. Immunol. 1995, 96, 588–596. [Google Scholar] [CrossRef]
- Tamay, Z.; Akcay, A.; Ergin, A.; Güler, N. Dietary habits and prevalence of allergic rhinitis in 6 to 7-year-old schoolchildren in Turkey. Allergol. Int. 2014, 63, 553–562. [Google Scholar] [CrossRef]
- Saadeh, D.; Salameh, P.; Caillaud, D.; Charpin, D.; De Blay, F.; Kopferschmitt, C.; Lavaud, F.; Annesi-Maesano, I.; Baldi, I.; Raherison, C. Prevalence and association of asthma and allergic sensitization with dietary factors in schoolchildren: Data from the french six cities study. BMC Public Health 2015, 15, 993. [Google Scholar] [CrossRef] [PubMed]
- Torres, R.L.; Torres, I.L.; Laste, G.; Ferreira, M.B.; Cardoso, P.F.G.; Belló-Klein, A. Effects of acute and chronic administration of methylprednisolone on oxidative stress in rat lungs. J. Bras. Pneumol. 2014, 40, 238–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, F.; Wang, Y.; Qi, H.-H.; Zhou, X.; Jin, X.-Q. Rapid non-genomic effects of glucocorticoids on oxidative stress in a guinea pig model of asthma. Respirology 2008, 13, 227–232. [Google Scholar] [CrossRef]
- Sadowska-Woda, I.; Bieszczad-Bedrejczuk, E.; Rachel, M. Influence of desloratadine on selected oxidative stress markers in patients between 3 and 10 years of age with allergic perennial rhinitis. Eur. J. Pharmacol. 2010, 640, 197–201. [Google Scholar] [CrossRef]
| Variables | Case (N: 226) | |
|---|---|---|
| Age (yr), mean ± SD | 8.96 ± 1.75 | |
| Sex, male:female (%) | 136:90 (60.2%:39.8%) | |
| Height (cm), mean ± SD | 137.21 ± 11.59 | |
| Weight (kg), mean ± SD | 35.45 ± 10.23 | |
| Grade (%) | 1st | 34 (15.0%) |
| 2nd | 27 (11.9%) | |
| 3rd | 33 (14.6%) | |
| 4th | 50 (22.1%) | |
| 5th | 33 (14.6%) | |
| 6th | 49 (21.7%) | |
| Skin prick test (%) | Positive | 127 (56.2%) |
| Negative | 99 (43.8%) | |
| Symptom Duration | N | TAS | TOS | ||
|---|---|---|---|---|---|
| Mean ± SD | p Value | Mean ± SD | p Value | ||
| <1 month | 35 | 1.41 ± 0.07 | 0.717 | 15.23 ± 9.79 | 0.283 |
| ≥1 month | 191 | 1.40 ± 0.07 | 13.45 ± 8.90 | ||
| <2 months | 88 | 1.40 ± 0.08 | 0.653 | 14.61 ± 9.01 | 0.240 |
| ≥2 months | 138 | 1.41 ± 0.07 | 13.16 ± 9.06 | ||
| <3 months | 134 | 1.40 ± 0.07 | 0.486 | 14.00 ± 9.01 | 0.577 |
| ≥3 months | 92 | 1.41 ± 0.07 | 13.31 ± 9.13 | ||
| <6 months | 184 | 1.40 ± 0.07 | 0.068 | 14.03 ± 9.09 | 0.271 |
| ≥6 months | 42 | 1.42 ± 0.08 | 12.33 ± 8.83 | ||
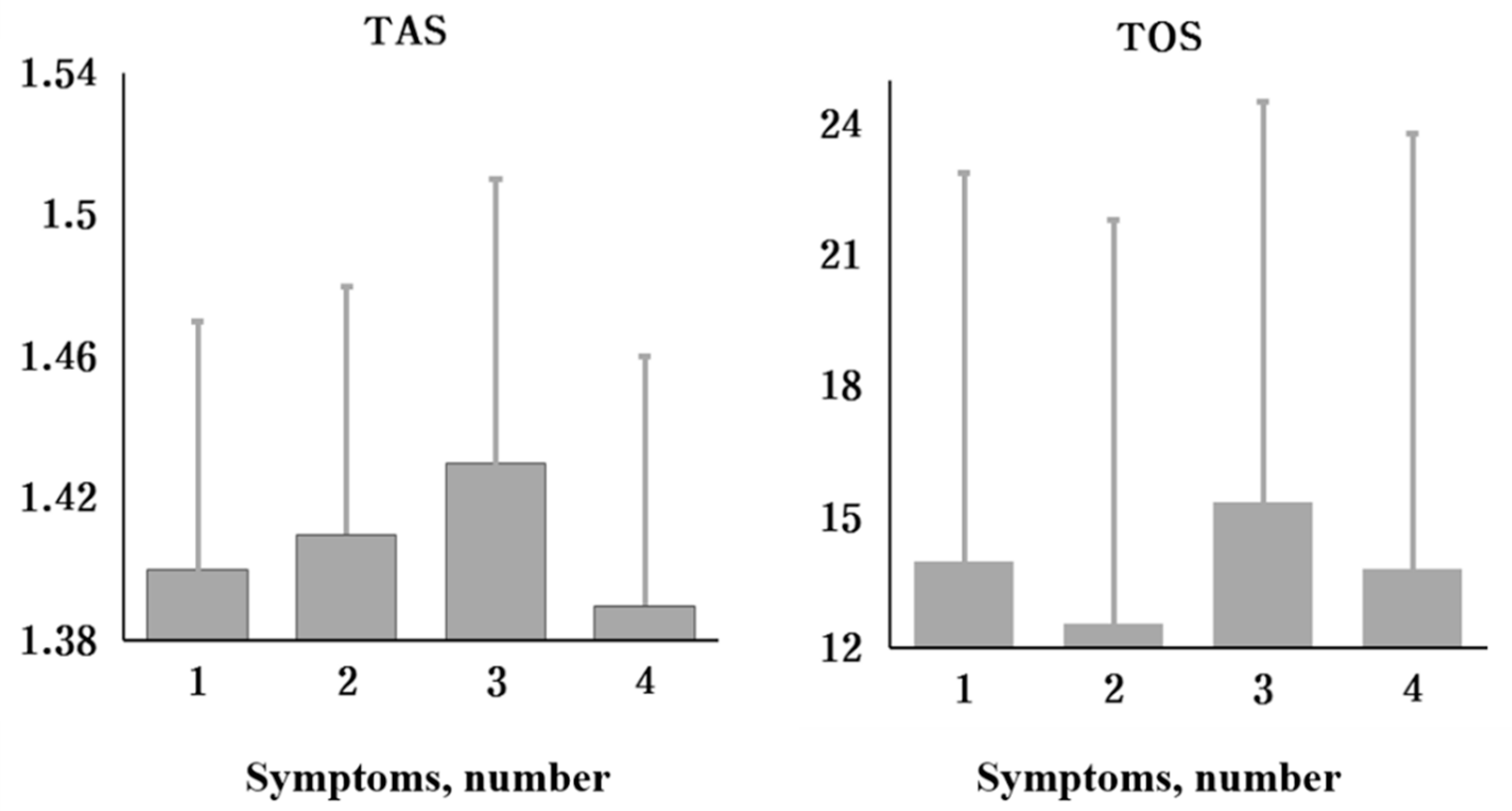
| Variables | I | II | III | IV | p Value * |
|---|---|---|---|---|---|
| TAS (mmol/L) Mean ± SD | 1.40 ± 0.07 | 1.41 ± 0.07 | 1.43 ± 0.08 | 1.39 ± 0.07 | 0.299 |
| TOS (µmol/L) Mean ± SD | 14.00 ± 8.90 | 12.57 ± 9.23 | 15.36 ± 9.18 | 13.84 ± 9.94 | 0.674 |
| Symptom Duration | Groups According to Number of Symptoms | p Value | ||||
|---|---|---|---|---|---|---|
| I | II | III | IV | |||
| <6 months | N | 127 | 41 | 8 | 8 | |
| TAS | 1.40 ± 0.07 | 1.41 ± 0.07 | 1.39 ± 0.04 | 1.38 ± 0.07 | 0.741 | |
| TOS | 13.97 ± 8.97 | 13.87 ± 9.55 | 15.12 ± 10.31 | 14.98 ± 9.00 | 0.975 | |
| ≥6 months | N | 11 | 16 | 7 | 8 | |
| TAS | 1.40 ± 0.08 | 1.42 ± 0.08 | 1.48 ± 0.08 | 1.40 ± 0.06 | 0.154 | |
| TOS | 14.45 ± 8.46 | 9.25 ± 7.65 | 15.64 ± 8.51 | 12.71 ± 11.30 | 0.320 | |
| Variable | Category | Number | p Value | Exp(B) | 95% CI | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Skin prick test | Negative | 99 | 0.056 | 2.052 | 0.982 | 4.288 |
| Positive | 127 | |||||
| Age | <10 years. | 131 | 0.366 | 0.715 | 0.346 | 1.479 |
| ≥10 years. | 95 | |||||
| Sex | Boys | 136 | 0.217 | 0.625 | 0.296 | 1.318 |
| Girls | 90 | |||||
| AR history of father | No | 158 | 0.816 | 1.094 | 0.513 | 2.332 |
| Yes | 68 | |||||
| AR history of mother | No | 142 | 0.887 | 1.053 | 0.515 | 2.155 |
| Yes | 84 | |||||
| TAS | 226 | 0.034 | 1.655 | 1.040 | 2.634 | |
| TOS | 226 | 0.163 | 0.972 | 0.935 | 1.011 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, H.; Sim, C.; Lee, J.; Oh, I.; An, T.; Lee, J. A Prospective Study on the Association between Oxidative Stress and Duration of Symptoms in Allergic Rhinitis. J. Pers. Med. 2021, 11, 1290. https://doi.org/10.3390/jpm11121290
Moon H, Sim C, Lee J, Oh I, An T, Lee J. A Prospective Study on the Association between Oxidative Stress and Duration of Symptoms in Allergic Rhinitis. Journal of Personalized Medicine. 2021; 11(12):1290. https://doi.org/10.3390/jpm11121290
Chicago/Turabian StyleMoon, Hyun, Changsun Sim, Jiho Lee, Inbo Oh, Taehoon An, and Jongcheol Lee. 2021. "A Prospective Study on the Association between Oxidative Stress and Duration of Symptoms in Allergic Rhinitis" Journal of Personalized Medicine 11, no. 12: 1290. https://doi.org/10.3390/jpm11121290
APA StyleMoon, H., Sim, C., Lee, J., Oh, I., An, T., & Lee, J. (2021). A Prospective Study on the Association between Oxidative Stress and Duration of Symptoms in Allergic Rhinitis. Journal of Personalized Medicine, 11(12), 1290. https://doi.org/10.3390/jpm11121290






