Results of the Second Phase of the GER-e-TEC Experiment concerning the Telemonitoring of Elderly Patients Affected by COVID-19 Disease to Detect the Exacerbation of Geriatric Syndromes
Abstract
:1. Introduction
2. Patients and Method
2.1. Objective
2.2. Patients
2.3. Study Outline
3. Experimental Protocol
4. The Remote Monitoring Platform
5. Parameters Evaluated and Statistical Analyses
6. Administrative Requirements
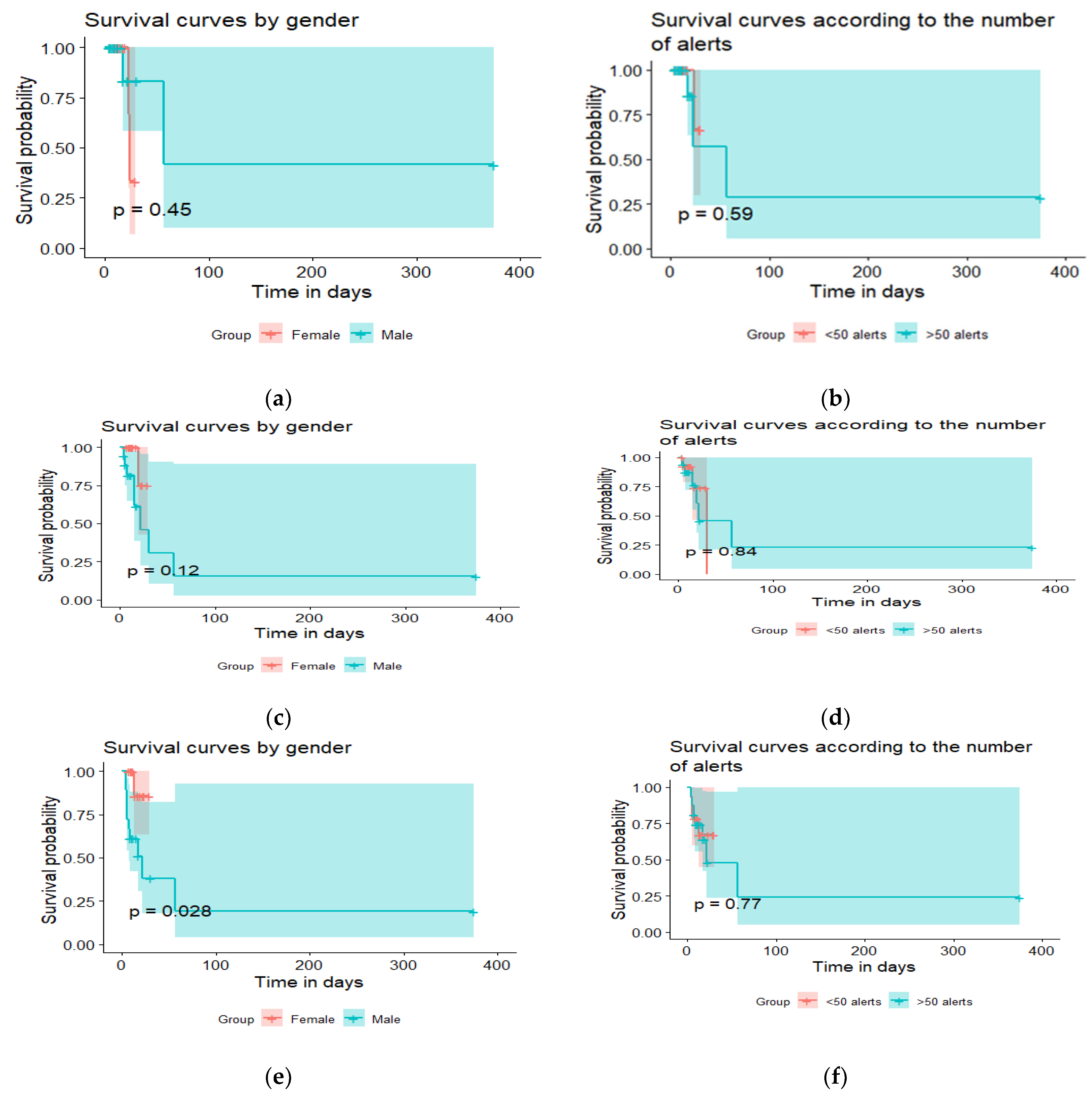
7. Results
Characteristics of Patients
8. Data from the Sensors/Questionnaires
9. Number of Alerts for Geriatric Syndromes/Chronic Diseases
10. Clinical Relevance of Alerts
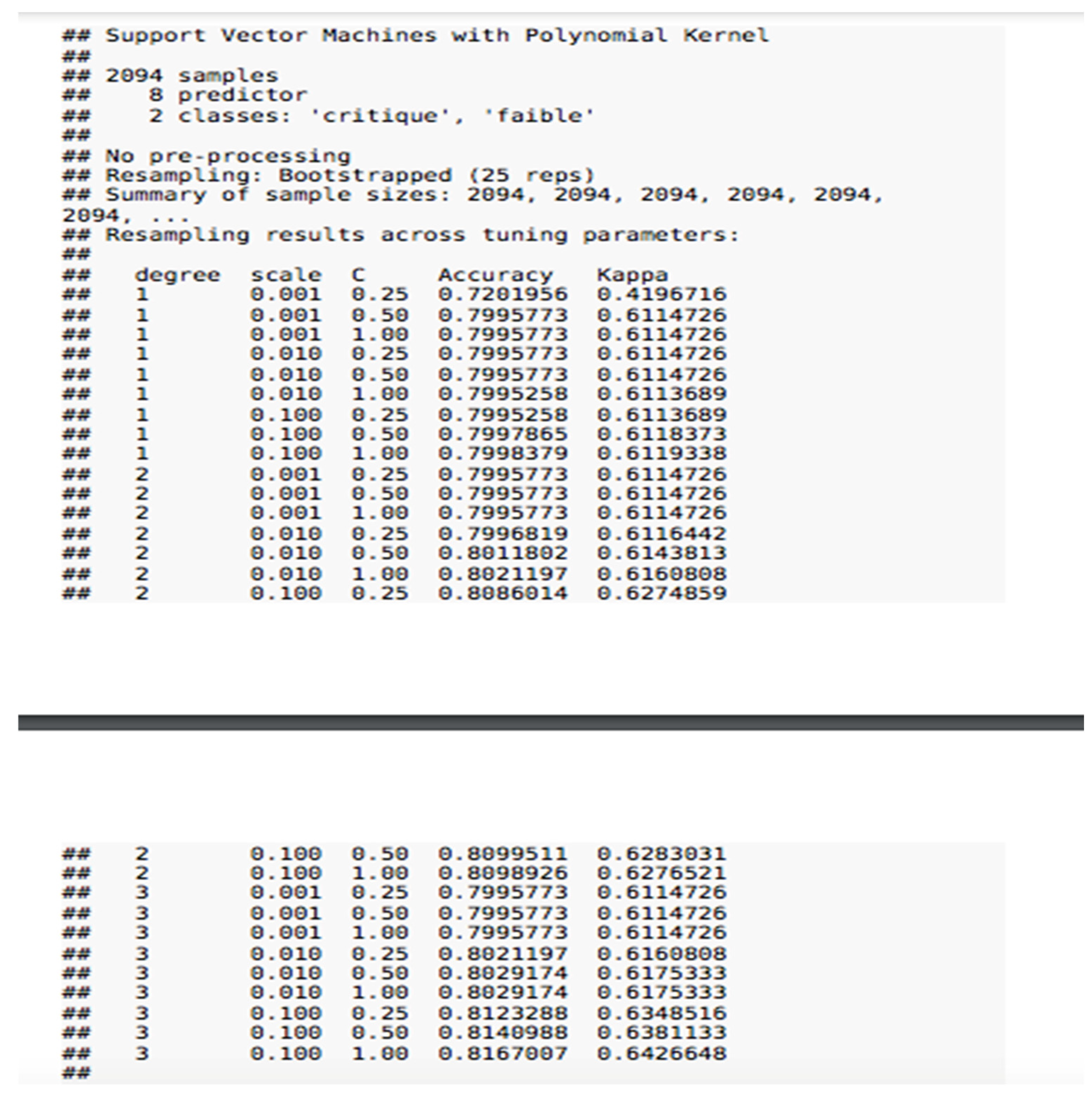
11. Multivariate Analysis and Alert Prediction
11.1. Logistic Regression
- .
- Accuracy88.7% of alerts are well classified.
- .
- errorThe model does not correctly predict critical or urgent alerts in 11.3% of cases.
- .
- SensitivityOf the predicted critical or urgent alerts, 98.7% are observed in the sample.
- .
- SpecificityOf the predicted low or moderate alerts, 75.4% are observed in the sample.
- .
- Positive predictive valueOf the critical or urgent alerts observed, 84.1% are predicted by the model.
- .
- Negative predictive valueOf the low or moderate alerts observed, 97.8% are predicted by the model.
11.2. SVM Modeling
- .
- Accuracy82.8% of alerts are well classified. We therefore have a lower accuracy rate than with logistic regression.
- .
- errorThe model does not correctly predict critical or urgent alerts in 17.2% of cases.
- .
- SensitivityOf the predicted critical or urgent alerts, 97% are observed in the sample.
- .
- SpecificityOf the predicted low or moderate alerts, 70.2% are observed in the sample.
- .
- Positive predictive valueOf the critical or urgent alerts observed, 74.4% are predicted by the model.
- .
- Negative predictive valueOf the low or moderate alerts observed, 96.3% are predicted by the model.
12. Discussion
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. China Novel Coronavirus Investigating and Research Team. A Nov-el Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Kaeuffer, C.; Le Hyaric, C.; Fabacher, T.; Mootien, J.; Dervieux, B.; Ruch, Y.; Hugerot, A.; Zhu, Y.-J.; Pointurier, V.; Clere-Jehl, R.; et al. Clinical characteristics and risk factors associated with severe COVID-19: Prospective analysis of 1,045 hospitalised cases in North-Eastern France, March 2020. Eurosurveillance 2020, 25, 2000895. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.; et al. China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19. March 2020. Available online: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19 (accessed on 11 March 2020).
- Weinstein, R.S.; Lopez, A.M.; Joseph, B.A.; Erps, K.A.; Holcomb, M.; Barker, G.P.; Krupinski, E.A. Telemedicine, telehealth, and mobile health applications that work: Opportunities and barriers. Am. J. Med. 2014, 127, 183–187. [Google Scholar] [CrossRef]
- Zulfiqar, A.-A.; Lorenzo-Villalba, N.; Zulfiqar, O.-A.; Hajjam, M.; Courbon, Q.; Esteoulle, L.; Geny, B.; Talha, S.; Letourneau, D.; Hajjam, J.; et al. e-Health: A Future Solution for Optimized Management of Elderly Patients. GER-e-TEC™ Project. Medicines 2020, 7, 41. [Google Scholar] [CrossRef]
- Zulfiqar, A.-A.; Vaudelle, O.; Hajjam, M.; Geny, B.; Talha, S.; Letourneau, D.; Hajjam, J.; Erve, S.; El Hassani, A.H.; Andrès, E. Results of the “GER-e-TEC” Experiment Involving the Use of an Automated Platform to Detect the Exacerbation of Geriatric Syndromes. J. Clin. Med. 2020, 9, 3836. [Google Scholar] [CrossRef] [PubMed]
- Khairat, S.; Meng, C.; Xu, Y.; Edson, B.; Gianforcaro, R. Interpreting COVID-19 and Virtual Care Trends: Cohort Study. JMIR Public Health Surveill. 2020, 6, e18811. [Google Scholar] [CrossRef] [Green Version]
- Rockwell, K.L.; Gilroy, A.S. Incorporating telemedicine as part of COVID-19 outbreak response systems. Am. J. Manag. Care 2020, 26, 147–148. [Google Scholar] [CrossRef]
- Lam, P.W.; Sehgal, P.; Andany, N.; Mubareka, S.; Simor, A.E.; Ozaldin, O.; Leis, J.A.; Daneman, N.; Chan, A.K. A virtual care program for outpatients diagnosed with COVID-19: A feasibility study. CMAJ Open 2020, 8, E407–E413. [Google Scholar] [CrossRef] [PubMed]
- Ferry, O.R.; Moloney, E.C.; Spratt, O.T.; Whiting, G.F.M.; Bennett, C.J. A Virtual Ward Model of Care for Patients with COVID-19: Retrospective Single-Center Clinical Study. J. Med. Internet Res. 2021, 23, e25518. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, M.; Bal-Alvarado, M.; Guerra, F.S.; Ares-Rico, R.; Suárez-Gil, R.; Rodríguez-Álvarez, A.; Pérez-López, A.; Casariego-Vales, E.; Rial, Á.F.; Rey, R.R.; et al. Monitoring of COVID-19 patients by telemedicine with telemonitoring. Rev. Clín. Esp. (Engl. Ed. ) 2020, 220, 472–479. [Google Scholar] [CrossRef]
- O’Keefe, J.B.; Tong, E.J.; O’Keefe, G.D.; Tong, D.C. Description of symptom course in a telemedicine monitoring clinic for acute symptomatic COVID-19: A retrospective cohort study. BMJ Open 2021, 11, e044154. [Google Scholar] [CrossRef] [PubMed]
- Sicsic, I., Jr.; Chacon, A.R.; Zaw, M.; Ascher, K.; Abreu, A.; Chediak, A. A case of SARS-CoV-2 reinfection in a patient with obstruc-tive sleep apnea managed with telemedicine. BMJ Case Rep. 2021, 14, e240496. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, O.R.; Dearing, C.; Jagers, D.; Shaw, M.J.; Raffan, F.; Jones, A.; Taggart, R.; Sinclair, T.; Anderson, T.; Ritchie, A.G. Virtual Health Care for Community Management of Patients With COVID-19 in Australia: Observational Cohort Study. J. Med. Internet Res. 2021, 23, e21064. [Google Scholar] [CrossRef] [PubMed]
- Motta, L.P.; Silva, P.P.F.D.; Borguezan, B.M.; Amaral, J.L.M.D.; Milagres, L.G.; Bóia, M.N.; Ferraz, M.R.; Mogami, R.; Nunes, R.A.; Melo, P.L.D. An emergency system for moni-toring pulse oximetry, peak expiratory flow, and body temperature of patients with COVID-19 at home: Development and preliminary application. PLoS ONE 2021, 16, e0247635. [Google Scholar] [CrossRef] [PubMed]
- Silven, A.V.; Petrus, A.H.J.; Villalobos-Quesada, M.; Dirikgil, E.; Oerlemans, C.R.; Landstra, C.P.; Boosman, H.; van Os, H.J.; Blanker, M.H.; Treskes, R.W.; et al. Telemonitoring for Patients With COVID-19: Recommendations for Design and Implementation. J. Med. Internet Res. 2020, 22, e20953. [Google Scholar] [CrossRef] [PubMed]

| Geriatric Risk | Connected Sensors/Questionnaires | Frequency |
|---|---|---|
| Hemodynamic data (hypertension/hypotension–tachycardia/bradycardia–oxygen desaturation/infections) | Sphygmomanometer–pulse oximeter–thermometer | Three times per day |
| Heart failure | Questionnaire | Daily |
| Constipation | Questionnaire | Twice a day |
| Risk of bed rest | Questionnaire Pedometer | Daily Daily |
| Pain | Questionnaire | Daily |
| Dehydration | Questionnaire Biological sensors (natremia–kaliemia–creatinine) | Daily Twice a week |
| Sleep quality | Pedometer | Day and night |
| Physical activity | Pedometer Questionnaire | Daily Daily |
| Diabetes | Glucometer | Three times per day |
| Iatrogenism | Questionnaire | On admission and once during hospitalization |
| Malnutrition | Balance Biological sensor (albumin) | Twice a week Once during hospitalization |
| Confusion | Questionnaire (CAM *) | Daily |
| Neuropsychiatric disorders | Questionnaire (NPI *) | Twice a day |
| Medical Characteristics (n, %) | |
|---|---|
| Medical History | |
| Heart deficiency | 15 (50%) |
| Arterial hypertension | 16 (53.3%) |
| Atrial fibrillation | 8 (26.7%) |
| Coronary syndrome | 9 (30%) |
| Pacemaker | 4 (13.3%) |
| Obliterating arteriopathy of the lower limbs | 1 (3.3%) |
| Sleep apnea syndrome | 1 (3.3%) |
| Phlebitis/pulmonary embolism | 5 (16.7%) |
| Dyslipidemia | 9 (30%) |
| Diabetes | 10 (33.3%) |
| Stroke | 7 (23.3%) |
| Chronic renal deficiency | 4 (13.3%) |
| COPD * | 5 (16.7%) |
| Solids neoplasms | 4 (13.3%) |
| Cirrhosis | 1 (3.3%) |
| Peptic ulcer | 2 (6.7%) |
| Hypothyroidism | 4 (13.3%) |
| Connectivities | 3 (10%) |
| Cognitive disorder | 15 (50%) |
| Treatment | |
| Beta blockers | 12 (40%) |
| ACE inhibitors, Sartan | 11 (36.7%) |
| Diuretics | 13 (43.3%) |
| Calcium channel blockers | 9 (30%) |
| Anticoagulants | 8 (26.7%) |
| Antiplatelet agents | 9 (30%) |
| Statins | 9 (30%) |
| Oral antidiabetics | 5 (16.7%) |
| Insulin therapy | 2 (6.7%) |
| Benzodiazepines | 14 (46.7%) |
| Antipsychotics | 5 (16.7%) |
| Antidepressant | 5 (16.7%) |
| Proton pump inhibitors | 13 (43.3%) |
| L-Thyroxin | 4 (13.3%) |
| Antiarrhythmics | 4 (13.3%) |
| Symptoms at onset of illness COVID-19 | |
| Fatigue | 12 (40%) |
| Confusion | 6 (20%) |
| Dehydratation | 7 (23.3%) |
| Dyspnoea | 22 (73.3%) |
| Fever | 12 (40%) |
| Cough | 11 (36.7%) |
| Diarrhoae | 4 (13.3%) |
| Acute heart failure | 3 (10%) |
| Pulmonary embolism/phlebitis | 3 (10%) |
| Arterial thrombosis | 2 (6.7%) |
| Asymptomatic | 1 (3.3%) |
| Total lung involvement Chest CT Findings in Coronavirus Disease-19 (COVID-19) | |
| Minimal (<25%) | 17 (56.7%) |
| Moderate (25–50%) | 7 (23.3%) |
| Severe to critical (>=75%) | 6 (20%) |
| General Data | First Phase GER-e-TEC (n = 36) [8] | Second Phase GER-e-TEC COVID (n = 30) | p |
|---|---|---|---|
| Age | 81.4 (±7.7) | 85.9 (±6.4) | 0.0053 |
| Average use of the telemedicine solution | 22.1 | 27.3 | 0.6575 |
| Average number of drug treatments | 8.5 (±4.2) | 8 (±4.1) | 0.6964 |
| Charlson score | 6.86 | 6 | 1 |
| Average measurements recorded per patient for geriatric disorders | 4476 | 4675 | 0.5408 |
| Average measurements recorded per patient per day | 226 | 319 | <2.2 × 10−16 |
| Data from Sensors and Questionnaires (Mean ± Standard Derivation) | p | ||
| Arterial pressure | 105.70 mm Hg (±8.1 mm Hg) | 105.72 mm Hg (±8.12 mm Hg) | 0.5023/0.9986 |
| Heart rate | 77.6 bpm (±15 bpm) | 80.4 bpm (±15.2 bpm) | 7.831 × 10−6 |
| Oxygen saturation | 96.5% (±21) | 94.2% (±4.1) | <2.2 × 10−16 |
| Blood glucose level | 124.3 mg/L (±86 mg/L) | 163.2 mg/L (±86.7 mg/L) | 4.196 × 10−6 |
| Weight | 75.1 kg (±23.1 kg) | 68.5 kg (±14.4 kg) | 0.9937 |
| Temperature | 36.7 °C (±0.6 °C) | 36.5 °C (±0.9 °C) | 3.162 × 10−9 |
| Physical activity (median) | 394 steps per day | 212.5 steps per day | 3.13 × 10−7 |
| Daily activity index | 13.9% (±14.1%) | 10.6% (±11.3%) | 0.01341 |
| Amount of sleep | 500.3 min per day (±206 min) | 492.5 min per day (±135.7 min | 0.188 |
| Amount of light sleep | 139.8 min per day (±144.4 min) | 124.1 min per day (±126.1 min) | 0.9495 |
| Amount of deep sleep | 358.8 min per day (±159.2 min) | 368.4 min per day (±137.8 min) | 0.02305 |
| Stool frequency | 0.6 stools per day (±0.6) | 0.4 stools per day (±0.6) | <2.2 × 10−16 |
| VAS pain score | 1.2 (±0.3) | 0.4 (±0.5) | <2.2 × 10−16 |
| VRS pain score | 0.6 (±0.2) | 0.1 (±1.2) | <2.2 × 10−16 |
| Algoplus | 7.5 (±2.3) | 0.9 (±1.7) | 0.5 |
| Albumin level | 35.2 g/L (4.1 g/L) | 36.2 g/L (3.8 g/L) | 0.074 |
| Natremia | 136.2 mmol/L (±3.6 mmol/L) | 140.3 mmol/L (±6.2 mmol/L) | 1.941 × 10−12 |
| Kalemia | 4.2 mEq/L (±0.6 mEq/L) | 4.1 mEq/L (±0.6 mEq/L) | 0.01169 |
| Creatinine level | 87.3 µmol/L (±30.2 µmol/L) | 80.8 µmol/L (±26.3 µmol/L) | 0.9487 |
| INR * | 2.5 (±1.4) | 3.4 (±2.4) | 0.6226 |
| Vitamin D | - | 29 ng/mL (±33.6) | - |
| General Data | Elderly Patients COVID-19 Alive (n = 19) | Elderly Patients COVID-19 Deceased (n = 11) | p |
|---|---|---|---|
| Age | 85.1 (±5.2) | 87.4 (±8.1) | 0.9074 |
| Average use of the telemedicine solution | 35.1 (±82.6) | 13.7 (±15.5) | 0.8569 |
| Average number of drug treatments | 7.9 (±4.6) | 8.1 (±3) | 0.5406 |
| Charlson score | 5.7 (±1.1) | 6.5 (±1.4) | 0.925 |
| Average measurements recorded per patient for geriatric disorders | 5015 | 3876 | 0.03176 |
| Average measurements recorded per patient per day | 322 | 302 | 0.9979 |
| Albumin level | 37 (±4.3) | 34.6 (±2.6) | 0.9857 |
| Natremia | 139.2 mmol/L (±5.8 mmol/L) | 142.2 mmol/L (±7 mmol/L) | 0.002776 |
| Kalemia | 4 mEq/L (±0.5 mEq/L) | 4.2 mEq/L (±0.7 mEq/L) | 0.8188 |
| Creatinine level | 80.5 µmol/L (±25.8 µmol/L) | 81.6 µmol/L (±27.9 µmol/L) | 0.5608 |
| INR | 2.9 (±1.8) | 4.1 (±3.2) | 0.6537 |
| Stool frequency | 0.5 stools per day (±0.6) | 0.2 stools per day (±0.5) | 1.047 × 10−6 |
| Arterial pressure | 107.72 mm Hg (±6.13 mm Hg) | 101.71 mm Hg (±9.12 mm Hg) | 6.607 × 10−5/0.08317 |
| Heart rate | 79.9 bpm (±14.5 bpm) | 82.4 bpm (±16.8 bpm) | 0.02348 |
| Oxygen saturation | 95.3 % (±2.6) | 92.2 % (±5.4) | <2.2 × 10−16 |
| Blood glucose level | 151.3 mg/L (±92.6 mg/L) | 210.8 mg/L (±60.6 mg/L) | 2.165 × 10−14 |
| Weight | 68 kg (±17.3 kg) | 70.2 kg (±10.3 kg) | 0.05735 |
| Temperature | 36.4 °C (±0.8 °C) | 36.4 °C (±0.9 °C) | 0.9151 |
| Physical activity | 840.1 steps per day (±1222.7) | 170.2 steps per day (±328.7) | 7.912 × 10−13 |
| Daily activity index | 12.9% (±13%) | 4.3% (±3.7%) | 8.58 × 10−5 |
| VAS pain score | 0.3 (±1) | 0.5 (±1.7) | 0.71 |
| VRS pain score | 0.1 (±0.4) | 0.2 (±0.6) | 0.5218 |
| Amount of sleep | 484 min per day (±123.1 min) | 497.3 min per day (±160.1 min) | 0.6248 |
| Amount of light sleep | 140.7 min per day (±112.5 min) | 90 min per day (±155.3 min) | 1 |
| Amount of deep sleep | 343.3 min per day (±140.4 min) | 407.3 min per day (±136.2 min) | 3.749 × 10−7 |
| Vitamin D | 33.4 ng/mL (±38.9) | 19.7 ng/mL (±15.2) | 0.8822 |
| Geriatric Syndromes | Alerts Total | Low Alerts | Moderate Alerts | Critical Alerts |
|---|---|---|---|---|
| Bed rest | 159 | 159 (100%) | 0 | 0 |
| Confusion | 9 | 0 | 0 | 9 (100%) |
| Constipation | 28 | 0 | 28 (100%) | 0 |
| Tachy–bradycardia | 11 | 0 | 0 | 11 (100%) |
| Malnutrition | 18 | 0 | 17 (94.6%) | 1 (5.5%) |
| Pain | 33 | 0 | 33 (100%) | 0 |
| Hyperthermia | 11 | 0 | 11 (100%) | 0 |
| Hypo- and hyperkalemia | 29 | 0 | 29 (100%) | 0 |
| Hypo- and hypernatremia | 18 | 0 | 18 (100%) | 0 |
| Hypo- and hypertension | 238 | 0 | 0 | 238 (100%) |
| Iatrogenesis | 244 | 44 (18%) | 58 (23.8%) | 142 (58.2%) |
| Heart failure | 413 | 0 | 126 (30.5%) | 287 (69.5%) |
| Hypertension | 238 | 0 | 0 | 238 (100%) |
| Dehydration | 34 | 0 | 34 (100%) | 0 |
| Diabetes | 142 | 69 (48.6%) | 38 (26.8%) | 35 (24.6%) |
| Decompensated Heart Failure | Pain | Dehydration | Brady-and Tachycardia | Constipation | Bed Rest | Malnutrition | Iatrogenia | Confusion | |
|---|---|---|---|---|---|---|---|---|---|
| Sensitivity | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% |
| Specificity | - | - | 50% | 50% | 50% | - | 49% | 49% | - |
| Positive predictive value | 100% | 100% | 58% | 73% | 34% | 100% | 29% | 42% | 100% |
| Negative predictive value | - | - | 100% | 100% | 100% | - | 100% | 100% | - |
| Fever | Hypo-and Hyperkalemia | Hypo-and Hypernatremia | Diabetes | Hypertension | Agitation/Aggression | Hallucinations | Anxiety | Apathy/Indifference | Delusion | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sensitivity | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% |
| Specificity | - | 49% | 50% | - | - | - | - | - | - | - |
| Positive predictive value | 100% | 43% | 35% | 100% | 100% | 100% | 100% | 100% | 100% | 100% |
| Negative predictive value | - | 100% | 100% | - | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zulfiqar, A.-A.; Massimbo, D.N.D.; Hajjam, M.; Geny, B.; Talha, S.; Hajjam, J.; Erve, S.; Hajjam, A.; Andrès, E. Results of the Second Phase of the GER-e-TEC Experiment concerning the Telemonitoring of Elderly Patients Affected by COVID-19 Disease to Detect the Exacerbation of Geriatric Syndromes. J. Pers. Med. 2021, 11, 1117. https://doi.org/10.3390/jpm11111117
Zulfiqar A-A, Massimbo DND, Hajjam M, Geny B, Talha S, Hajjam J, Erve S, Hajjam A, Andrès E. Results of the Second Phase of the GER-e-TEC Experiment concerning the Telemonitoring of Elderly Patients Affected by COVID-19 Disease to Detect the Exacerbation of Geriatric Syndromes. Journal of Personalized Medicine. 2021; 11(11):1117. https://doi.org/10.3390/jpm11111117
Chicago/Turabian StyleZulfiqar, Abrar-Ahmad, Delwende Noaga Damien Massimbo, Mohamed Hajjam, Bernard Geny, Samy Talha, Jawad Hajjam, Sylvie Erve, Amir Hajjam, and Emmanuel Andrès. 2021. "Results of the Second Phase of the GER-e-TEC Experiment concerning the Telemonitoring of Elderly Patients Affected by COVID-19 Disease to Detect the Exacerbation of Geriatric Syndromes" Journal of Personalized Medicine 11, no. 11: 1117. https://doi.org/10.3390/jpm11111117







