Pharmacogenetically Guided Escitalopram Treatment for Pediatric Anxiety Disorders: Protocol for a Double-Blind Randomized Trial
Abstract
:1. Introduction
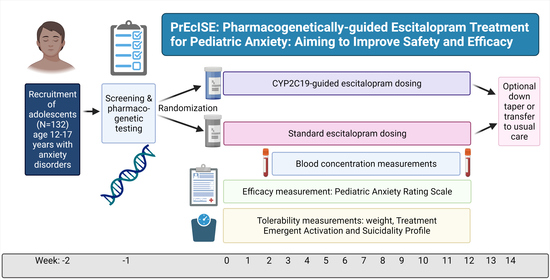
2. Study Protocol
2.1. Setting and Study Population
2.2. Randomization
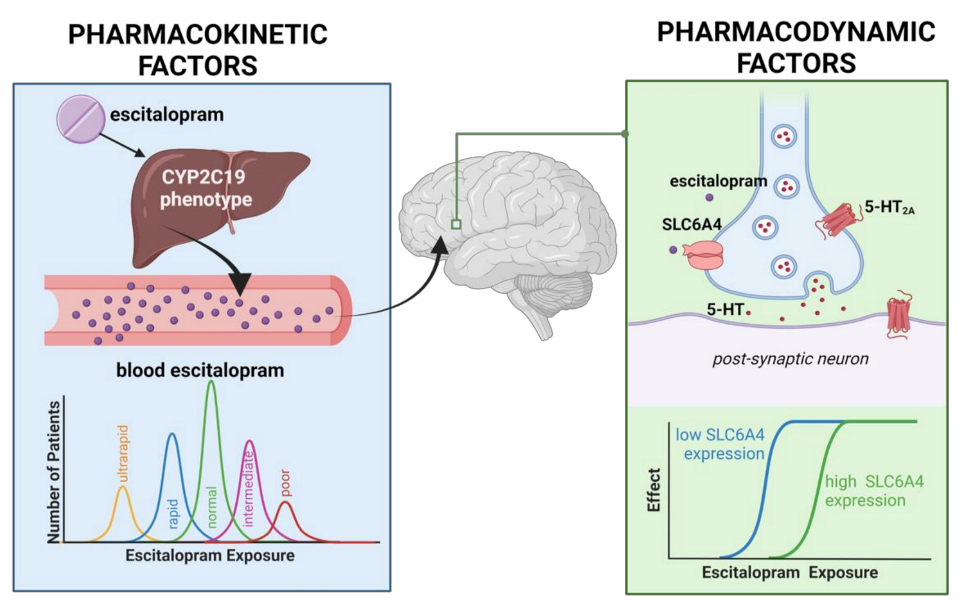
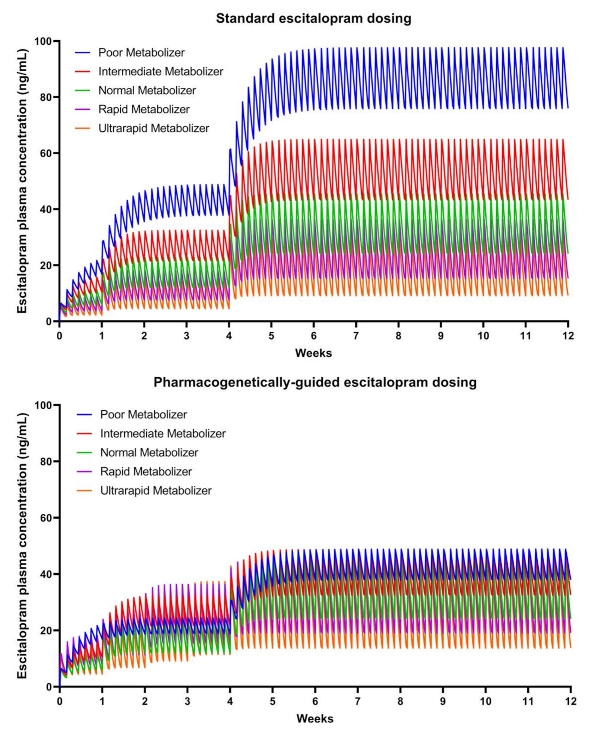
2.3. Medication and Dosing
2.4. Primary Outcome
2.5. Secondary Outcomes
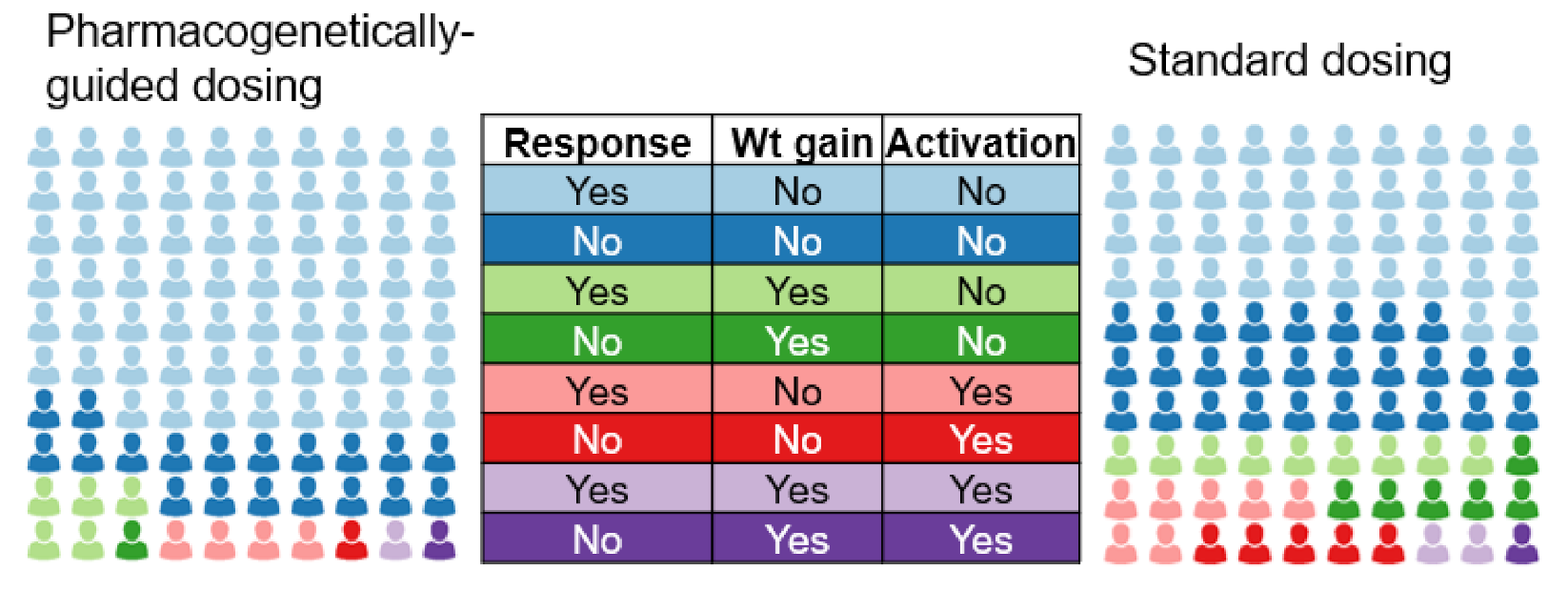
2.6. Hypotheses and Statistical Analyses
3. Impact of the Study
4. Ethics Statement
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Baxter, A.J.; Vos, T.; Scott, K.M.; Ferrari, A.J.; Whiteford, H.A. The global burden of anxiety disorders in 2010. Psychol. Med. 2014, 44, 2363–2374. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Berglund, P.; Demler, O.; Jin, R.; Walters, E.E. Lifetime prevalence and age-of-onset distributions’ of DSM-IV disorders in the national comorbidity survey replication. Arch. Gen. Psychiatry 2005, 62, 593–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costello, E.J.; Angold, A.; Burns, B.J.; Stangl, D.K.; Tweed, D.L.; Erkanli, A.; Worthman, C.M. The Great Smoky Mountains Study of Youth. Goals, design, methods, and the prevalence of DSM-III-R disorders. Arch. Gen. Psychiatry 1996, 53, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Asselmann, E.; Wittchen, H.-U.; Lieb, R.; Höfler, M.; Beesdo-Baum, K. Associations of fearful spells and panic attacks with incident anxiety, depressive, and substance use disorders: A 10-year prospective-longitudinal community study of adolescents and young adults. J. Psychiatr. Res. 2014, 55, 8–14. [Google Scholar] [CrossRef]
- Beesdo, K.; Bittner, A.; Pine, D.S.; Stein, M.B.; Höfler, M.; Lieb, R.; Wittchen, H.-U. Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Arch. Gen. Psychiatry 2007, 64, 903–912. [Google Scholar] [CrossRef] [Green Version]
- Connolly, S.D.; Bernstein, G.A. Practice parameter for the assessment and treatment of children and adolescents with anxiety disorders. J. Am. Acad. Child Adolesc. Psychiatry 2007, 46, 267–283. [Google Scholar] [CrossRef]
- Mohatt, J.; Bennett, S.M.; Walkup, J.T. Treatment of Separation, Generalized, and Social Anxiety Disorders in Youths. Am. J. Psychiatry 2014, 171, 741–748. [Google Scholar] [CrossRef]
- Strawn, J.R.; Geracioti, L.; Rajdev, N.; Clemenza, K.; Levine, A. Pharmacotherapy for generalized anxiety disorder in adult and pediatric patients: An evidence-based treatment review. Expert Opin. Pharmacother. 2018, 19, 1057–1070. [Google Scholar] [CrossRef]
- Strawn, J.R.; Mills, J.A.; Sauley, B.A.; Welge, J.A. The Impact of Antidepressant Dose and Class on Treatment Response in Pediatric Anxiety Disorders: A Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 2018, 57, 235–244. [Google Scholar] [CrossRef]
- Walkup, J.T.; Albano, A.M.; Piacentini, J.; Birmaher, B.; Compton, S.N.; Sherrill, J.T.; Ginsburg, G.S.; Rynn, M.A.; McCracken, J.; Waslick, B.; et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N. Engl. J. Med. 2008, 359, 2753–2766. [Google Scholar] [CrossRef]
- Aldrich, S.L.; Poweleit, E.A.; Prows, C.A.; Martin, L.J.; Strawn, J.R.; Ramsey, L.B. Influence of CYP2C19 Metabolizer Status on Escitalopram/Citalopram Tolerability and Response in Youth With Anxiety and Depressive Disorders. Front. Pharmacol. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Sakolsky, D.J.; Perel, J.M.; Emslie, G.J.; Clarke, G.N.; Wagner, K.D.; Vitiello, B.; Keller, M.B.; Birmaher, B.; Asarnow, J.R.; Ryan, N.D.; et al. Antidepressant exposure as a predictor of clinical outcomes in the Treatment of Resistant Depression in Adolescents (TORDIA) study. J. Clin. Psychopharmacol. 2011, 31, 92–97. [Google Scholar] [CrossRef]
- Olesen, O.V.; Linnet, K. Studies on the stereoselective metabolism of citalopram by human liver microsomes and cDNA-expressed cytochrome P450 enzymes. Pharmacology 1999, 59, 298–309. [Google Scholar] [CrossRef]
- Lloret-Linares, C.; Bosilkovska, M.; Daali, Y.; Gex-Fabry, M.; Heron, K.; Bancila, V.; Michalopoulos, G.; Perroud, N.; Richard-Lepouriel, H.; Aubry, J.M.; et al. Phenotypic assessment of drug metabolic pathways and P-glycoprotein in patients treated with antidepressants in an ambulatory setting. J. Clin. Psychiatry 2018, 79. [Google Scholar] [CrossRef]
- Hicks, J.K.; Bishop, J.R.; Sangkuhl, K.; Muller, D.J.; Ji, Y.; Leckband, S.G.; Leeder, J.S.; Graham, R.L.; Chiulli, D.L.; LLerena, A.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin. Pharmacol. Ther. 2015, 98, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Jukić, M.M.; Haslemo, T.; Molden, E.; Ingelman-Sundberg, M. Impact of CYP2C19 genotype on escitalopram exposure and therapeutic failure: A retrospective study based on 2,087 patients. Am. J. Psychiatry 2018, 175, 463–470. [Google Scholar] [CrossRef] [Green Version]
- Steere, B.; Baker, J.A.R.; Hall, S.D.; Guo, Y. Prediction of in vivo clearance and associated variability of CYP2C19 substrates by genotypes in populations utilizing a pharmacogenetics-based mechanistic model. Drug Metab. Dispos. 2015, 43, 870–883. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.; Schaid, D.J.; Desta, Z.; Kubo, M.; Batzler, A.J.; Snyder, K.; Mushiroda, T.; Kamatani, N.; Ogburn, E.; Hall-Flavin, D.; et al. Citalopram and escitalopram plasma drug and metabolite concentrations: Genome-wide associations. Br. J. Clin. Pharmacol. 2014, 78, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Uher, R.; Tansey, K.E.; Rietschel, M.; Henigsberg, N.; Maier, W.; Mors, O.; Hauser, J.; Placentino, A.; Souery, D.; Farmer, A.; et al. Common genetic variation and antidepressant efficacy in major depressive disorder: A meta-analysis of three genome-wide pharmacogenetic studies. Am. J. Psychiatry 2013, 170, 207–217. [Google Scholar] [CrossRef]
- Strawn, J.R.; Mills, J.A.; Schroeder, H.; Mossman, S.A.; Varney, S.T.; Ramsey, L.B.; Poweleit, E.A.; Desta, Z.; Cecil, K.M.; Delbello, M.P. Escitalopram in adolescents with generalized anxiety disorder. J. Clin. Psychiatry 2020, 81, e1–e9. [Google Scholar] [CrossRef]
- Cipriani, A.; Zhou, X.; Del Giovane, C.; Hetrick, S.E.; Qin, B.; Whittington, C.; Coghill, D.; Zhang, Y.; Hazell, P.; Leucht, S.; et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: A network meta-analysis. Lancet 2016, 388, 881–890. [Google Scholar] [CrossRef]
- Tulisiak, A.K.; Klein, J.A.; Harris, E.; Luft, M.J.; Schroeder, H.K.; Mossman, S.A.; Varney, S.T.; Keeshin, B.R.; Cotton, S.; Strawn, J.R. Antidepressant Prescribing by Pediatricians: A Mixed-Methods Analysis. Curr. Probl. Pediatr. Adolesc. Health Care 2017, 47, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Reinblatt, S.P.; Dosreis, S.; Walkup, J.T.; Riddle, M.A. Activation Adverse Events Induced by the Selective Serotonin Reuptake Inhibitor Fluvoxamine in Children and Adolescents. J. Child Adolesc. Psychopharmacol. 2009, 19, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Safer, D.J.; Zito, J.M. Treatment-Emergent Adverse Events from Selective Serotonin Reuptake Inhibitors by Age Group: Children versus Adolescents. J. Child Adolesc. Psychopharmacol. 2006, 16, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Safer, D.J. Raising the minimum effective dose of serotonin reuptake inhibitor antidepressants. J. Clin. Psychopharmacol. 2016, 36, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, K.; Tansey, K.E.; Uher, R.; Dernovšek, M.Z.; Mors, O.; Hauser, J.; Souery, D.; Maier, W.; Henigsberg, N.; Rietschel, M.; et al. Exploring the role of drug-metabolising enzymes in antidepressant side effects. Psychopharmacology 2015, 232, 2609–2617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tibaldi, G.; Munizza, C.; Bollini, P.; Pampallona, S.; Kupelnick, B. Effectiveness of antidepressants. Meta-analysis of dose-effect relationships in randomised clinical trials. Br. J. Psychiatry 1999, 174, 297–303. [Google Scholar] [CrossRef]
- Rynn, M.A.; Walkup, J.T.; Compton, S.N.; Sakolsky, D.J.; Sherrill, J.T.; Shen, S.; Kendall, P.C.; McCracken, J.; Albano, A.M.; Piacentini, J.; et al. Child/Adolescent anxiety multimodal study: Evaluating safety. J. Am. Acad. Child Adolesc. Psychiatry 2015, 54, 180–190. [Google Scholar] [CrossRef] [Green Version]
- Luft, M.J.; Lamy, M.; DelBello, M.P.; McNamara, R.K.; Strawn, J.R. Antidepressant-Induced Activation in Children and Adolescents: Risk, Recognition and Management. Curr. Probl. Pediatr. Adolesc. Health Care 2018, 48, 50–62. [Google Scholar] [CrossRef]
- Zuckerman, M.L.; Vaughan, B.L.; Whitney, J.; Dodds, A.; Yakhkind, A.; MacMillan, C.; Raches, D.; Pravdova, I.; DeMaso, D.R.; Beardslee, W.R.; et al. Tolerability of selective serotonin reuptake inhibitors in thirty-nine children under age seven: A retrospective chart review. J. Child Adolesc. Psychopharmacol. 2007, 17, 165–174. [Google Scholar] [CrossRef]
- Reid, A.M.; McNamara, J.P.H.; Murphy, T.K.; Guzick, A.G.; Storch, E.A.; Geffken, G.R.; Bussing, R. Side-effects of SSRIs disrupt multimodal treatment for pediatric OCD in a randomized-controlled trial. J. Psychiatr. Res. 2015, 71, 140–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilens, T.E.; Biederman, J.; Kwon, A.; Chase, R.; Greenberg, L.; Mick, E.; Spencer, T.J.; Wilens, T.E.; Biederman, J.; Kwon, A.; et al. A systematic chart review of the nature of psychiatric adverse events in children and adolescents treated with selective serotonin reuptake inhibitors. J. Child Adolesc. Psychopharmacol. 2003, 13, 143–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calarge, C.A.; Mills, J.A.; Janz, K.F.; Burns, T.L.; Coryell, W.H.; Zemel, B.S. Body Composition in Adolescents During Treatment With Selective Serotonin Reuptake Inhibitors. Pediatrics 2017, 140, e20163943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramsey, L.B.; Aldrich, S.L.; Poweleit, E.; Prows, C.A.; Martin, L.J.; Strawn, J.R. Racial Differences in Escitalopram/Citalopram-Related Weight Gain in Children and Adolescents: A Natural Language Processing-Based Electronic Medical Record Study. J. Child Adolesc. Psychopharmacol. 2019, 29, 162–163. [Google Scholar] [CrossRef]
- Isolan, L.; Pheula, G.; Salum, G.A., Jr.; Oswald, S.; Rohde, L.A.; Manfro, G.G. An open-label trial of escitalopram in children and adolescents with social anxiety disorder. J Child Adolesc Psychopharmacol 2007, 17, 751–760. [Google Scholar] [CrossRef]
- Emslie, G.J.; Ventura, D.; Korotzer, A.; Tourkodimitris, S. Escitalopram in the treatment of adolescent depression: A randomized placebo-controlled multisite trial. J. Am. Acad. Child Adolesc. Psychiatry 2009, 48, 721–729. [Google Scholar] [CrossRef] [Green Version]
- Green, D.J.; Mummaneni, P.; Kim, I.W.; Oh, J.M.; Pacanowski, M.; Burckart, G.J. Pharmacogenomics in the assessment of therapeutic risks versus benefits: Inside the United States Food and Drug Administration. Clin. Pharmacol. Ther. 2016, 99, 622–632. [Google Scholar] [CrossRef]
- Maruf, A.A.; Greenslade, A.; Arnold, P.D.; Bousman, C. Antidepressant pharmacogenetics in children and young adults: A systematic review. J. Affect. Disord. 2019, 254, 98–108. [Google Scholar] [CrossRef]
- Porcelli, S.; Fabbri, C.; Serretti, A. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with antidepressant efficacy. Eur. Neuropsychopharmacol. 2012, 22, 239–258. [Google Scholar] [CrossRef]
- Huezo-Diaz, P.; Uher, R.; Smith, R.; Rietschel, M.; Henigsberg, N.; Marušič, A.; Mors, O.; Maier, W.; Hauser, J.; Souery, D.; et al. Moderation of antidepressant response by the serotonin transporter gene. Br. J. Psychiatry 2009, 195, 30–38. [Google Scholar] [CrossRef] [Green Version]
- Tomita, T.; Yasui-Furukori, N.; Nakagami, T.; Tsuchimine, S.; Ishioka, M.; Kaneda, A.; Sugawara, N.; Kaneko, S. The influence of 5-HTTLPR genotype on the association between the plasma concentration and therapeutic effect of paroxetine in patients with major depressive disorder. PLoS ONE 2014, 9, 1–6. [Google Scholar] [CrossRef]
- Odgerel, Z.; Talati, A.; Hamilton, S.P.; Levinson, D.F.; Weissman, M.M. Genotyping serotonin transporter polymorphisms 5-HTTLPR and rs25531 in European- and African-American subjects from the National Institute of Mental Health’s Collaborative Center for Genomic Studies. Transl. Psychiatry 2013, 3, e307-6. [Google Scholar] [CrossRef]
- Hu, X.-Z.; Lipsky, R.H.; Zhu, G.; Akhtar, L.A.; Taubman, J.; Greenberg, B.D.; Xu, K.; Arnold, P.D.; Richter, M.A.; Kennedy, J.L.; et al. Serotonin Transporter Promoter Gain-of-Function Genotypes Are Linked to Obsessive-Compulsive Disorder. Am. J. Hum. Genet. 2006, 78, 815–826. [Google Scholar] [CrossRef] [Green Version]
- Mrazek, D.A.; Rush, A.J.; Biernacka, J.M.; O’Kane, D.J.; Cunningham, J.M.; Wieben, E.D.; Schaid, D.J.; Drews, M.S.; Courson, V.L.; Snyder, K.A.; et al. SLC6A4 variation and citalopram response. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2009, 150, 341–351. [Google Scholar] [CrossRef] [Green Version]
- Biernacka, J.M.; Sangkuhl, K.; Jenkins, G.; Whaley, R.M.; Barman, P.; Batzler, A.; Altman, R.B.; Arolt, V.; Brockmöller, J.; Chen, C.H.; et al. The International SSRI Pharmacogenomics Consortium (ISPC): A genome-wide association study of antidepressant treatment response. Transl. Psychiatry 2015, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Horstmann, S.; Lucae, S.; Menke, A.; Hennings, J.M.; Ising, M.; Roeske, D.; Müller-Myhsok, B.; Holsboer, F.; Binder, E.B. Polymorphisms in GRIK4, HTR2A, and FKBP5 show interactive effects in predicting remission to antidepressant treatment. Neuropsychopharmacology 2010, 35, 727–740. [Google Scholar] [CrossRef] [Green Version]
- Niitsu, T.; Fabbri, C.; Bentini, F.; Serretti, A. Pharmacogenetics in major depression: A comprehensive meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 45, 183–194. [Google Scholar] [CrossRef]
- Keeling, N.J.; Rosenthal, M.M.; West-Strum, D.; Patel, A.S.; Haidar, C.E.; Hoffman, J.M. Preemptive pharmacogenetic testing: Exploring the knowledge and perspectives of US payers. Genet. Med. 2019, 21, 1224–1232. [Google Scholar] [CrossRef] [Green Version]
- Czaja, A.S.; Valuck, R.J.; Anderson, H.D. Comparative safety of selective serotonin reuptake inhibitors among pediatric users with respect to adverse cardiac events. Pharmacoepidemiol. Drug Saf. 2013, 22, 607–614. [Google Scholar] [CrossRef]
- Leeder, J.S.; Brown, J.T.; Soden, S.E. Individualizing the use of medications in children: Making goldilocks happy. Clin. Pharmacol. Ther. 2014, 96, 304–306. [Google Scholar] [CrossRef]
- Compton, S.N.; Walkup, J.T.; Albano, A.M.; Piacentini, J.C.; Birmaher, B.; Sherrill, J.T.; Ginsburg, G.S.; Rynn, M.A.; McCracken, J.T.; Waslick, B.D.; et al. Child/Adolescent Anxiety Multimodal Study (CAMS): Rationale, design, and methods. Child Adolesc. Psychiatry Ment. Health 2010, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheehan, D.V.; Sheehan, K.H.; Shytle, R.D.; Janavs, J.; Bannon, Y.; Rogers, J.E.; Milo, K.M.; Stock, S.L.; Wilkinson, B. Reliability and validity of the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID). J. Clin. Psychiatry 2010, 71, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Caudle, K.E.; Dunnenberger, H.M.; Freimuth, R.R.; Peterson, J.F.; Burlison, J.D.; Whirl-Carrillo, M.; Scott, S.A.; Rehm, H.L.; Williams, M.S.; Klein, T.E.; et al. Standardizing terms for clinical pharmacogenetic test results: Consensus terms from the Clinical Pharmacogenetics Implementation Consortium (CPIC). Genet. Med. 2017, 19, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Strawn, J.R.; Lu, L.; Peris, T.S.; Levine, A.; Walkup, J.T. Research Review: Peadiatric Anxiety Disorders: What have we learnt in the last 10 years? J. Child Psychol. Psychiatry 2020. [Google Scholar] [CrossRef]
- Periclou, A.; Rao, N.; Sherman, T.; Ventura, D.; Abramowitz, W. Single-dose pharmacokinetic study of escitalopram in adolescents and adults. In Proceedings of the Annual Meeting of the American College of Clinical Pharmacy, Atlanta, GA, USA, 2–5 November 2003. [Google Scholar]
- Chang, M.; Tybring, G.; Dahl, M.L.; Lindh, J.D. Impact of Cytochrome P450 2C19 Polymorphisms on Citalopram/Escitalopram Exposure: A Systematic Review and Meta-Analysis. Clin. Pharmacokinet. 2014, 53, 801–811. [Google Scholar] [CrossRef] [Green Version]
- Strawn, J.R.; Poweleit, E.A.; Ramsey, L.B. CYP2C19-Guided Escitalopram and Sertraline Dosing in Pediatric Patients: A Pharmacokinetic Modeling Study. J. Child Adolesc. Psychopharmacol. 2019, 29, 340–347. [Google Scholar] [CrossRef] [Green Version]
- Strawn, J.R.; Poweleit, E.A.; Uppugunduri, C.R.S.; Ramsey, L.B. Pediatric Therapeutic Drug Monitoring for Selective Serotonin Reuptake Inhibitors. Front. Pharmacol. 2021, 12, 1–9. [Google Scholar] [CrossRef]
- Riddle, M.A.; Ginsburg, G.S.; Walkup, J.T.; Labelarte, M.J.; Pine, D.S.; Davies, M.; Greenhill, L.; Sweeney, M.; Klein, R.; Abikoff, H.; et al. The Pediatric Anxiety Rating Scale (PARS): Development and psychometric properties. J. Am. Acad. Child Adolesc. Psychiatry 2002, 41, 1061–1069. [Google Scholar]
- Guy, W. CGI Clinical Global Impressions. In ECDEU Assessment Manual; U.S. Department of Health, Education, and Welfare: Rockville, MD, USA, 1976; pp. 217–222. [Google Scholar]
- Rynn, M.A.; Riddle, M.A.; Yeung, P.P.; Kunz, N.R. Efficacy and safety of extended-release venlafaxine in the treatment of generalized anxiety disorder in children and adolescents: Two placebo-controlled trials. Am. J. Psychiatry 2007, 164, 290–300. [Google Scholar] [CrossRef]
- Fluvoxamine for the Treatment of Anxiety Disorders in Children and Adolescents. The Research Unit on Pediatric Psychopharmacology Anxiety Study Group. N. Engl. J. Med. 2001, 344, 1279–1285. [Google Scholar] [CrossRef]
- Strawn, J.R.; Prakash, A.; Zhang, Q.; Pangallo, B.A.; Stroud, C.E.; Cai, N.; Findling, R.L. A randomized, placebo-controlled study of duloxetine for the treatment of children and adolescents with generalized anxiety disorder. J. Am. Acad. Child Adolesc. Psychiatry 2015, 54, 283–293. [Google Scholar] [CrossRef]
- Strawn, J.R.; Compton, S.N.; Robertson, B.; Albano, A.M.; Hamdani, M.; Rynn, M.A. Extended Release Guanfacine in Pediatric Anxiety Disorders: A Pilot, Randomized, Placebo-Controlled Trial. J. Child Adolesc. Psychopharmacol. 2017, 27, 29–37. [Google Scholar] [CrossRef]
- Caporino, N.E.; Sakolsky, D.; Brodman, D.M.; McGuire, J.F.; Piacentini, J.; Peris, T.S.; Ginsburg, G.S.; Walkup, J.T.; Iyengar, S.; Kendall, P.C.; et al. Establishing Clinical Cutoffs for Response and Remission on the Screen for Child Anxiety Related Emotional Disorders (SCARED). J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 696–702. [Google Scholar] [CrossRef]
- Caporino, N.E.; Brodman, D.M.; Kendall, P.C.; Albano, A.M.; Sherrill, J.; Piacentini, J.; Sakolsky, D.; Birmaher, B.; Compton, S.N.; Ginsburg, G.; et al. Defining Treatment Response and Remission in Child Anxiety: Signal Detection Analysis Using the Pediatric Anxiety Rating Scale. J. Am. Acad. Child Adolesc. Psychiatry 2013, 52, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Bussing, R.; Murphy, T.K.; Storch, E.A.; McNamara, J.P.H.; Reid, A.M.; Garvan, C.W.; Goodman, W.K. Psychometric properties of the Treatment-Emergent Activation and Suicidality Assessment Profile (TEASAP) in youth with OCD. Psychiatry Res. 2013, 205, 253–261. [Google Scholar] [CrossRef] [Green Version]
- Posner, K.; Brown, G.K.; Stanley, B.; Brent, D.A.; Yershova, K.V.; Oquendo, M.A.; Currier, G.W.; Melvin, G.A.; Greenhill, L.; Shen, S.; et al. The Columbia-Suicide Severity Rating Scale: Initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am. J. Psychiatry 2011, 168, 1266–1277. [Google Scholar] [CrossRef] [Green Version]
- Findling, R.L.; McCusker, E.; Strawn, J.R. A Randomized, Double-Blind, Placebo-Controlled Trial of Vilazodone in Children and Adolescents with Major Depressive Disorder with Twenty-Six-Week Open-Label Follow-Up. J. Child Adolesc. Psychopharmacol. 2020, 30, 355–365. [Google Scholar] [CrossRef]
- Strawn, J.R.; Welge, J.A.; Wehry, A.M.; Keeshin, B.; Rynn, M.A. Efficacy and tolerability of antidepressants in pediatric anxiety disorders: A systematic review and meta-analysis. Depress. Anxiety 2015, 32, 149–157. [Google Scholar] [CrossRef]
- Dobson, E.T.; Bloch, M.H.; Strawn, J.R. Efficacy and tolerability of pharmacotherapy in pediatric anxiety disorders: A network meta-analysis. J. Clin. Psychiatry 2019, 80, 17r12064. [Google Scholar] [CrossRef]
- Mansoor, B.; Rengasamy, M.; Hilton, R.; Porta, G.; He, J.; Spirito, A.; Emslie, G.J.; Mayes, T.L.; Clarke, G.; Wagner, K.D.; et al. The Bidirectional Relationship Between Body Mass Index and Treatment Outcome in Adolescents with Treatment-Resistant Depression. J. Child Adolesc. Psychopharmacol. 2013, 23, 458–467. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strawn, J.R.; Poweleit, E.A.; Mills, J.A.; Schroeder, H.K.; Neptune, Z.A.; Specht, A.M.; Farrow, J.E.; Zhang, X.; Martin, L.J.; Ramsey, L.B. Pharmacogenetically Guided Escitalopram Treatment for Pediatric Anxiety Disorders: Protocol for a Double-Blind Randomized Trial. J. Pers. Med. 2021, 11, 1188. https://doi.org/10.3390/jpm11111188
Strawn JR, Poweleit EA, Mills JA, Schroeder HK, Neptune ZA, Specht AM, Farrow JE, Zhang X, Martin LJ, Ramsey LB. Pharmacogenetically Guided Escitalopram Treatment for Pediatric Anxiety Disorders: Protocol for a Double-Blind Randomized Trial. Journal of Personalized Medicine. 2021; 11(11):1188. https://doi.org/10.3390/jpm11111188
Chicago/Turabian StyleStrawn, Jeffrey R., Ethan A. Poweleit, Jeffrey A. Mills, Heidi K. Schroeder, Zoe A. Neptune, Ashley M. Specht, Jenni E. Farrow, Xue Zhang, Lisa J. Martin, and Laura B. Ramsey. 2021. "Pharmacogenetically Guided Escitalopram Treatment for Pediatric Anxiety Disorders: Protocol for a Double-Blind Randomized Trial" Journal of Personalized Medicine 11, no. 11: 1188. https://doi.org/10.3390/jpm11111188
APA StyleStrawn, J. R., Poweleit, E. A., Mills, J. A., Schroeder, H. K., Neptune, Z. A., Specht, A. M., Farrow, J. E., Zhang, X., Martin, L. J., & Ramsey, L. B. (2021). Pharmacogenetically Guided Escitalopram Treatment for Pediatric Anxiety Disorders: Protocol for a Double-Blind Randomized Trial. Journal of Personalized Medicine, 11(11), 1188. https://doi.org/10.3390/jpm11111188