COVID-19 Vaccine Does Not Increase the Risk of Disease Flare-Ups among Patients with Autoimmune and Immune-Mediated Diseases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Data Sources/Measurement
2.3. Sample Size
2.4. Data Analysis
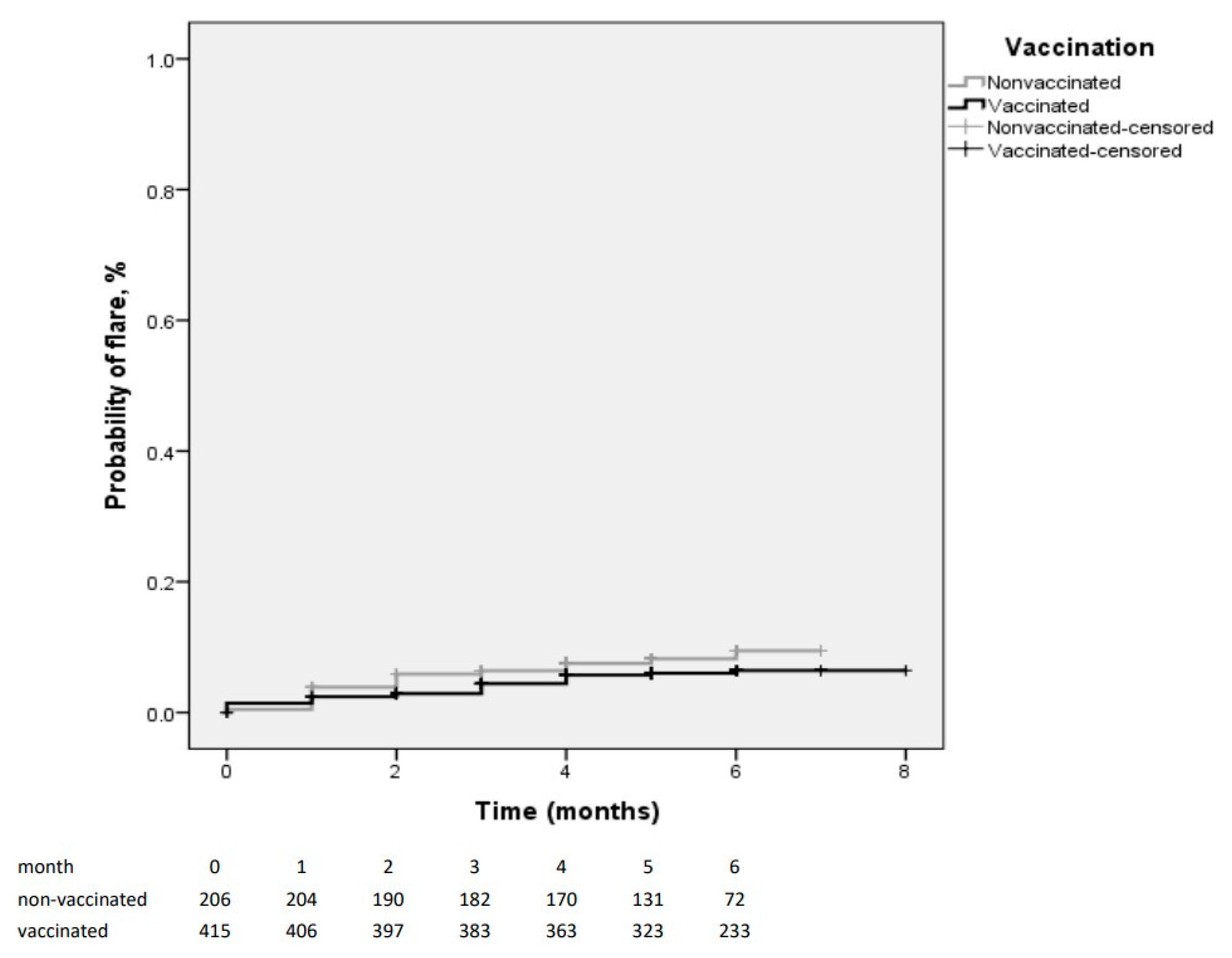
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Furer, V.; Rondaan, C.; Heijstek, M.W.; Agmon-Levin, N.; Van Assen, S.; Bijl, M.; Breedveld, F.C.; D’Amelio, R.; Dougados, M.; Kapetanovic, M.C.; et al. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann. Rheum. Dis. 2019, 79, 39–52. [Google Scholar] [CrossRef]
- Ng, B.; McBain, L.; Grainger, R. Rheumatologists fail to advise people with RA to get immunised, which matters if you are under 65: An audit in a New Zealand rheumatology service. N. Z. Med. J. 2016, 129, 27906921. [Google Scholar]
- Dudley, M.Z.; Halsey, N.A.; Omer, S.B.; Orenstein, W.A.; O’Leary, S.T.; Limaye, R.J.; Salmon, D.A. The state of vaccine safety science: Systematic reviews of the evidence. Lancet Infect. Dis. 2020, 20, e80–e89. [Google Scholar] [CrossRef]
- Segal, Y.; Calabrò, M.; Kanduc, D.; Shoenfeld, Y. Human papilloma virus and lupus: The virus, the vaccine and the disease. Curr. Opin. Rheumatol. 2017, 29, 331–342. [Google Scholar] [CrossRef]
- Baker, B.; Guimarães, L.E.; Tomljenovic, L.; Agmon-Levin, N.; Shoenfeld, Y. The safety of human papilloma virus-blockers and the risk of triggering autoimmune diseases. Expert Opin. Drug Saf. 2015, 14, 1387–1394. [Google Scholar] [CrossRef]
- Ishay, Y.; Kenig, A.; Tsemach-Toren, T.; Amer, R.; Rubin, L.; Hershkovitz, Y.; Kharouf, F. Autoimmune phenomena following SARS-CoV-2 vaccination. Int. Immunopharmacol. 2021, 99, 107970. [Google Scholar] [CrossRef]
- Baimukhamedov, C. Arthritis of the left elbow joint after vaccination against SARS-CoV-2 infection. Int. J. Rheum. Dis. 2021, 24, 1218–1220. [Google Scholar] [CrossRef] [PubMed]
- Terracina, K.A.; Tan, F.K. Flare of rheumatoid arthritis after COVID-19 vaccination. Lancet Rheumatol. 2021, 3, e469–e470. [Google Scholar] [CrossRef]
- Li, X.; Tong, X.; Yeung, W.W.Y.; Kuan, P.; Yum, S.H.H.; Chui, C.S.L.; Lai, F.T.T.; Wan, E.Y.F.; Wong, C.K.H.; Chan, E.W.Y.; et al. Two-dose COVID-19 vaccination and possible arthritis flare among patients with rheumatoid arthritis in Hong Kong. Ann. Rheum. Dis. 2021. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Obser-vational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann. Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef] [Green Version]
- Katz, M.H. Outcome variables in multivariable analysis. In Multivariable Analysis; Cambridge University Press (CUP): Cambridge, UK, 2011; pp. 25–73. [Google Scholar]
- Selected Adverse Events Reported after COVID-19 Vaccination|CDC. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html (accessed on 22 September 2021).
- Baicus, C.; Delcea, C.; Pinte, L.; Dan, G.A. Hyper-inflammation after COVID-19 mARN vaccination: At the crossroads of multisystem inflammatory disease and adult-onset Still’s disease. Does terminology matter? Romanian J. Intern. Med. 2021. Epub ahead of print. [Google Scholar] [CrossRef]
- Negahdaripour, M.; Shafiekhani, M.; Moezzi, S.M.I.; Amiri, S.; Rasekh, S.; Bagheri, A.; Mosaddeghi, P.; Vazin, A. Administration of COVID-19 Vaccines in ImmunocompromisedPatients. Int. Immunopharmacol. 2021, 99, 108021. [Google Scholar] [CrossRef]
- Machado, P.M.; Lawson-Tovey, S.; Hyrich, K.; Carmona, L.; Gossec, L.; Mateus, E.; Strangfeld, A.; Raffeiner, B.; Goulenok, T.; Brocq, O.; et al. LB0002 COVID-19 Vaccine Safety in Patients with Rheumatic and Musculoskeletal Disease. Ann. Rheum. Dis. 2021, 80, 199–200. [Google Scholar] [CrossRef]
- Furer, V.; Eviatar, T.; Zisman, D.; Peleg, H.; Paran, D.; Levartovsky, D.; Zisapel, M.; Elalouf, O.; Kaufman, I.; Meidan, R.; et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult pa-tients with autoimmune inflammatory rheumatic diseases and in the general population: A multicentre study. Ann. Rheum. Dis. 2021, 80, 1330–1338. [Google Scholar] [CrossRef]
- Geisen, U.M.; Berner, D.K.; Tran, F.; Sümbül, M.; Vullriede, L.; Ciripoi, M.; Reid, H.M.; Schaffarzyk, A.; Longardt, A.C.; Franzenburg, J.; et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann. Rheum. Dis. 2021, 80, 1306–1311. [Google Scholar] [CrossRef] [PubMed]
- Cherian, S.; Paul, A.; Ahmed, S.; Alias, B.; Manoj, M.; Santhosh, A.K.; Varghese, D.R.; Krishnan, N.; Shenoy, P. Safety of the ChAdOx1 nCoV-19 and the BBV152 vaccines in 724 patients with rheumatic diseases: A post-vaccination cross-sectional survey. Rheumatol. Int. 2021, 41, 1441–1445. [Google Scholar] [CrossRef]
- Di Filippo, M.; Cordioli, C.; Malucchi, S.; Annovazzi, P.; Cavalla, P.; Clerici, V.T.; Ragonese, P.; Nociti, V.; Radaelli, M.; Laroni, A.; et al. mRNA COVID-19 vaccines do not increase the short-term risk of clinical relapses in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2021. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Watad, A.; De Marco, G.; Mahajna, H.; Druyan, A.; Eltity, M.; Hijazi, N.; Haddad, A.; Elias, M.; Zisman, D.; Naffaa, M.; et al. Immune-Mediated Disease Flares or New-Onset Disease in 27 Subjects Following mRNA/DNA SARS-CoV-2 Vaccination. Vaccines 2021, 9, 435. [Google Scholar] [CrossRef] [PubMed]
- Barbhaiya, M.; Levine, J.M.; Bykerk, V.P.; Jannat-Khah, D.; Mandl, L.A. Systemic rheumatic disease flares after SARS-CoV-2 vaccination among rheumatology outpatients in New York City. Ann. Rheum. Dis. 2021, 80, 1352–1354. [Google Scholar] [CrossRef]
- Wells, G.; Becker, J.-C.; Teng, J.; Dougados, M.; Schiff, M.; Smolen, J.; Aletaha, D.; Van Riel, P.L.C.M. Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann. Rheum. Dis. 2008, 68, 954–960. [Google Scholar] [CrossRef]
- Arora, S.; Isenberg, D.A.; Castrejon, I. Measures of Adult Systemic Lupus Erythematosus: Disease Activity and Damage. Arthritis Rheum. 2020, 72, 27–46. [Google Scholar] [CrossRef]
- Seror, R.; Ravaud, P.; Mariette, X.; Bootsma, H.; Theander, E.; Hansen, A.; Ramos-Casals, M.; Doerner, T.; Bombardieri, S.; Hachulla, E.; et al. EULAR Sjögren’s Syndrome Patient Reported Index (ESSPRI): Development of a consensus patient index for primary Sjögren’s syndrome. Ann. Rheum. Dis. 2011, 70, 968–972. [Google Scholar] [CrossRef]
- Medscape Home Page. Available online: https://reference.medscape.com/calculator/299/basdai (accessed on 27 October 2021).
- Louden, B.A.; Pearce, D.J.; Lang, W.; Feldman, S.R. A simplified psoriasis area severity index (SPASI) for rating psoriasis severity in clinic patients. Dermatol. Online J. 2004, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Schoels, M.M.; Aletaha, D.; Alasti, F.; Smolen, J.S. Disease activity in psoriatic arthritis (PsA): Defining remission and treatment success using the DAPSA score. Ann. Rheum. Dis. 2015, 75, 811–818. [Google Scholar] [CrossRef]
- Valentini, G.; Bencivelli, W.; Bombardieri, S.; D’Angelo, S.; Della Rossa, A.; Silman, A.J.; Black, C.M.; Czirjak, L.; Nielsen, H.; Vlachoyiannopoulos, P.G. European Scleroderma Study Group to define disease activity criteria for systemic sclerosis. III. Assessment of the construct validity of the preliminary activity criteria. Ann. Rheum. Dis. 2003, 62, 901–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Vasculitis Society Home Page. Available online: https://vasculitis.org/disease-scoring/ (accessed on 2 November 2021).
- Burns, T.M.; Conaway, M.; Sanders, D.B. The MG Composite. Neurology 2010, 74, 1434–1440. [Google Scholar] [CrossRef] [Green Version]
- Kaunzner, U.W.; Al-Kawaz, M.; Gauthier, S.A. Defining Disease Activity and Response to Therapy in MS. Curr. Treat. Options Neurol. 2017, 19, 20. [Google Scholar] [CrossRef] [PubMed]

| Patients with and without Flare | Vaccinated and Non-Vaccinated Patients | |||||||
|---|---|---|---|---|---|---|---|---|
| Flare-Up (n = 42) | Non-Flare-Up (n = 581) | p Value | Missing | Vaccinated (n = 416) | Non-Vaccinated (n = 207) | p Value | Missing | |
| Enrolled online | 31 (73.8%) | 30 (63.7%) | 0.243 | 299 (71.9%) | 102 (49.3%) | <0.001 | ||
| Vaccinared | 25 (59.5%) | 391 (67.3%) | 0.312 | - | - | - | ||
| Gender (F) | 37 (88.1%) | 485 (83.5%) | 0.522 | 339 (81.5%) | 183 (88.4%) | 0.028 | ||
| Age (y) | 46 (21, 83) | 49 (19, 88) | 0.126 | 50 (21, 88) | 48 (19, 73) | 0.290 | ||
| Immune disease and comorbidities | ||||||||
| Charlson index | 2 (0, 6) | 1 (0, 10) | 0.512 | 1 (0, 9) | 1 (0, 10) | 0.354 | ||
| More than one immune disease | 16 (38.1%) | 118 (20.3%) | 0.011 | 83 (20%) | 51 (24.6%) | 0.180 | ||
| Number of immune diseases/patient | 1 (1, 4) | 1 (1, 6) | 0.006 | 1 (1, 4) | 1 (1, 6) | 0.142 | ||
| Immune pulmonary involvement | 6 (14.3%) | 59 (10.2%) | 0.429 | 40 (9.6%) | 25 (12.1%) | 0.404 | ||
| AIRD 1 | 26 (61.9%) | 369 (63.5%) | 0.869 | 239 (57.5%) | 156 (75.4%) | <0.001 | ||
| Rheumatoid arthritis | 7 (16.7%) | 91 (15.7%) | 0.827 | 59 (14.2%) | 39 (18.8%) | 0.161 | ||
| Systemic lupus erythematosus | 9 (21.4%) | 88 (15.1%) | 0.273 | 48 (11.5%) | 49 (23.7%) | <0.001 | ||
| Sjögren’s syndrome/Sicca 2 | 7 (16.7%) | 71 (12.2%) | 0.466 | 50 (12%) | 28 (13.5%) | 0.608 | ||
| Ankylosing spondylitis | 2 (4.8%) | 63 (10.8%) | 0.298 | 48 (11.5%) | 17 (8.2%) | 0.214 | ||
| Psoriatic arthritis/psoriasis | 4 (9.5%) | 49 (8.4%) | 0.774 | 32 (7.7%) | 21 (10.1%) | 0.360 | ||
| Systemic sclerosis/limited scleroderma | 1 (2.4%) | 30 (5.2%) | 0.714 | 14 (3.4%) | 17 (8.2%) | 0.011 | ||
| Antiphospholipid syndrome | 3 (7.1%) | 22 (3.8%) | 0.234 | 12 (2.9%) | 13 (6.3%) | 0.051 | ||
| Systemic vasculitis | 2 (4.8%) | 15 (2.6%) | 0.320 | 8 (1.9%) | 9 (4.3%) | 0.114 | ||
| Other AIRD 3 | 1 (2.4%) | 14 (2.4%) | 1 | 9 (2.2%) | 6 (2.9%) | 0.586 | ||
| Non-AIRD | 16 (38.1%) | 212 (36.5%) | 0.869 | 177 (42.5%) | 51 (24.6%) | <0.001 | ||
| Inflammatory bowel disease | 5 (11.9%) | 39 (6.7%) | 0.207 | 34 (8.2%) | 10 (4.8%) | 0.138 | ||
| Celiac disease | 1 (2.4%) | 18 (3.1%) | 1 | 14 (3.4%) | 5 (2.4%) | 0.626 | ||
| Primary biliary cholangitis | 1 (2.4%) | 8 (1.4%) | 0.469 | 4 (1%) | 5 (2.4%) | 0.167 | ||
| Autoimmune hepatitis | 0 (0%) | 11 (1.9%) | 1 | 8 (1.9%) | 3 (1.4%) | 1 | ||
| Myasthenia gravis | 6 (14.3%) | 22 (3.8%) | 0.008 | 15 (3.6%) | 13 (6.3%) | 0.151 | ||
| Multiple sclerosis | 1 (2.4%) | 24 (4.1%) | 1 | 17 (4.1%) | 8 (3.9%) | 1 | ||
| Hematological diseases 4 | 1 (2.4%) | 4 (0.7%) | 0.295 | 4 (1%) | 1 (0.5%) | 1 | ||
| Cutaneous diseases 5 | 1 (2.4%) | 16 (2.8%) | 1 | 12 (2.9%) | 5 (2.4%) | 1 | ||
| Autoimmune thyroid disease | 9 (21.4%) | 128 (22%) | 1 | 109 (26.2%) | 28 (13.5%) | <0.001 | ||
| Other non-AIRD 6 | 6 (14.3%) | 44 (6.7%) | 0.136 | 40 (9.6%) | 10 (4.8%) | 0.042 | ||
| Treatment | ||||||||
| Corticosteroids | 16 (38.1%) | 120 (20.7%) | 0.012 | 71 (17.1%) | 65 (31.4%) | <0.001 | ||
| Corticosteroid dose (mg) | 10 (5, 45) | 10 (2,5, 40) | 0.015 | 29 | 6.25 (5, 20) | 10 (3, 45) | <0.001 | 29 |
| Synthetic DMARDs 7 | 12 (28.6%) | 226 (38.9%) | 0.194 | 143 (34.4%) | 95 (45.9%) | 0.007 | ||
| Hydroxychloroquine | 8 (19%) | 125 (21.5%) | 0.846 | 81 (19.5%) | 52 (25.1%) | 0.119 | ||
| Methotrexate | 2 (4.8%) | 64 (11%) | 0.298 | 42 (10.1%) | 24 (11.6%) | 0.582 | ||
| Sulfasalazine | 2 (4.8%) | 48 (8.3%) | 0.566 | 34 (8.2%) | 16 (7.7%) | 1 | ||
| Leflunomide | 1 (2.4%) | 20 (3.4%) | 1 | 10 (2.4%) | 11 (5.3%) | 0.096 | ||
| Biologic DMARDs 7 | 10 (23.8%) | 119 (20.6%) | 0.561 | 89 (21.4%) | 40 (19.4%) | 0.600 | ||
| Mycophenolate mofetil | 1 (2.4%) | 17 (2.9%) | 1 | 13 (3.1%) | 5 (2.4%) | 0.801 | ||
| Azathioprine | 5 (11.9%) | 40 (6.9%) | 0.216 | 23 (5.5%) | 22 (10.6%) | 0.031 | ||
| Flare-up-related variables | ||||||||
| Disease diagnosis-enrollement/vaccine (years) | 11 (1, 40) | 9 (0, 56) | 0.608 | 29 | 9 (0, 50) | 10 (0, 56) | 0.999 | 29 |
| Flare-up in the year prior enrolement/vaccination | 22 (73.3%) | 189 (47.4%) | 0.007 | 194 | 130 (46.8%) | 81 (53.6%) | 0.189 | 194 |
| Enrollment/vaccination-flare-up timelaps (months) | - | - | - | 6.13 (0, 8) | 5.57 (1, 8) | <0.001 | ||
| COVID-19 8 prior enrollement/vaccine | 4 (9.5%) | 81 (13.9%) | 0.640 | 75 (18%) | 10 (4.8%) | <0.001 | ||
| Adverse events after dose 1 | 24 (92.3%) | 283 (72.4%) | 0.022 | 307/416 | - | - | ||
| Adverse events after dose 2 | 21 (80.8%) | 252 (64.5%) | 0.134 | 273/416 | - | - | ||
| Treatment adjustment before vaccination | 4 (16%) | 27 (6.9%) | 0.105 | 31/416 | - | - | ||
| Treatment adjustment before flare | 3/42 (7.1%) | - | - | 1 (4%) | 2 (11.8%) | 0.556 | ||
| Average length of flare-up (days) | 30 (4, 164) | - | - | 4 | 30 (4, 149) | 27.5 (14, 164) | 0.803 | 4 |
| Still having flare-up at the last assessment | 19 (45.2%) | 18 (3.2%) | <0.001 | 21 | 18 (4.5%) | 19 (9.6%) | 0.018 | 21 |
| Flare-up management | ||||||||
| Hospitalization | 15/42 | - | - | 10/25 | 5/17 | - | ||
| Treatment adjustment | 36/42 | - | - | 21/25 | 15/17 | - | ||
| 1st Dose (n = 416) | 2nd Dose (n = 407) | |||||||
|---|---|---|---|---|---|---|---|---|
| Non-AIRD 1 (n = 134, 75.7%) | AIRD (n = 173, 72.4%) | p Value (0.499) | Total Adverse Events (n = 307) | Non-AIRD (n = 125, 70.6%) | AIRD (n = 148, 61.9%) | p Value (0.076) | Total Adverse Events (n = 273) | |
| Pain in injection site | 81 (45.3%) | 99 (41.4%) | 0.485 | 180 | 86 (48%) | 82 (34.3%) | 0.005 | 168 |
| Local swelling | 9 (5%) | 6 (2.5%) | 0.192 | 15 | 6 (3.4%) | 4 (1.7%) | 0.338 | 10 |
| Local redness | 22 (12.3%) | 23 (9.6%) | 0.427 | 45 | 8 (4.5%) | 7 (2.9%) | 0.435 | 15 |
| Fatigue | 46 (25.7%) | 51 (21.3%) | 0.349 | 97 | 41 (22.9%) | 52 (21.8%) | 0.813 | 93 |
| Headache | 33 (18.4%) | 44 (18.4%) | 1 | 77 | 24 (13.4%) | 25 (10.5%) | 0.361 | 49 |
| Myalgia | 24 (13.4%) | 35 (14.6) | 0.777 | 59 | 25 (14%) | 25 (10.5%) | 0.289 | 50 |
| New/increased joint pain | 25 (14%) | 35 (14.7%) | 0.888 | 60 | 10 (5.6%) | 20 (8.4%) | 0.340 | 30 |
| Chills | 24 (13.4%) | 35 (14.6%) | 0.777 | 59 | 28 (15.6%) | 34 (14.5%) | 0.782 | 62 |
| Fever | 8 (4.5%) | 13 (5.4%) | 0.822 | 21 | 22 (12.3%) | 24 (10%) | 0.528 | 46 |
| Allergic reactions | 3 (1.7%) | 2 (0.8%) | 0.655 | 5 | 1 (0.6%) | 0 (0%) | 0.427 | 1 |
| Skin rash | 3 (1.7%) | 3 (1.3%) | 1 | 6 | 1 (0.6%) | 0(0%) | 0.428 | 1 |
| Anaphylactic shock | 0 (0%) | 0 (0%) | 0 | 0 | 0 (0%) | 1 (0.4%) | 1 | 1 |
| Lymphadenopathy | 4 (2.2%) | 5 (2.1%) | 1 | 9 | 5 (2.9%) | 4 (1.7%) | 0.501 | 9 |
| Paresthesia | 1 (0.6%) | 6 (2.5%) | 0.247 | 7 | 2 (1.2%) | 5 (2.1%) | 0.704 | 7 |
| Digestive symptoms | 4 (2.2%) | 5 (2.1%) | 1 | 9 | 7 (4%) | 12 (5%) | 0.813 | 19 |
| Dizziness | 2 (1.1%) | 7 (2.9%) | 0.311 | 9 | 6 (3.5%) | 6(2.5%) | 0.569 | 12 |
| Cough | 3 (1.7%) | 0 (0%) | 0.078 | 3 | 1 (0.6%) | 0 (0%) | 0.420 | 1 |
| Variable | B | p | OR with 95%CI 1 |
|---|---|---|---|
| More than one immune disease | 0.667 | 0.079 | 1.9 (0.92–4.09) |
| Corticotherapy | 0.438 | 0.255 | 1.5 (0.73–3.3) |
| Vaccine | −0.630 | 0.094 | 0.53 (0.25–1.11) |
| Flare-up during the previous year | 0.972 | 0.019 | 2.64 (1.17–5.97) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinte, L.; Negoi, F.; Ionescu, G.D.; Caraiola, S.; Balaban, D.V.; Badea, C.; Mazilu, D.; Dumitrescu, B.; Mateescu, B.; Ionescu, R.; et al. COVID-19 Vaccine Does Not Increase the Risk of Disease Flare-Ups among Patients with Autoimmune and Immune-Mediated Diseases. J. Pers. Med. 2021, 11, 1283. https://doi.org/10.3390/jpm11121283
Pinte L, Negoi F, Ionescu GD, Caraiola S, Balaban DV, Badea C, Mazilu D, Dumitrescu B, Mateescu B, Ionescu R, et al. COVID-19 Vaccine Does Not Increase the Risk of Disease Flare-Ups among Patients with Autoimmune and Immune-Mediated Diseases. Journal of Personalized Medicine. 2021; 11(12):1283. https://doi.org/10.3390/jpm11121283
Chicago/Turabian StylePinte, Larisa, Florentina Negoi, Georgeta Daniela Ionescu, Simona Caraiola, Daniel Vasile Balaban, Camelia Badea, Diana Mazilu, Bianca Dumitrescu, Bogdan Mateescu, Ruxandra Ionescu, and et al. 2021. "COVID-19 Vaccine Does Not Increase the Risk of Disease Flare-Ups among Patients with Autoimmune and Immune-Mediated Diseases" Journal of Personalized Medicine 11, no. 12: 1283. https://doi.org/10.3390/jpm11121283
APA StylePinte, L., Negoi, F., Ionescu, G. D., Caraiola, S., Balaban, D. V., Badea, C., Mazilu, D., Dumitrescu, B., Mateescu, B., Ionescu, R., Parvu, M. I., & Baicus, C. (2021). COVID-19 Vaccine Does Not Increase the Risk of Disease Flare-Ups among Patients with Autoimmune and Immune-Mediated Diseases. Journal of Personalized Medicine, 11(12), 1283. https://doi.org/10.3390/jpm11121283








