A Cost–Consequence Analysis of Preemptive SLCO1B1 Testing for Statin Myopathy Risk Compared to Usual Care
Abstract
:1. Introduction
2. Materials and Methods
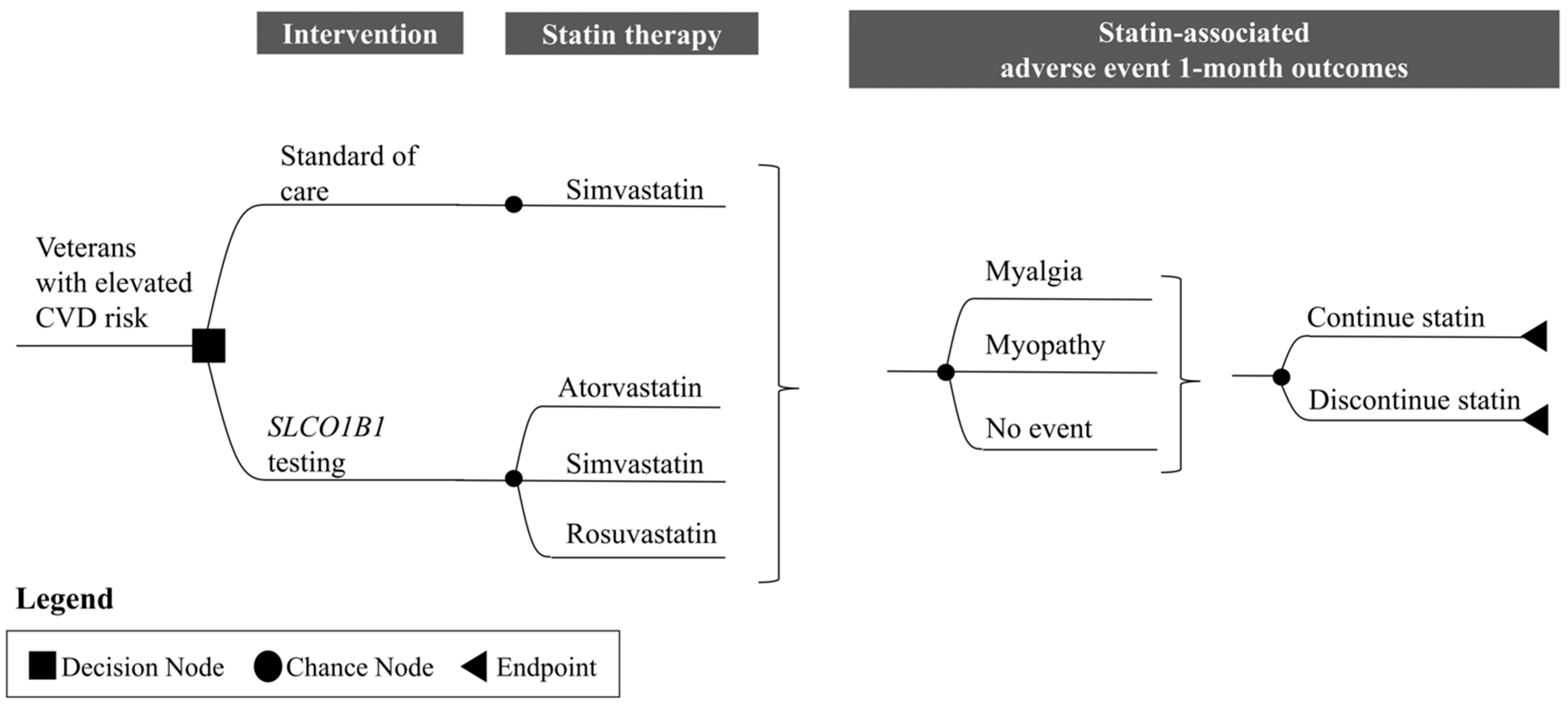
2.1. I-PICC Study Overview
2.2. Cost–Consequence Analysis
Overall Approach
2.3. VHA Clinical, Service Utilization, and Cost Data
2.4. Statistical Analysis
2.5. Scenario and Sensitivity Analyses
2.6. Projected Cost and Health Outcomes
3. Results
3.1. I-PICC Study Participant Characteristics
3.2. Clinical Outcomes in I-PICC Study Cohort
3.3. Health Care Service Utilization and Costs in I-PICC Study Cohort
3.4. Projected Cost and Health Outcomes in Hypothetical Veteran Patient Cohort
3.5. Scenario and Sensitivity Analyses for I-PICC Study Cohort
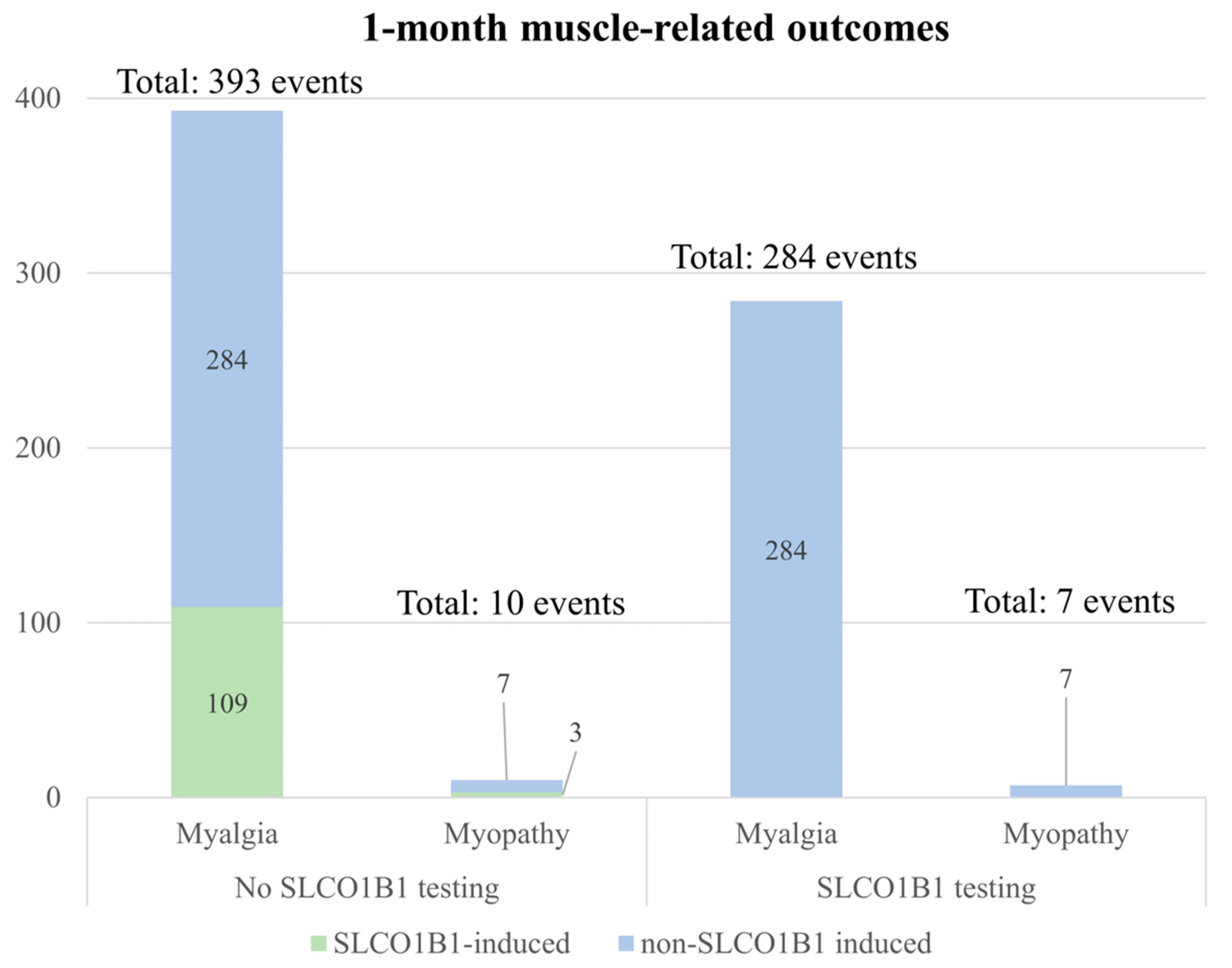
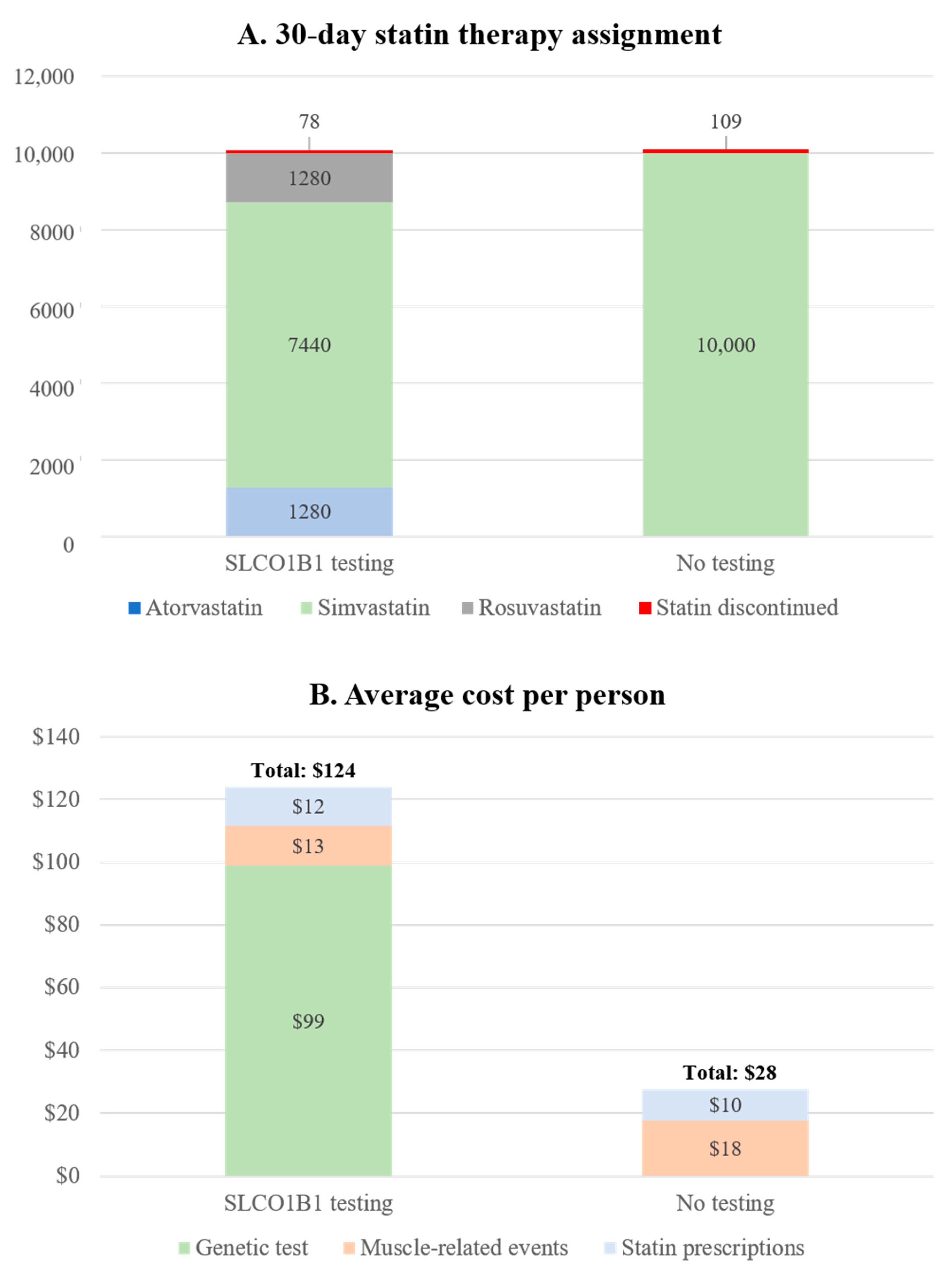
3.6. Scenario and Sensitivity Analysis for Projected Cost and Health Outcomes in Hypothetical Veteran Patient Cohort
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Relling, M.V.; Evans, W.E. Pharmacogenomics in the clinic. Nature 2015, 526, 343–350. [Google Scholar] [CrossRef] [Green Version]
- Roden, D.M.; McLeod, H.L.; Relling, M.V.; Williams, M.S.; Mensah, G.A.; Peterson, J.F.; Van Driest, S.L. Pharmacogenomics. Lancet 2019, 394, 521–532. [Google Scholar] [CrossRef]
- The Pharmacogenomics Knowledge Base (PharmGKB). Available online: https://www.pharmgkb.org/ (accessed on 3 December 2020).
- Volpi, S.; Bult, C.J.; Chisholm, R.L.; Deverka, P.A.; Ginsburg, G.S.; Jacob, H.J.; Kasapi, M.; McLeod, H.L.; Roden, D.M.; Williams, M.S.; et al. Research Directions in the Clinical Implementation of Pharmacogenomics: An Overview of US Programs and Projects. Clin. Pharm. Ther. 2018, 103, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Keeling, N.J.; Rosenthal, M.M.; West-Strum, D.; Patel, A.S.; Haidar, C.E.; Hoffman, J.M. Preemptive pharmacogenetic testing: Exploring the knowledge and perspectives of US payers. Genet. Med. 2019, 21, 1224–1232. [Google Scholar] [CrossRef] [Green Version]
- The SEARCH Collaborative Group. SLCO1B1 Variants and Statin-Induced Myopathy—A Genomewide Study. N. Engl. J. Med. 2008, 359, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Carr, D.F.; Francis, B.; Jorgensen, A.L.; Zhang, E.; Chinoy, H.; Heckbert, S.R.; Bis, J.C.; Brody, J.A.; Floyd, J.S.; Psaty, B.M.; et al. Genomewide Association Study of Statin-Induced Myopathy in Patients Recruited Using the UK Clinical Practice Research Datalink. Clin. Pharm. Ther. 2019, 106, 1353–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kee, P.S.; Chin, P.K.L.; Kennedy, M.A.; Maggo, S.D.S. Pharmacogenetics of Statin-Induced Myotoxicity. Front. Genet. 2020, 11, 575678. [Google Scholar] [CrossRef]
- Ramsey, L.B.; Johnson, S.G.; Caudle, K.E.; Haidar, C.E.; Voora, D.; Wilke, R.A.; Maxwell, W.D.; McLeod, H.L.; Krauss, R.M.; Roden, D.M.; et al. The Clinical Pharmacogenetics Implementation Consortium Guideline for SLCO1B1 and Simvastatin-Induced Myopathy: 2014 Update. Clin. Pharmacol. Ther. 2014, 96, 423–428. [Google Scholar] [CrossRef]
- Turongkaravee, S.; Jittikoon, J.; Lukkunaprasit, T.; Sangroongruangsri, S.; Chaikledkaew, U.; Thakkinstian, A. A systematic review and meta-analysis of genotype-based and individualized data analysis of SLCO1B1 gene and statin-induced myopathy. Pharm. J. 2021, 21, 296–307. [Google Scholar] [CrossRef]
- Lu, B.; Sun, L.; Seraydarian, M.; Hoffmann, T.J.; Medina, M.W.; Risch, N.; Iribarren, C.; Krauss, R.M.; Oni-Orisan, A. Effect of SLCO1B1 T521C on Statin-Related Myotoxicity With Use of Lovastatin and Atorvastatin. Clin. Pharm. Ther. 2021, 110, 733–740. [Google Scholar] [CrossRef]
- Hopewell, J.C.; Offer, A.; Haynes, R.; Bowman, L.; Li, J.; Chen, F.; Bulbulia, R.; Lathrop, M.; Baigent, C.; Landray, M.J.; et al. Independent risk factors for simvastatin-related myopathy and relevance to different types of muscle symptom. Eur. Heart J. 2020, 41, 3336–3342. [Google Scholar] [CrossRef]
- Chanfreau-Coffinier, C.; Hull, L.E.; Lynch, J.A.; DuVall, S.L.; Damrauer, S.M.; Cunningham, F.E.; Voight, B.F.; Matheny, M.E.; Oslin, D.W.; Icardi, M.S.; et al. Projected Prevalence of Actionable Pharmacogenetic Variants and Level A Drugs Prescribed Among US Veterans Health Administration Pharmacy Users. JAMA Netw. Open 2019, 2, e195345. [Google Scholar] [CrossRef] [PubMed]
- Armitage, J.; Baigent, C.; Barnes, E.; Betteridge, D.J.; Blackwell, L.; Blazing, M.; Bowman, L.; Braunwald, E.; Byington, R.; Cannon, C.; et al. Efficacy and safety of statin therapy in older people: A meta-analysis of individual participant data from 28 randomised controlled trials. Lancet 2019, 393, 407–415. [Google Scholar] [CrossRef] [Green Version]
- Kohli-Lynch, C.N.; Bellows, B.K.; Thanassoulis, G.; Zhang, Y.; Pletcher, M.J.; Vittinghoff, E.; Pencina, M.J.; Kazi, D.; Sniderman, A.D.; Moran, A.E. Cost-effectiveness of Low-density Lipoprotein Cholesterol Level-Guided Statin Treatment in Patients With Borderline Cardiovascular Risk. JAMA Cardiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Plutzky, J.; Skentzos, S.; Morrison, F.; Mar, P.; Shubina, M.; Turchin, A. Discontinuation of statins in routine care settings: A cohort study. Ann. Intern. Med. 2013, 158, 526–534. [Google Scholar] [CrossRef] [Green Version]
- Stroes, E.S.; Thompson, P.D.; Corsini, A.; Vladutiu, G.D.; Raal, F.J.; Ray, K.K.; Roden, M.; Stein, E.; Tokgozoglu, L.; Nordestgaard, B.G.; et al. Statin-associated muscle symptoms: Impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur. Heart J. 2015, 36, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Navar, A.M.; Peterson, E.D.; Li, S.; Robinson, J.G.; Roger, V.L.; Goldberg, A.C.; Virani, S.; Wilson, P.W.F.; Nanna, M.G.; Lee, L.V.; et al. Prevalence and Management of Symptoms Associated With Statin Therapy in Community Practice: Insights From the PALM (Patient and Provider Assessment of Lipid Management) Registry. Circ. Cardiovasc. Qual. Outcomes 2018, 11, e004249. [Google Scholar] [CrossRef] [PubMed]
- Bradley, C.K.; Wang, T.Y.; Li, S.; Robinson, J.G.; Roger, V.L.; Goldberg, A.C.; Virani, S.S.; Louie, M.J.; Lee, L.V.; Peterson, E.D.; et al. Patient-Reported Reasons for Declining or Discontinuing Statin Therapy: Insights From the PALM Registry. J. Am. Heart Assoc. 2019, 8, e011765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soran, H.; France, M.; Adam, S.; Iqbal, Z.; Ho, J.H.; Durrington, P.N. Quantitative evaluation of statin effectiveness versus intolerance and strategies for management of intolerance. Atherosclerosis 2020, 306, 33–40. [Google Scholar] [CrossRef]
- Graham, J.H.; Sanchez, R.J.; Saseen, J.J.; Mallya, U.G.; Panaccio, M.P.; Evans, M.A. Clinical and economic consequences of statin intolerance in the United States: Results from an integrated health system. J. Clin. Lipidol. 2017, 11, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Brunham, L.R.; Baker, S.; Mammen, A.; Mancini, G.B.J.; Rosenson, R.S. Role of genetics in the prediction of statin-associated muscle symptoms and optimization of statin use and adherence. Cardiovasc. Res. 2018, 114, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Peyser, B.; Perry, E.P.; Singh, K.; Gill, R.D.; Mehan, M.R.; Haga, S.B.; Musty, M.D.; Milazzo, N.A.; Savard, D.; Li, Y.J.; et al. Effects of Delivering SLCO1B1 Pharmacogenetic Information in Randomized Trial and Observational Settings. Circ. Genom. Precis. Med. 2018, 11, e002228. [Google Scholar] [CrossRef] [Green Version]
- Vassy, J.L.; Gaziano, J.M.; Green, R.C.; Ferguson, R.E.; Advani, S.; Miller, S.J.; Chun, S.; Hage, A.K.; Seo, S.-J.; Majahalme, N.; et al. Effect of Pharmacogenetic Testing for Statin Myopathy Risk vs Usual Care on Blood Cholesterol: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2027092. [Google Scholar] [CrossRef] [PubMed]
- Vassy, J.L.; Chun, S.; Advani, S.; Ludin, S.A.; Smith, J.G.; Alligood, E.C. Impact of SLCO1B1 Pharmacogenetic Testing on Patient and Healthcare Outcomes: A Systematic Review. Clin. Pharm. Ther. 2018, 106, 360–373. [Google Scholar] [CrossRef]
- Parthan, A.; Leahy, K.J.; O’Sullivan, A.K.; Iakoubova, O.A.; Bare, L.A.; Devlin, J.J.; Weinstein, M.C. Cost effectiveness of targeted high-dose atorvastatin therapy following genotype testing in patients with acute coronary syndrome. Pharmacoeconomics 2013, 31, 519–531. [Google Scholar] [CrossRef]
- Mitchell, D.; Guertin, J.R.; Iliza, A.C.; Fanton-Aita, F.; LeLorier, J. Economic Evaluation of a Pharmacogenomics Test for Statin-Induced Myopathy in Cardiovascular High-Risk Patients Initiating a Statin. Mol. Diagn. Ther. 2017, 21, 95–105. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, D.; Guertin, J.R.; Dubois, A.; Dube, M.P.; Tardif, J.C.; Iliza, A.C.; Fanton-Aita, F.; Matteau, A.; LeLorier, J. A Discrete Event Simulation Model to Assess the Economic Value of a Hypothetical Pharmacogenomics Test for Statin-Induced Myopathy in Patients Initiating a Statin in Secondary Cardiovascular Prevention. Mol. Diagn. Ther 2018, 22, 241–254. [Google Scholar] [CrossRef]
- Shi, Y.; Graves, J.A.; Garbett, S.P.; Zhou, Z.; Marathi, R.; Wang, X.; Harrell, F.E.; Lasko, T.A.; Denny, J.C.; Roden, D.M.; et al. A Decision-Theoretic Approach to Panel-Based, Preemptive Genotyping. MDM Policy Pract. 2019, 4. [Google Scholar] [CrossRef] [Green Version]
- Dong, O.M.; Wheeler, S.B.; Cruden, G.; Lee, C.R.; Voora, D.; Dusetzina, S.B.; Wiltshire, T. Cost-Effectiveness of Multigene Pharmacogenetic Testing in Patients With Acute Coronary Syndrome After Percutaneous Coronary Intervention. Value Health 2020, 23, 61–73. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Moriarty, J.P.; Swanson, K.M.; Takahashi, P.Y.; Bielinski, S.J.; Weinshilboum, R.; Wang, L.; Borah, B.J. A model-based cost-effectiveness analysis of pharmacogenomic panel testing in cardiovascular disease management: Preemptive, reactive, or none? Genet. Med. 2020, 23, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Swanson, K.M.; Rojas, R.L.; Wang, Z.; St Sauver, J.L.; Visscher, S.L.; Prokop, L.J.; Bielinski, S.J.; Wang, L.; Weinshilboum, R.; et al. Systematic review of the evidence on the cost-effectiveness of pharmacogenomics-guided treatment for cardiovascular diseases. Genet. Med. 2020, 22, 475–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vassy, J.L.; Brunette, C.A.; Majahalme, N.; Advani, S.; MacMullen, L.; Hau, C.; Zimolzak, A.J.; Miller, S.J. The Integrating Pharmacogenetics in Clinical Care (I-PICC) Study: Protocol for a point-of-care randomized controlled trial of statin pharmacogenetics in primary care. Contemp. Clin. Trials 2018, 75, 40–50. [Google Scholar] [CrossRef]
- Brunette, C.A.; Miller, S.J.; Majahalme, N.; Hau, C.; MacMullen, L.; Advani, S.; Ludin, S.A.; Zimolzak, A.J.; Vassy, J.L. Pragmatic Trials in Genomic Medicine: The Integrating Pharmacogenetics In Clinical Care (I-PICC) Study. Clin. Transl. Sci. 2020, 13, 381–390. [Google Scholar] [CrossRef]
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Bairey Merz, C.N.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Lloyd-Jones, D.M.; et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, S1–45. [Google Scholar] [CrossRef] [Green Version]
- Mauskopf, J.A.; Paul, J.E.; Grant, D.M.; Stergachis, A. The role of cost-consequence analysis in healthcare decision-making. Pharmacoeconomics 1998, 13, 277–288. [Google Scholar] [CrossRef]
- Drummond, M.; Sculpher, M.J.; Claxton, K.; Stoddart, G.L.; Torrance, G.W. Methods for the Economic Evaluation of Health Care Programmes, 4th ed.; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Neumann, P.J.; Ganiats, T.G.; Russell, L.B.; Sanders, G.D.; Siegel, J.E. Cost-Effectiveness in Health and Medicine, 2nd ed.; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Ramsey, S.D.; Willke, R.J.; Glick, H.; Reed, S.D.; Augustovski, F.; Jonsson, B.; Briggs, A.; Sullivan, S.D. Cost-Effectiveness Analysis Alongside Clinical Trials II—An ISPOR Good Research Practices Task Force Report. Value Health 2015, 18, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Sanders, G.D.; Neumann, P.J.; Basu, A.; Brock, D.W.; Feeny, D.; Krahn, M.; Kuntz, K.M.; Meltzer, D.O.; Owens, D.K.; Prosser, L.A.; et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA 2016, 316, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Neumann, P.J.; Sanders, G.D. Cost-Effectiveness Analysis 2.0. N. Engl. J. Med. 2017, 376, 203–205. [Google Scholar] [CrossRef] [Green Version]
- Health Economic Resource Center (HERC). Measuring Costs for Cost-Effectiveness Analysis. Available online: https://www.herc.research.va.gov/include/page.asp?id=measure-costs-cea (accessed on 20 January 2021).
- United States Bureau of Labor Statistics. Consumer Price Index for All Urban Consumers (CPI-U). Available online: https://data.bls.gov/cgi-bin/surveymost?cu (accessed on 31 December 2020).
- Husereau, D.; Drummond, M.; Petrou, S.; Carswell, C.; Moher, D.; Greenberg, D.; Augustovski, F.; Briggs, A.H.; Mauskopf, J.; Loder, E.; et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)—Explanation and elaboration: A report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health 2013, 16, 231–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, L.E.; Shea, K.; Gephart, S. The Veterans Affairs’s Corporate Data Warehouse: Uses and Implications for Nursing Research and Practice. Nurs. Adm. Q. 2015, 39, 311–318. [Google Scholar] [CrossRef]
- Barnett, P.G. Determination of VA health care costs. Med. Care Res. Rev. 2003, 60, 124S–141S. [Google Scholar] [CrossRef] [PubMed]
- Fortney, J.C.; Maciejewski, M.L.; Tripathi, S.P.; Deen, T.L.; Pyne, J.M. A budget impact analysis of telemedicine-based collaborative care for depression. Med. Care 2011, 49, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Phibbs, C.S.; Barnett, P.G.; Fan, A. Research Guide to the Managerial Cost Accounting National Cost Extracts; VA Paolo Alto, Health Economic Resource Center: Menlo Park, CA, USA, 2015. [Google Scholar]
- Pence, B.W.; Gaynes, B.N.; Thielman, N.M.; Heine, A.; Mugavero, M.J.; Turner, E.L.; Quinlivan, E.B. Balancing Contamination and Referral Bias in a Randomized Clinical Trial: An Application of Pseudo-Cluster Randomization. Am. J. Epidemiol. 2015, 182, 1039–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, K.-Y.; Zeger, S.L. Longitudinal Data Analysis Using Generalized Linear Models. Biometrika 1986, 73, 13. [Google Scholar] [CrossRef]
- Scott, L.Z.; Kung-Yee, L. Longitudinal Data Analysis for Discrete and Continuous Outcomes. Biometrics 1986, 42, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Teerenstra, S.; Moerbeek, M.; Melis, R.J.; Borm, G.F. A comparison of methods to analyse continuous data from pseudo cluster randomized trials. Stat. Med. 2007, 26, 4100–4115. [Google Scholar] [CrossRef]
- Stedman, M.R.; Gagnon, D.R.; Lew, R.A.; Solomon, D.H.; Brookhart, M.A. An evaluation of statistical approaches for analyzing physician-randomized quality improvement interventions. Contemp. Clin. Trials 2008, 29, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Chu, R.; Thabane, L.; Ma, J.; Holbrook, A.; Pullenayegum, E.; Devereaux, P.J. Comparing methods to estimate treatment effects on a continuous outcome in multicentre randomized controlled trials: A simulation study. BMC Med. Res. Methodol. 2011, 11, 21. [Google Scholar] [CrossRef]
- Kahan, B.C.; Harhay, M.O. Many multicenter trials had few events per center, requiring analysis via random-effects models or GEEs. J. Clin. Epidemiol. 2015, 68, 1504–1511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davison, A.C.; Hinkley, D.V. Bootstrap Methods and their Application; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Ren, S.; Lai, H.; Tong, W.; Aminzadeh, M.; Hou, X.; Lai, S. Nonparametric bootstrapping for hierarchical data. J. Appl. Stat. 2010, 37, 1487–1498. [Google Scholar] [CrossRef]
- Harden, J.J. Improving Statistical Inference with Clustered Data. Stat. Politics Policy 2012, 3. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; 4.0.2; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Halekoh, U.; Højsgaard, S.; Yan, J. The R Package geepack for Generalized Estimating Equations. J. Stat. Softw. 2006, 15, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Cary, V.J. Gee: Generalized Estimation Equation Solver. Ported to R by Thomas Lumley and Brian Ripley. 4.13-20; 2019; Available online: https://rdrr.io/cran/gee/ (accessed on 9 October 2021).
- Kuhn, M.; Chow, F.; Wickham, H. Rsample: General Resampling Infrastructure; 0.0.8; RStudio: Boston, MA, USA, 2020. [Google Scholar]
- Jacobs, J.C.; Barnett, P.G. Emergent Challenges in Determining Costs for Economic Evaluations. Pharmacoeconomics 2017, 35, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.; Reith, C.; Emberson, J.; Armitage, J.; Baigent, C.; Blackwell, L.; Blumenthal, R.; Danesh, J.; Smith, G.D.; DeMets, D.; et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016, 388, 2532–2561. [Google Scholar] [CrossRef] [Green Version]
- Newman, C.B.; Preiss, D.; Tobert Jonathan, A.; Jacobson Terry, A.; Page Robert, L.; Goldstein Larry, B.; Chin, C.; Tannock Lisa, R.; Miller, M.; Raghuveer, G.; et al. Statin Safety and Associated Adverse Events: A Scientific Statement From the American Heart Association. Arteriosc. Thromb. Vasc. Biol. 2019, 39, e38–e81. [Google Scholar] [CrossRef] [Green Version]
- Maciejewski, M.L.; Perkins, M.; Li, Y.F.; Chapko, M.; Fortney, J.C.; Liu, C.F. Utilization and expenditures of veterans obtaining primary care in community clinics and VA medical centers: An observational cohort study. BMC Health Serv. Res. 2007, 7, 56. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-F.; Chapko, M.; Bryson, C.L.; Burgess Jr, J.F.; Fortney, J.C.; Perkins, M.; Sharp, N.D.; Maciejewski, M.L. Use of Outpatient Care in Veterans Health Administration and Medicare among Veterans Receiving Primary Care in Community-Based and Hospital Outpatient Clinics. Health Serv. Res. 2010, 45, 1268–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, J.; Vanneman, M.E.; Dally, S.K.; Trivedi, A.N.; Phibbs, C.S. Use of Veterans Affairs and Medicaid Services for Dually Enrolled Veterans. Health Serv. Res. 2018, 53, 1539–1561. [Google Scholar] [CrossRef] [PubMed]
- Nichols, G.A.; Philip, S.; Reynolds, K.; Granowitz, C.B.; O’Keeffe-Rosetti, M.; Fazio, S. Comparison of Medical Care Utilization and Costs Among Patients With Statin-Controlled Low-Density Lipoprotein Cholesterol With Versus Without Hypertriglyceridemia. Am. J. Cardiol. 2018, 122, 1128–1132. [Google Scholar] [CrossRef] [Green Version]
- Christensen, K.D.; Vassy, J.L.; Phillips, K.A.; Blout, C.L.; Azzariti, D.R.; Lu, C.Y.; Robinson, J.O.; Lee, K.; Douglas, M.P.; Yeh, J.M.; et al. Short-term costs of integrating whole-genome sequencing into primary care and cardiology settings: A pilot randomized trial. Genet. Med. 2018, 20, 1544–1553. Available online: https://www.nature.com/articles/gim201835#supplementary-information (accessed on 22 February 2021). [CrossRef]
- The Management of Dyslipidemia for Cardiovascular Risk Reduction Work Group; Department of Veterans Affairs & Department of Defense. VA/DoD Clinical Practice Guideline for the Management of Dyslipidemia for Cardiovascular Risk Reduction (Version 3). Available online: https://www.healthquality.va.gov/guidelines/CD/lipids/VADoDDyslipidemiaCPG.pdf (accessed on 4 August 2020).
- The Management of Dyslipidemia for Cardiovascular Risk Reduction Work Group; Department of Veterans Affairs & Department of Defense. VA/DoD Clinical Practice Guideline for the Management of Dyslipidemia for Cardiovascular Risk Reduction (Version 4). Available online: https://www.healthquality.va.gov/guidelines/CD/lipids/VADoDDyslipidemiaCPG5087212020.pdf (accessed on 4 August 2020).
- Turner, R.M.; Pirmohamed, M. Statin-Related Myotoxicity: A Comprehensive Review of Pharmacokinetic, Pharmacogenomic and Muscle Components. J. Clin. Med. 2019, 9, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arca, M.; Pigna, G. Treating statin-intolerant patients. Diabetes Metab. Syndr. Obes. 2011, 4, 155–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saxon, D.R.; Eckel, R.H. Statin Intolerance: A Literature Review and Management Strategies. Prog. Cardiovasc. Dis. 2016, 59, 153–164. [Google Scholar] [CrossRef]
- Davis, J.W.; Weller, S.C. Intensity of statin therapy and muscle symptoms: A network meta-analysis of 153,000 patients. BMJ Open 2021, 11, e043714. [Google Scholar] [CrossRef] [PubMed]
- Tobert, J.A.; Newman, C.B. The nocebo effect in the context of statin intolerance. J. Clin. Lipidol. 2016, 10, 739–747. [Google Scholar] [CrossRef]
- Hope, H.F.; Binkley, G.M.; Fenton, S.; Kitas, G.D.; Verstappen, S.M.M.; Symmons, D.P.M. Systematic review of the predictors of statin adherence for the primary prevention of cardiovascular disease. PLoS ONE 2019, 14, e0201196. [Google Scholar] [CrossRef] [PubMed]
- Markovitz, A.A.; Holleman, R.G.; Hofer, T.P.; Kerr, E.A.; Klamerus, M.L.; Sussman, J.B. Effects of Guideline and Formulary Changes on Statin Prescribing in the Veterans Affairs. Health Serv. Res. 2017, 52, 1996–2017. [Google Scholar] [CrossRef] [PubMed]
- Al-Salameh, A.; Danchin, N.; Verstuyft, C.; Kotti, S.; Puymirat, E.; Ferrieres, J.; Schiele, F.; Coste, P.; Lemesle, G.; Cayla, G.; et al. Association between rs4149056 variant in SLCO1B1 and early discontinuation of statin after acute myocardial infarction. Pharmacogenomics 2020, 21, 163–172. [Google Scholar] [CrossRef]
- Lamoureux, F.; Duflot, T.; French Network of, P. Pharmacogenetics in cardiovascular diseases: State of the art and implementation-recommendations of the French National Network of Pharmacogenetics (RNPGx). Therapie 2017, 72, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Picard, N.; Boyer, J.C.; Etienne-Grimaldi, M.C.; Barin-Le Guellec, C.; Thomas, F.; Loriot, M.A.; French National Network of, P. Pharmacogenetics-based personalized therapy: Levels of evidence and recommendations from the French Network of Pharmacogenetics (RNPGx). Therapie 2017, 72, 185–192. [Google Scholar] [CrossRef]
- Weitzel, K.W.; Cavallari, L.H.; Lesko, L.J. Preemptive Panel-Based Pharmacogenetic Testing: The Time is Now. Pharm. Res. 2017, 34, 1551–1555. [Google Scholar] [CrossRef] [PubMed]
- Plumpton, C.O.; Pirmohamed, M.; Hughes, D.A. Cost-Effectiveness of Panel Tests for Multiple Pharmacogenes Associated with Adverse Drug Reactions: An Evaluation Framework. Clin. Pharm. Ther. 2019, 105, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
| PGx+ (n = 193) | PGx− (n = 215) | Total (n = 408) | |
|---|---|---|---|
| Age at enrollment, mean (SD), years | 64.2 (7.8) | 63.9 (7.7) | 64.1 (7.78) |
| Women, n (%) | 9 (4.7) | 16 (7.4) | 25 (6.1) |
| Non-white race, n (%) | 30 (15.5) | 26 (12.1) | 56 (13.7) |
| Hispanic ethnicity, n (%) | 2 (1.0) | 6 (2.8) | 8 (2.0) |
| Smoker, n (%) | 59 (30.1) | 78 (36.3) | 137 (33.6) |
| Baseline LDL-C, mean (SD), mg/dL | 106 (32.0) | 109 (28.0) | 108 (30.0) |
| Meeting ACC/AHA statin criteria *, n (%) | |||
| ASCVD | 52 (26.9) | 46 (21.4) | 98 (24.0) |
| LDL-C > 190 mg/dL | 5 (2.6) | 6 (2.8) | 11 (2.7) |
| Diabetes | 47 (24.4) | 51 (23.7) | 98 (24.0) |
| 10-year ASCVD risk ≥7.5% | 171 (88.6) | 196 (91.2) | 367 (90.0) |
| SLCO1B1 Genotype | |||
| Reduced function T/C or C/C genotype, n (%) | 45 (23.3) | 75 (34.9) | 120 (29.4) |
| Unadjusted Estimate | Adjusted Difference ^ | 95% CI | p | ||
|---|---|---|---|---|---|
| 12-month outcomes | PGx+ (n = 193) | PGx− (n = 215) | |||
| Lipid prescriptions, n (%) USD | |||||
| Offered statin | 65 (33.7) | 69 (32.1) | 1.9% | −8.4%, 11.8% | 0.687 |
| Prescribed statin ~ | 26 (13.5) | 24 (11.2) | 2.9% | −4.2%, 9.0% | 0.336 |
| Atorvastatin * | 19 (73.1) | 19 (79.2) | −3.4% | −27.2%, 20.4% | 0.779 |
| Rosuvastatin * | 0 | 3 (12.5) | −13.6% | −23.3%, −3.8% | 0.006 |
| Simvastatin * | 7 (26.9) | 2 (0.1) | 17.1% | −3.3%, 37.6% | 0.101 |
| Provider-documented SAMS among statin users, n (%) * | 2 (7.7) | 3 (12.5) | −5.5% | −22.6%, 11.7% | 0.533 |
| Statin discontinuations * | 3 (11.5) | 4 (16.7) | −3.7% | −23.2, 15.8% | 0.709 |
| Other lipid medications | 14 (7.3) | 12 (5.6) | 1.7% | −2.3%, 5.7% | 0.421 |
| Utilization, mean (SD) | |||||
| Inpatient stays | 0.4 (1.3) | 0.4 (1.4) | −0.1 | −0.3, 0.2 | 0.694 |
| Inpatient length of stay, days | 4.8 (21.4) | 4.5 (24.8) | 0.3 | −5.0, 4.9 | 0.892 |
| Outpatient encounters, days | 40.1 (31.0) | 38.9 (27.2) | 1.2 | −4.1, 6.7 | 0.654 |
| Primary care visits | 3.9 (3.7) | 4.7 (10.4) | −0.5 | −3.8, 0.4 | 0.659 |
| Cardiology visits | 0.6 (1.3) | 0.9 (1.9) | −0.2 | −0.6, 0.1 | 0.131 |
| Costs, mean (SD), US dollars | |||||
| Directly attributable costs | 5648 (3122) | 6407 (10,746) | −1004 | −2684, 1009 | 0.284 |
| SLCO1B1 PGx testing | 99 | 0 | 99 | − | |
| Lipid medications | 17 (74) | 8 (27) | 10 | −1, 23 | 0.140 |
| Statins | 6 (23) | 4 (19) | 2 | −2, 6 | 0.291 |
| Primary care | 2955 (2802) | 3400 (8950) | −445 | −1414, 450 | 0.394 |
| Cardiology | 316 (901) | 640 (2571) | −324 | −915, 34 | 0.431 |
| Imaging | 1243 (2998) | 1331 (3509) | −90 | −701, 666 | 0.786 |
| Laboratory | 1015 (1574) | 1027 (1386) | −11 | −331, 366 | 0.946 |
| SAMS-related care * | 3 (27) | 0 (0) | 3 | 0, 5 | 0.045 |
| Other outpatient services | 8748 (15,263) | 9381 (12,236) | −544 | −3314, 3292 | 0.719 |
| Physical inpatient stays | 6107 (27,836) | 4926 (18,887) | 880 | −4142, 6618 | 0.732 |
| Total costs | 20,497 (38,216) | 20,706 (30,769) | −52 | −6660, 8475 | 0.990 |
| Unadjusted Mean (SD), US Dollars | Adjusted Mean Difference and 95% CI ^ | p | ||
|---|---|---|---|---|
| PGx+ (n = 193) | PGx− (n = 215) | |||
| PGx, Statins, SAMS | 108 (39) | 4 (19) | 104 (97, 112) | 0.001 |
| PGx, Lipid Rx, SAMS | 119 (80) | 8 (27) | 110 (98, 123) | <0.001 |
| PGx, Lipid Rx, SAMS, Cardiology + | 434 (911) | 649 (2573) | −215 (−710, 133) | 0.307 |
| PGx, Lipid Rx, SAMS, Primary Care + | 3074 (2810) | 3409 (8952) | −335 (−1295, 518) | 0.585 |
| PGx, Lipid Rx, SAMS, Cardiology, Primary Care + | 3389 (3122) | 4048 (9378) | −659 (−1687, 389) | 0.243 |
| PGx, Lipid Rx, SAMS, Cardiology, Primary Care, Laboratory, Imaging | 5648 (5681) | 6407 (10,746) | −1004 (−2684, 1009) | 0.284 |
| Immediate Cost Mean Difference, 95% CI ^ | Attributable Cost Mean Difference, 95% CI ^ | Total Cost Mean Difference, 95% CI ^ | |
|---|---|---|---|
| Base case, USD | 110 (98, 123) *** | −1004 (−2684, 1009) | −52 (−6660, 8475) |
| Cost of PGx testing | |||
| No cost (−100%) | 13 (1, 25) * | −1092 (−2714, 879) | −154 (−6761, 8371) |
| Lower bound (−25%) | 85 (75, 97) ** | −1026 (−2633, 949) | −78 (−6685, 8449) |
| Upper bound (+25%) | 135 (123, 148) ** | −981 (−2576, 952) | −26 (−6634, 8501) |
| Cost of statin prescription | |||
| Lower bound (−50%) | 109 (98, 120) ** | −1004 (−2605, 1084) | −52 (−6659, 8475) |
| Upper bound (+200%) | 112 (98, 126) ** | −1003 (−2604, 1019) | −50 (−6661, 8475) |
| Statin users | |||
| T/C or C/C genotype USD ~ | 74 (29, 119) ** | 4377 (−5061, 13,815) | 3297 (−22,444, 29,039) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunette, C.A.; Dong, O.M.; Vassy, J.L.; Danowski, M.E.; Alexander, N.; Antwi, A.A.; Christensen, K.D. A Cost–Consequence Analysis of Preemptive SLCO1B1 Testing for Statin Myopathy Risk Compared to Usual Care. J. Pers. Med. 2021, 11, 1123. https://doi.org/10.3390/jpm11111123
Brunette CA, Dong OM, Vassy JL, Danowski ME, Alexander N, Antwi AA, Christensen KD. A Cost–Consequence Analysis of Preemptive SLCO1B1 Testing for Statin Myopathy Risk Compared to Usual Care. Journal of Personalized Medicine. 2021; 11(11):1123. https://doi.org/10.3390/jpm11111123
Chicago/Turabian StyleBrunette, Charles A., Olivia M. Dong, Jason L. Vassy, Morgan E. Danowski, Nicholas Alexander, Ashley A. Antwi, and Kurt D. Christensen. 2021. "A Cost–Consequence Analysis of Preemptive SLCO1B1 Testing for Statin Myopathy Risk Compared to Usual Care" Journal of Personalized Medicine 11, no. 11: 1123. https://doi.org/10.3390/jpm11111123
APA StyleBrunette, C. A., Dong, O. M., Vassy, J. L., Danowski, M. E., Alexander, N., Antwi, A. A., & Christensen, K. D. (2021). A Cost–Consequence Analysis of Preemptive SLCO1B1 Testing for Statin Myopathy Risk Compared to Usual Care. Journal of Personalized Medicine, 11(11), 1123. https://doi.org/10.3390/jpm11111123







