Unmanaged Pharmacogenomic and Drug Interaction Risk Associations with Hospital Length of Stay among Medicare Advantage Members with COVID-19: A Retrospective Cohort Study
Abstract
:1. Introduction
2. Methods
2.1. Ethics Approval Statement
2.2. Data Source and Study Population
2.3. Independent and Outcome Variables
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COVID-19 | Coronavirus-induced disease 2019 |
| CPIC | Clinical Pharmacogenetics Implementation Consortium |
| DDI | Drug–drug interaction |
| FDA | U.S. Food and Drug Administration |
| HCC | Hierarchical conditions category |
| LOS | Length of stay |
| PIP | Pharmacogenetic interaction probability |
| RAF | Risk adjustment factor |
| SNP | Special needs plan |
References
- Mitchell, E.M. Concentration of Health Expenditures and Selected Characteristics of High Spenders, U.S. Civilian Noninstitutionalized Population, 2016. In Statistical Brief (Medical Expenditure Panel Survey (US)); Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2019. [Google Scholar]
- Bueno, H. Trends in Length of Stay and Short-term Outcomes Among Medicare Patients Hospitalized for Heart Failure, 1993–2006. JAMA 2010, 303, 2141–2147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, M.; Sajobi, T.; Lucyk, K.; Lorenzetti, D.L.; Quan, H. Systematic Review of Risk Adjustment Models of Hospital Length of Stay (LOS). Med. Care 2015, 53, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Salim, S.A.; Elmaraezy, A.; Pamarthy, A.; Thongprayoon, C.; Cheungpasitporn, W.; Palabindala, V. Impact of hospitalists on the efficiency of inpatient care and patient satisfaction: A systematic review and meta-analysis. J. Community Hosp. Intern. Med. Perspect. 2019, 9, 121–134. [Google Scholar] [CrossRef] [Green Version]
- Ward, C.; Patel, V.; Elsaid, M.I.; Jaisinghani, P.; Sharma, R. A case-control study of length of stay outliers. Am. J. Manag. Care 2021, 27, e66–e71. [Google Scholar] [CrossRef]
- Lazarou, J.; Pomeranz, B.H.; Corey, P.N. Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of pro-spective studies. JAMA 1998, 279, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Sultana, J.; Cutroneo, P.; Trifirò, G. Clinical and economic burden of adverse drug reactions. J. Pharmacol. Pharmacother. 2013, 4 (Suppl. S1), 73–77. [Google Scholar] [CrossRef] [Green Version]
- Formica, D.; Sultana, J.; Cutroneo, P.M.; Lucchesi, S.; Angelica, R.; Crisafulli, S.; Ingrasciotta, Y.; Salvo, F.; Spina, E.; Trifirò, G. The economic burden of preventable adverse drug reactions: A systematic review of observational studies. Expert Opin. Drug Saf. 2018, 17, 681–695. [Google Scholar] [CrossRef]
- Ji, Y.; Skierka, J.M.; Blommel, J.H.; Moore, B.E.; VanCuyk, D.L.; Bruflat, J.K.; Peterson, L.M.; Veldhuizen, T.L.; Fadra, N.; Peterson, S.E.; et al. Preemptive Pharmacogenomic Testing for Precision Medicine: A Comprehensive Anal-ysis of Five Actionable Pharmacogenomic Genes Using Next-Generation DNA Sequencing and a Customized CYP2D6 Genotyping Cascade. J. Mol. Diagn. 2016, 18, 438–445. [Google Scholar] [CrossRef] [Green Version]
- Chanfreau-Coffinier, C.; Hull, L.E.; Lynch, J.A.; Duvall, S.L.; Damrauer, S.M.; Cunningham, F.E.; Voight, B.F.; Matheny, M.; Oslin, D.W.; Icardi, M.S.; et al. Projected Prevalence of Actionable Pharmacogenetic Variants and Level A Drugs Prescribed Among US Veterans Health Administration Pharmacy Users. JAMA Netw. Open 2019, 2, e195345. [Google Scholar] [CrossRef]
- McInnes, G.; Lavertu, A.; Sangkuhl, K.; Klein, T.E.; Whirl-Carrillo, M.; Altman, R.B. Pharmacogenetics at Scale: An Analysis of the UK Biobank. Clin. Pharmacol. Ther. 2021, 109, 1528–1537. [Google Scholar] [CrossRef]
- Ruaño, G.; Szarek, B.L.; Villagra, D.; Gorowski, K.; Kocherla, M.; Seip, R.L.; Goethe, J.W.; Schwartz, H.I. Length of psychiatric hospitalization is correlated with CYP2D6 functional status in inpatients with major depressive disorder. Biomark. Med. 2013, 7, 429–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finkelstein, J.; Zhang, F.; Cabrera, M. Association Between Number of Actionable Pharmacogenetic Variants and Length of Hospital Stay. Stud. Health Technol. Inform. 2020, 272, 195–198. [Google Scholar]
- Shadmi, E.; Chen, Y.; Dourado, I.; Faran-Perach, I.; Furler, J.; Hangoma, P.; Hanvoravongchai, P.; Obando, C.; Petrosyan, V.; Rao, K.D.; et al. Health equity and COVID-19: Global perspectives. Int. J. Equity Health 2020, 19, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lal, A.; Erondu, N.A.; Heymann, D.L.; Gitahi, G.; Yates, R. Fragmented health systems in COVID-19: Rectifying the misalignment between global health security and universal health coverage. Lancet 2021, 397, 61–67. [Google Scholar] [CrossRef]
- Grasselli, G.; Pesenti, A.; Cecconi, M. Critical Care Utilization for the COVID-19 Outbreak in Lombardy, Italy: Early Experi-ence and Forecast During an Emergency Response. JAMA 2020, 323, 1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iacobucci, G. Covid-19: All non-urgent elective surgery is suspended for at least three months in England. BMJ 2020, 368, m1106. [Google Scholar] [CrossRef] [Green Version]
- Kerlin, M.P.; Costa, D.K.; Davis, B.S.; Admon, A.J.; Vranas, K.C.; Kahn, J.M. Actions Taken by US Hospitals to Prepare for Increased Demand for Intensive Care During the First Wave of COVID-19: A national survey. Chest 2021, 160, 519–528. [Google Scholar] [CrossRef]
- Centers for Medicare and Medicaid Services. Preliminary Medicare COVID-19 Data Snapshot. 19 March 2021. Available online: https://www.cms.gov/research-statistics-data-systems/preliminary-medicare-covid-19-data-snapshot (accessed on 27 April 2021).
- Di Fusco, M.; Shea, K.M.; Lin, J.; Nguyen, J.L.; Angulo, F.J.; Benigno, M.; Malhotra, D.; Emir, B.; Sung, A.H.; Hammond, J.L.; et al. Health outcomes and economic burden of hospitalized COVID-19 patients in the United States. J. Med. Econ. 2021, 24, 308–317. [Google Scholar] [CrossRef]
- Data Note: Prescription Drugs and Older Adults. 9 August 2019. Available online: https://www.kff.org/health-reform/issue-brief/data-note-prescription-drugs-and-older-adults/ (accessed on 27 January 2021).
- Beijer, H.; De Blaey, C. Hospitalisations caused by adverse drug reactions (ADR): A meta-analysis of observational studies. Pharm. World Sci. 2002, 24, 46–54. [Google Scholar] [CrossRef]
- McQueenie, R.; Foster, H.M.E.; Jani, B.D.; Katikireddi, S.V.; Sattar, N.; Pell, J.P.; Ho, F.K.; Niedzwiedz, C.L.; Hastie, C.E.; Anderson, J.; et al. Multimorbidity, polypharmacy, and COVID-19 infection within the UK Biobank cohort. PLoS ONE 2020, 15, e0238091. [Google Scholar] [CrossRef]
- Stevenson, J.M.; Alexander, G.C.; Palamuttam, N.; Mehta, H.B. Projected Utility of Pharmacogenomic Testing Among Individuals Hospitalized With COVID-19: A Retrospective Multicenter Study in the United States. Clin. Transl. Sci. 2021, 14, 153–162. [Google Scholar] [CrossRef]
- SARS-CoV-2 and COVID-19 Related LOINC Terms. Available online: https://loinc.org/sars-cov-2-and-covid-19/ (accessed on 26 February 2021).
- Coleman, H.C.; Oesterheld, J.; Patterson, R.D. Genetic Data Analysis and Database Tools. U.S. Patent 8,099,298, 17 January 2012. Available online: https://patentimages.storage.googleapis.com/25/02/74/0495cac7195d3a/US8099298.pdf (accessed on 11 January 2021).
- Coleman, H.C.; Patterson, R.D.; Oesterheld, J.; Pany, R.V.; Ashcraft, K. Systems and Methods for Quantification and Presentation of Medical Risk Arising from Unknown Factors. U.S. Patent 2019/0164637 A1, 30 May 2019. Available online: https://patentimages.storage.googleapis.com/2b/e7/da/75f4be1d8818f2/US20190164637A1.pdf (accessed on 11 January 2021).
- Office of Enterprise Data and Analytics, Centers for Medicare & Medicaid Services (CMS). Chronic Conditions among Medicare Beneficiaries. 2018. Available online: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Chronic-Conditions/Chartbook_Charts (accessed on 27 January 2021).
- Hierarchical Condition Category Coding. Available online: https://www.aafp.org/family-physician/practice-and-career/getting-paid/coding/hierarchical-condition-category.html (accessed on 29 January 2021).
- Special Needs Plans. Available online: https://www.cms.gov/Medicare/Health-Plans/SpecialNeedsPlans (accessed on 29 January 2021).
- Tibshirani, R. Regression Shrinkage and Selection via the Lasso. J. R. Stat. Soc. Ser. B (Methodol.) 1996, 58, 267–288. Available online: https://www.jstor.org/stable/2346178?seq=1 (accessed on 26 February 2021). [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; Version 3.6.1; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Moreno, R.; Díez, J.; Diarte, J.; Macaya, F.; Hernández, J.T.; Rodríguez-Leor, O.; Trillo, R.; Alonso-Briales, J.; Amat-Santos, I.; Romaguera, R.; et al. Consequences of canceling elective invasive cardiac procedures during Covid-19 outbreak. Catheter. Cardiovasc. Interv. 2021, 97, 927–937. [Google Scholar] [CrossRef]
- Ueda, M.; Martins, R.; Hendrie, P.C.; McDonnell, T.; Crews, J.R.; Wong, T.L.; McCreery, B.; Jagels, B.; Crane, A.; Byrd, D.R.; et al. Managing Cancer Care During the COVID-19 Pandemic: Agility and Collaboration Toward a Common Goal. J. Natl. Compr. Cancer Netw. 2020, 18, 366–369. [Google Scholar] [CrossRef] [Green Version]
- Bennett, S.; Søreide, K.; Gholami, S.; Pessaux, P.; Teh, C.; Segelov, E.; Kennecke, H.; Prenen, H.; Myrehaug, S.; Callegaro, D.; et al. Strategies for the Delay of Surgery in the Management of Resectable Hepatobiliary Malignancies during the COVID-19 Pandemic. Curr. Oncol. 2020, 27, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Medicare Advantage versus the Traditional Medicare Program: Costs of Inpatient Stays, 2009–2017 #262. Available online: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb262-Medicare-Advantage-Costs-2009-2017.jsp (accessed on 19 March 2021).
- Lee, H.; Ryu, K.; Sohn, Y.; Kim, J.; Suh, G.Y.; Kim, E. Impact on Patient Outcomes of Pharmacist Participation in Multidisciplinary Critical Care Teams: A Systematic Review and Meta-Analysis. Crit. Care Med. 2019, 47, 1243–1250. [Google Scholar] [CrossRef]
- Williams, T.; Ho, K.; Dobb, G.; Finn, J.; Knuiman, M.; Webb, S. Effect of length of stay in intensive care unit on hospital and long-term mortality of critically ill adult patients. Br. J. Anaesth. 2010, 104, 459–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marfil-Garza, B.A.; Belaunzarán-Zamudio, P.F.; Gulias-Herrero, A.; Zuñiga, A.C.; Caro-Vega, Y.; Kershenobich-Stalnikowitz, D.; Sifuentes-Osornio, J. Risk factors associated with prolonged hospital length-of-stay: 18-year retrospective study of hospitalizations in a tertiary healthcare center in Mexico. PLoS ONE 2018, 13, e0207203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, A.M.; Lin, A.; Fu, R.; McConnell, K.J.; Sun, B. Associations of Emergency Department Length of Stay with Publicly Reported Quality-of-care Measures. Acad. Emerg. Med. 2017, 24, 246–250. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, J.H.; McInnis, T.; Hirsch, J.D. Cost of Prescription Drug–Related Morbidity and Mortality. Ann. Pharmacother. 2018, 52, 829–837. [Google Scholar] [CrossRef]
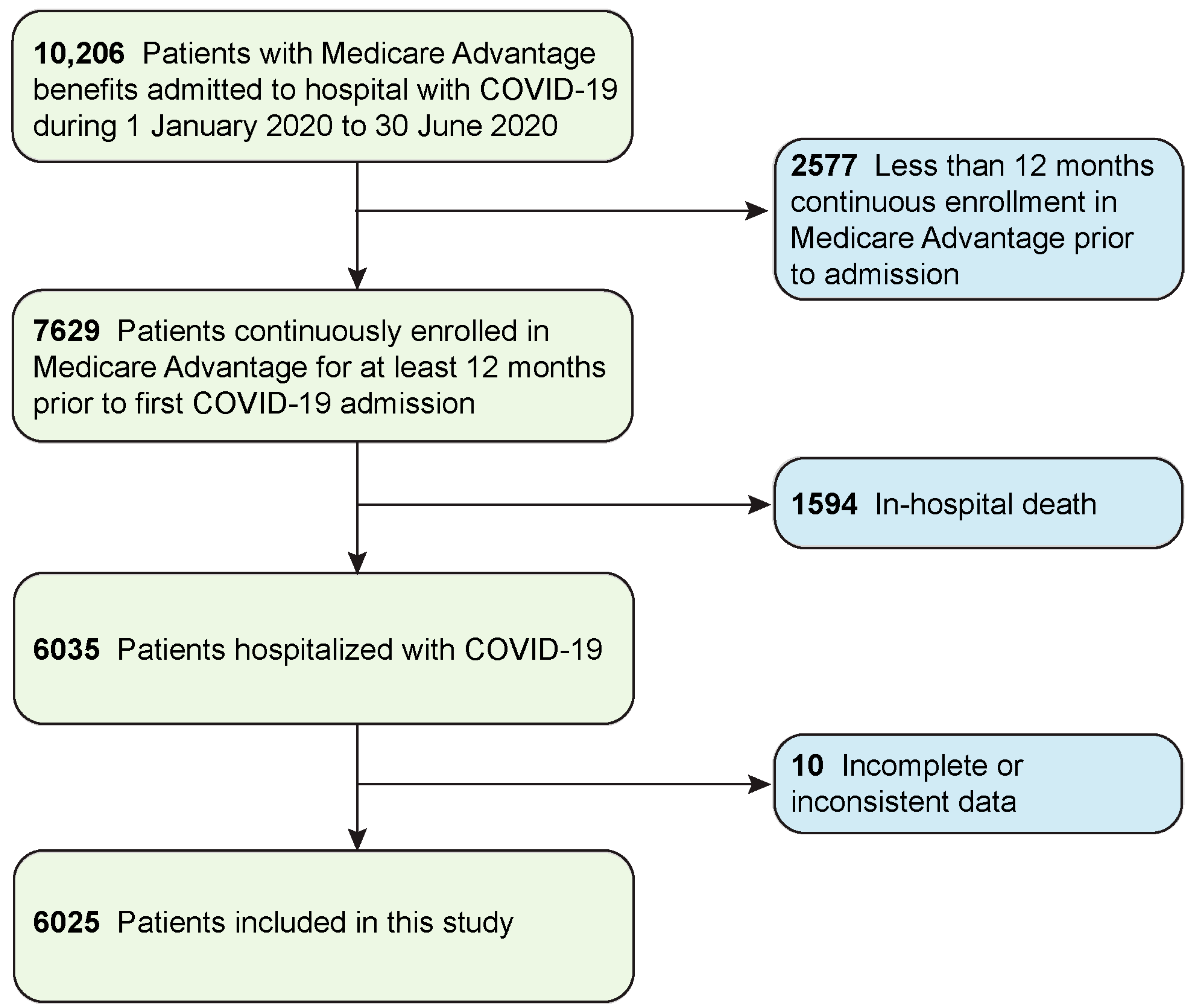
| Variable | Category | Value |
|---|---|---|
| Demographic/socioeconomic factors | ||
| Gender | Female, No. (%) | 3653 (61) |
| Age | Mean (SD) | 77 (11) |
| Race/ethnicity | White (non-Hispanic), No. (%) | 3753 (62) |
| Black (non-Hispanic), No. (%) | 1791 (30) | |
| Hispanic/Latino, No. (%) | 191 (3) | |
| Other, No. (%) | 140 (2) | |
| Asian/Pacific Islander, No. (%) | 99 (2) | |
| Unknown, No. (%) | 51 (1) | |
| Residential location | Urban, No. (%) | 2853 (47) |
| Suburban, No. (%) | 2200 (37) | |
| Rural, No. (%) | 972 (16) | |
| Median income | Mean (SD) | USD63,027 (USD17,435) |
| Plan and clinical characteristics | ||
| C-SNP | Enrolled, No. (%) | 268 (4) |
| D-SNP | Enrolled, No. (%) | 332 (6) |
| I-SNP | Enrolled, No. (%) | 2061 (34) |
| HCC count | 0 or 1, No. (%) | 1460 (24) |
| 2 or 3, No. (%) | 1991 (33) | |
| 4 or 5, No. (%) | 1182 (20) | |
| 6 or more, No. (%) | 1392 (23) | |
| Chronic conditions | COPD, No. (%) | 1450 (24) |
| Diabetes, No. (%) | 3104 (52) | |
| Hyperlipidemia, No. (%) | 3499 (58) | |
| Hypertension, No. (%) | 1902 (32) | |
| COVID-19 hospitalization (in days) | Mean LOS (SD) | 12.6 (11) |
| PIP | Low, ≤25%, No. (%) | 3514 (58) |
| Moderate, 26–50%, No. (%) | 1784 (30) | |
| High, >50%, No. (%) | 727 (12) | |
| DDI | Minimal or minor, No. (%) | 3110 (52) |
| Moderate, No. (%) | 983 (16) | |
| Major, No. (%) | 1542 (25) | |
| Contraindicated, No. (%) | 390 (7) | |
| Population | Moderate PIP (26% to 50%) * | High PIP (>50%) * | Moderate, Major, or Contraindicated DDI ** | |||
|---|---|---|---|---|---|---|
| Rate Ratio (95% CI) | p-Value | Rate Ratio (95% CI) | p-Value | Rate Ratio (95% CI) | p-Value | |
| Total cohort | 1.09 (1.04, 1.14) | <0.001 | 1.16 (1.09, 1.24) | <0.001 | 1.04 (1.00, 1.09) | 0.066 |
| HCC count | ||||||
| 0 or 1 | 1.15 (1.04, 1.28) | 0.007 | 1.39 (1.15, 1.67) | <0.001 | 0.91 (0.82, 1.00) | 0.045 |
| 2 or 3 | 1.03 (0.95, 1.11) | 0.522 | 1.08 (0.96, 1.21) | 0.204 | 1.10 (1.02, 1.18) | 0.010 |
| 4 or 5 | 1.13 (1.02, 1.25) | 0.019 | 1.13 (1.00, 1.29) | 0.057 | 1.07 (0.97, 1.17) | 0.179 |
| 6 or more | 1.08 (0.98, 1.18) | 0.112 | 1.16 (1.03, 1.31) | 0.014 | 1.01 (0.93, 1.11) | 0.786 |
| Chronic conditions | ||||||
| COPD | 1.13 (1.03, 1.24) | 0.009 | 1.18 (1.05, 1.34) | 0.006 | 1.01 (0.93, 1.10) | 0.796 |
| Diabetes | 1.05 (0.99, 1.12) | 0.119 | 1.15 (1.05, 1.25) | 0.002 | 1.07 (1.01, 1.13) | 0.031 |
| Hyperlipidemia | 1.08 (1.02, 1.15) | 0.013 | 1.12 (1.02, 1.22) | 0.014 | 1.05 (0.99, 1.11) | 0.096 |
| Hypertension | 1.03 (0.95, 1.12) | 0.478 | 1.22 (1.10, 1.35) | <0.001 | 1.02 (0.95, 1.1) | 0.522 |
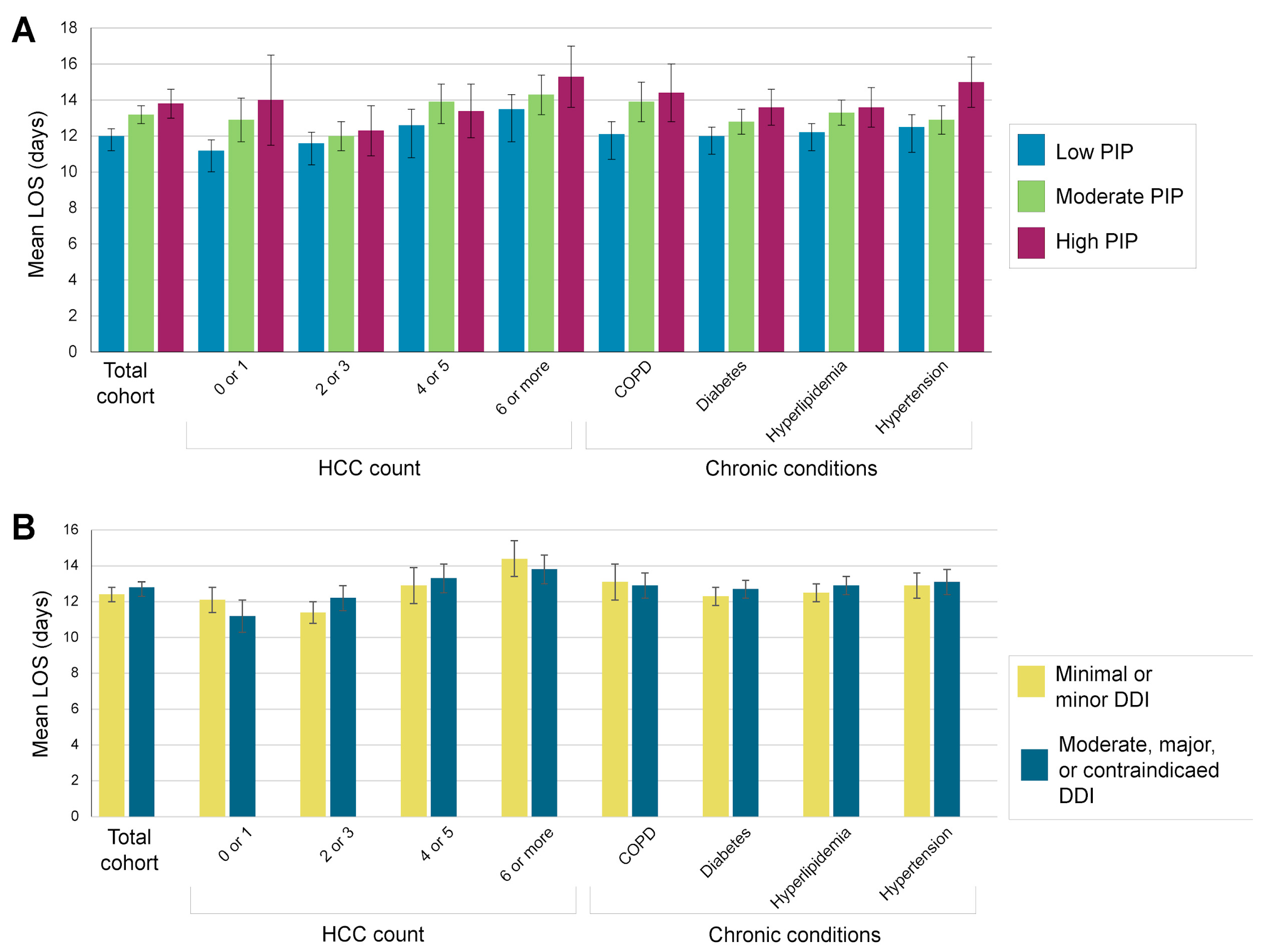
| Mean LOS (95% CI) by PIP | Mean LOS (95% CI) by DDI | |||||
|---|---|---|---|---|---|---|
| Chronic Condition | Total Subpopulation, Mean LOS (95% CI) | Low (≤25%) | Moderate (26–50%) | High (>50%) | Minimal or Minor | Moderate, Major, or Contraindicated |
| COPD | 13.0 (12.4, 13.6) | 12.1 (11.4, 12.8) | 13.9 (12.8, 15) | 14.4 (12.8, 16) | 13.1 (12.1, 14.1) | 12.9 (12.2, 13.6) |
| Diabetes | 12.5 (12.1, 12.9) | 12 (11.5, 12.5) | 12.8 (12.1, 13.5) | 13.6 (12.6, 14.6) | 12.3 (11.8, 12.8) | 12.7 (12.2, 13.2) |
| Hyperlipidemia | 12.7 (12.3, 13.1) | 12.2 (11.7, 12.7) | 13.3 (12.6, 14) | 13.6 (12.5, 14.7) | 12.5 (12, 13) | 12.9 (12.4, 13.4) |
| Hypertension | 13.0 (12.5, 13.5) | 12.5 (11.8, 13.2) | 12.9 (12.1, 13.7) | 15 (13.6, 16.4) | 12.9 (12.2, 13.6) | 13.1 (12.4, 13.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashcraft, K.; Moretz, C.; Schenning, C.; Rojahn, S.; Vines Tanudtanud, K.; Magoncia, G.O.; Reyes, J.; Marquez, B.; Guo, Y.; Erdemir, E.T.; et al. Unmanaged Pharmacogenomic and Drug Interaction Risk Associations with Hospital Length of Stay among Medicare Advantage Members with COVID-19: A Retrospective Cohort Study. J. Pers. Med. 2021, 11, 1192. https://doi.org/10.3390/jpm11111192
Ashcraft K, Moretz C, Schenning C, Rojahn S, Vines Tanudtanud K, Magoncia GO, Reyes J, Marquez B, Guo Y, Erdemir ET, et al. Unmanaged Pharmacogenomic and Drug Interaction Risk Associations with Hospital Length of Stay among Medicare Advantage Members with COVID-19: A Retrospective Cohort Study. Journal of Personalized Medicine. 2021; 11(11):1192. https://doi.org/10.3390/jpm11111192
Chicago/Turabian StyleAshcraft, Kristine, Chad Moretz, Chantelle Schenning, Susan Rojahn, Kae Vines Tanudtanud, Gwyn Omar Magoncia, Justine Reyes, Bernardo Marquez, Yinglong Guo, Elif Tokar Erdemir, and et al. 2021. "Unmanaged Pharmacogenomic and Drug Interaction Risk Associations with Hospital Length of Stay among Medicare Advantage Members with COVID-19: A Retrospective Cohort Study" Journal of Personalized Medicine 11, no. 11: 1192. https://doi.org/10.3390/jpm11111192
APA StyleAshcraft, K., Moretz, C., Schenning, C., Rojahn, S., Vines Tanudtanud, K., Magoncia, G. O., Reyes, J., Marquez, B., Guo, Y., Erdemir, E. T., & Hall, T. O. (2021). Unmanaged Pharmacogenomic and Drug Interaction Risk Associations with Hospital Length of Stay among Medicare Advantage Members with COVID-19: A Retrospective Cohort Study. Journal of Personalized Medicine, 11(11), 1192. https://doi.org/10.3390/jpm11111192






