Machine Learning-Based Radiomics of the Optic Chiasm Predict Visual Outcome Following Pituitary Adenoma Surgery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Enrollment
2.2. Ophthalmological Evaluation
2.3. MRI Acquisition
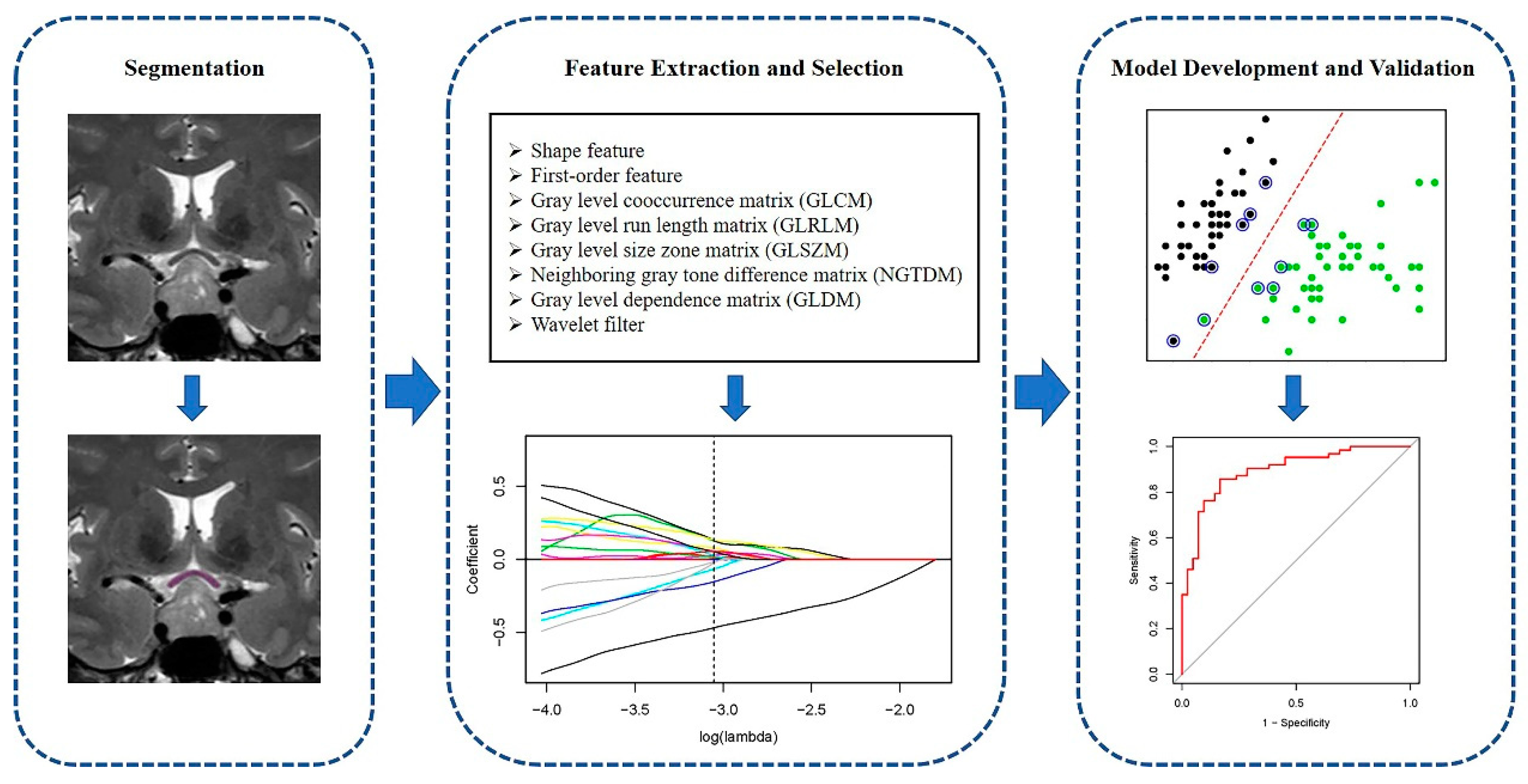
2.4. Optic Chiasm Segmentation
2.5. Radiomic Feature Extraction
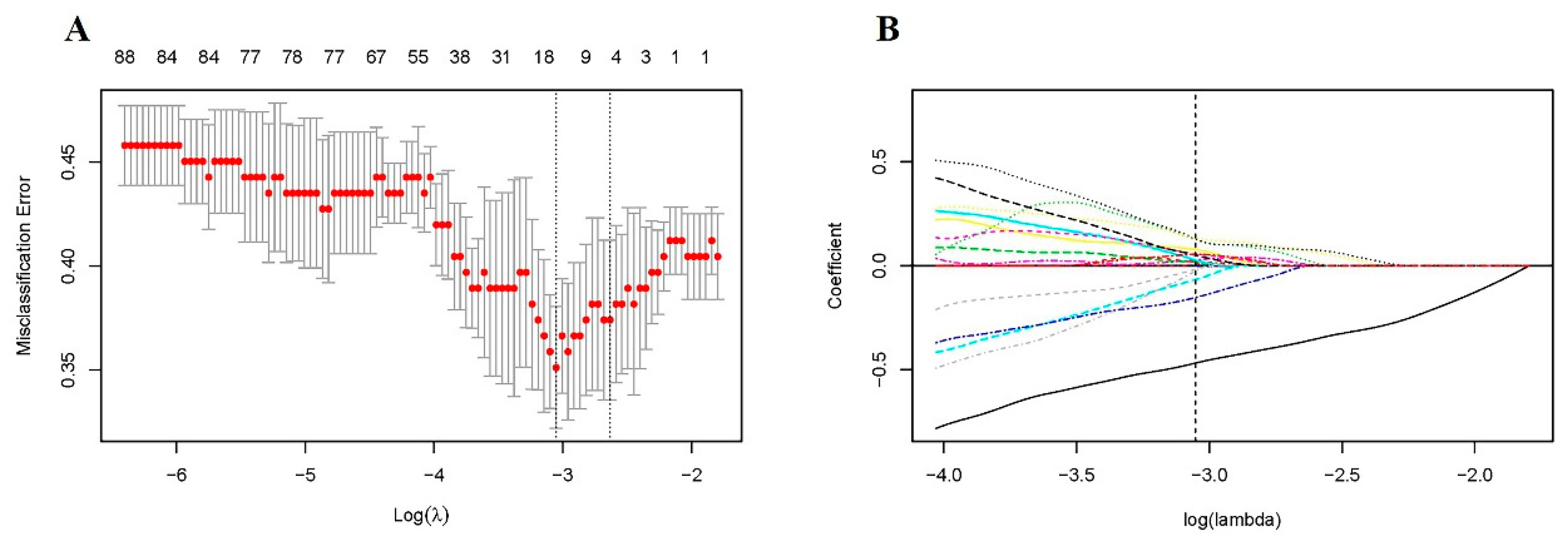
2.6. Feature Selection and Modeling
3. Results
3.1. Patient Characteristics
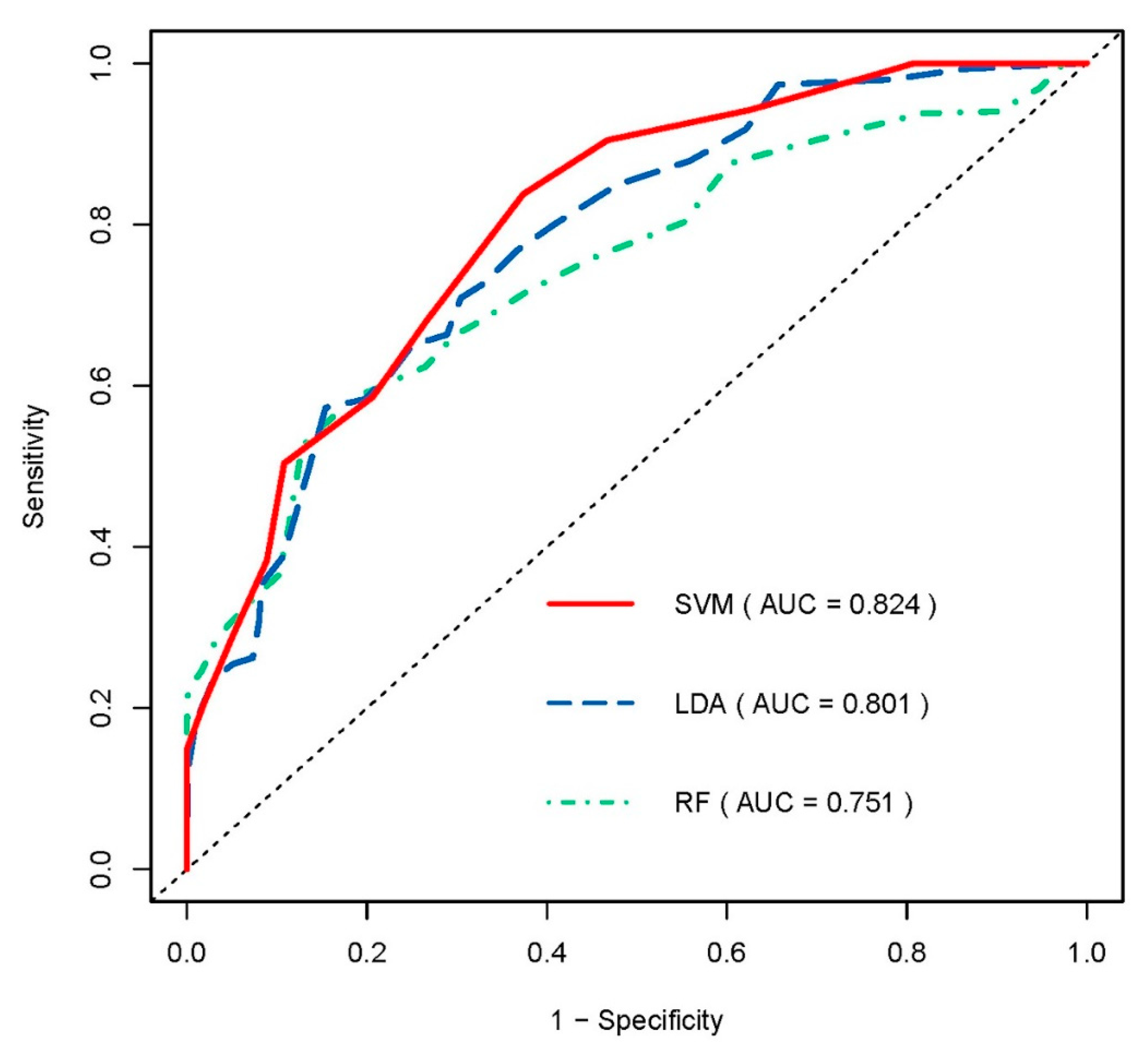
3.2. Predictive Performance of Radiomic Model
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Danesh-Meyer, H.V.; Yoon, J.J.; Lawlor, M.; Savino, P.J. Visual loss and recovery in chiasmal compression. Prog. Retin. Eye Res. 2019, 73, 100765. [Google Scholar] [CrossRef]
- Muskens, I.S.; Zamanipoor Najafabadi, A.H.; Briceno, V.; Lamba, N.; Senders, J.T.; van Furth, W.R.; Verstegen, M.J.T.; Smith, T.R.S.; Mekary, R.A.; Eenhorst, C.A.E.; et al. Visual outcomes after endoscopic endonasal pituitary adenoma resection: A systematic review and meta-analysis. Pituitary 2017, 20, 539–552. [Google Scholar] [CrossRef]
- Ho, R.W.; Huang, H.M.; Ho, J.T. The influence of pituitary adenoma size on vision and visual outcomes after trans-sphenoidal adenectomy: A report of 78 cases. J. Korean Neurosurg. Soc. 2015, 57, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kang, M.S.; Kim, S.Y.; Lee, J.E.; Shin, J.H.; Choi, H.; Kim, S.J. Analysis of factors affecting visual field recovery following surgery for pituitary adenoma. Int. Ophthalmol. 2021, 41, 2019–2026. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.I.; Son, K.Y.; Park, K.A.; Kong, D.S.; Oh, S.Y. Longitudinal changes in the retinal microstructures of eyes with chiasmal compression. Neurology 2020, 96, e131–e140. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, W.L.; Hosny, A.; Schabath, M.B.; Giger, M.L.; Birkbak, N.J.; Mehrtash, A.; Allison, T.; Arnaout, O.; Abbosh, C.; Dunn, I.F.; et al. Artificial intelligence in cancer imaging: Clinical challenges and applications. CA Cancer J. Clin. 2019, 69, 127–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosny, A.; Parmar, C.; Quackenbush, J.; Schwartz, L.H.; Aerts, H. Artificial intelligence in radiology. Nat. Rev. Cancer 2018, 18, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Liu, Z.; Hou, B.; Li, L.; Liu, X.; Liu, Z.; Wang, R.; Lin, Y.; Feng, F.; Tian, J.; et al. Development and validation of an MRI-based radiomic signature for the preoperative prediction of treatment response in patients with invasive functional pituitary adenoma. Eur. J. Radiol. 2019, 121, 108647. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.H.; Chen, T.Y.; Lim, S.W.; Wu, T.C.; Kuo, Y.T.; Ko, C.-C.; Su, M.-Y. Radiomics approach for prediction of recurrence in skull base meningiomas. Neuroradiology 2019, 61, 1355–1364. [Google Scholar] [CrossRef]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; LaRue, R.T.; Even, A.J.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef]
- Limkin, E.J.; Sun, R.; Dercle, L.; Zacharaki, E.I.; Robert, C.; Reuzé, S.; Schernberg, A.; Paragios, N.; Deutsch, E.; Ferté, C. Promises and challenges for the implementation of computational medical imaging (radiomics) in oncology. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 1191–1206. [Google Scholar] [CrossRef]
- Cellina, M.; Pirovano, M.; Ciocca, M.; Gibelli, D.; Floridi, C.; Oliva, G. Radiomic analysis of the optic nerve at the first episode of acute optic neuritis: An indicator of optic nerve pathology and a predictor of visual recovery? La Radiologia Medica 2021, 126, 698–706. [Google Scholar] [CrossRef]
- Liu, H.J.; Zhou, H.F.; Zong, L.X.; Liu, M.Q.; Wei, S.H.; Chen, Z.Y. MRI Histogram Texture Feature Analysis of the Optic Nerve in the Patients with Optic Neuritis. Chin. Med Sci. J. 2019, 34, 18–23. [Google Scholar]
- Chung, Y.S.; Na, M.; Yoo, J.; Kim, W.; Jung, I.H.; Moon, J.H.; Lee, J.; Kim, S.H.; Kim, E.H. Optical Coherent Tomography Predicts Long-Term Visual Outcome of Pituitary Adenoma Surgery: New Perspectives From a 5-Year Follow-up Study. Neurosurgery 2020, 88, 106–112. [Google Scholar] [CrossRef]
- Yoo, Y.J.; Hwang, J.M.; Yang, H.K.; Joo, J.D.; Kim, Y.H.; Kim, C.Y. Prognostic value of macular ganglion cell layer thickness for visual outcome in parasellar tumors. J. Neurol. Sci. 2020, 414, 116823. [Google Scholar] [CrossRef]
- Wang, M.T.M.; King, J.; Symons, R.C.A.; Stylli, S.S.; Meyer, J.; Daniell, M.D.; Savino, P.J.; Kaye, A.H.; Danesh-Meyer, H.V. Prognostic Utility of Optical Coherence Tomography for Long-Term Visual Recovery Following Pituitary Tumor Surgery. Am. J. Ophthalmol. 2020, 218, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, S.W.; Kim, D.W.; Shin, J.Y.; Choi, M.; Oh, M.C.; Kim, S.M.; Kim, E.H.; Kim, S.H.; Byeon, S.H. Predictive model for recovery of visual field after surgery of pituitary adenoma. J. Neurooncol. 2016, 130, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006, 31, 1116–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.; Fillion-Robin, J.-C.; Pieper, S.; Aerts, H.J. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joo, L.; Park, J.E.; Park, S.Y.; Nam, S.J.; Kim, Y.H.; Kim, J.H.; Kim, H.S. Extensive peritumoral edema and brain-to-tumor interface MRI features enable prediction of brain invasion in meningioma: Development and validation. Neuro-Oncology 2021, 23, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.W.; Zhu, F.P.; Xu, Q.; Wang, K.; Wu, M.Y.; Tang, W.W.; Li, X.-C.; Wang, X.-H. Radiomic Features at Contrast-enhanced CT Predict Recurrence in Early Stage Hepatocellular Carcinoma: A Multi-Institutional Study. Radiology 2020, 294, 568–579. [Google Scholar] [CrossRef]
- Cai, J.; Zheng, J.; Shen, J.; Yuan, Z.; Xie, M.; Gao, M.; Tan, H.; Liang, Z.-G.; Rong, X.; Li, Y.; et al. A Radiomics Model for Predicting the Response to Bevacizumab in Brain Necrosis after Radiotherapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 5438–5447. [Google Scholar] [CrossRef]
- Ngiam, K.Y.; Khor, I.W. Big data and machine learning algorithms for health-care delivery. Lancet Oncol. 2019, 20, e262–e273. [Google Scholar] [CrossRef]
- Lin, A.; Dey, D. CT-based radiomics and machine learning for the prediction of myocardial ischemia: Toward increasing quantification. J. Nucl. Cardiol. Off. Publ. Am. Soc. Nucl. Cardiol. 2020. [Google Scholar] [CrossRef]
- Hofmeister, J.; Bernava, G.; Rosi, A.; Vargas, M.I.; Carrera, E.; Montet, X.; Burgermeister, S.; Poletti, P.-A.; Platon, A.; Lovblad, K.-O.; et al. Clot-Based Radiomics Predict a Mechanical Thrombectomy Strategy for Successful Recanalization in Acute Ischemic Stroke. Stroke 2020, 51, 2488–2494. [Google Scholar] [CrossRef]
- Li, H.; Zhu, Y.; Burnside, E.S.; Drukker, K.; Hoadley, K.A.; Fan, C.; Conzen, S.D.; Whitman, G.J.; Sutton, E.J.; Net, J.M.; et al. MR Imaging Radiomics Signatures for Predicting the Risk of Breast Cancer Recurrence as Given by Research Versions of MammaPrint, Oncotype DX, and PAM50 Gene Assays. Radiology 2016, 281, 382–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dercle, L.; Lu, L.; Schwartz, L.H.; Qian, M.; Tejpar, S.; Eggleton, P.; Zhao, B.; Piessevaux, H. Radiomics Response Signature for Identification of Metastatic Colorectal Cancer Sensitive to Therapies Targeting EGFR Pathway. J. Natl. Cancer Inst. 2020, 112, 902–912. [Google Scholar] [CrossRef]
- Kondo, Y.; Ramaker, J.M.; Radcliff, A.B.; Baldassari, S.; Mayer, J.A.; Ver Hoeve, J.N.; Zhang, C.-L.; Chiu, S.-Y.; Colello, R.J.; Duncn, I.D. Spontaneous optic nerve compression in the osteopetrotic (op/op) mouse: A novel model of myelination failure. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 3514–3525. [Google Scholar] [CrossRef] [Green Version]
- Ji, G.W.; Zhu, F.P.; Xu, Q.; Wang, K.; Wu, M.Y.; Tang, W.W.; Li, X.-C.; Wang, X.-H. Machine-learning analysis of contrast-enhanced CT radiomics predicts recurrence of hepatocellular carcinoma after resection: A multi-institutional study. EBioMedicine 2019, 50, 156–165. [Google Scholar] [CrossRef] [Green Version]
- Papp, L.; Pötsch, N.; Grahovac, M.; Schmidbauer, V.; Woehrer, A.; Preusser, M.; Mitterhauser, M.; Kiesel, B.; Wadsak, W.; Beyer, T.; et al. Glioma Survival Prediction with Combined Analysis of In Vivo (11)C-MET PET Features, Ex Vivo Features, and Patient Features by Supervised Machine Learning. J. Nucl. Med. 2018, 59, 892–899. [Google Scholar] [CrossRef] [Green Version]
- Trebeschi, S.; Drago, S.G.; Birkbak, N.J.; Kurilova, I.; Cǎlin, A.M.; Delli Pizzi, A.; Lalezari, F.; Lambregts, D.M.J.; Rohaan, M.W.; Parmar, C.; et al. Predicting response to cancer immunotherapy using noninvasive radiomic biomarkers. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 998–1004. [Google Scholar] [CrossRef] [Green Version]
- Khorrami, M.; Prasanna, P.; Gupta, A.; Patil, P.; Velu, P.D.; Thawani, R.; Corredor, G.; Alilou, M.; Bera, K.; Fu, P.; et al. Changes in CT Radiomic Features Associated with Lymphocyte Distribution Predict Overall Survival and Response to Immunotherapy in Non-Small Cell Lung Cancer. Cancer Immunol. Res. 2020, 8, 108–119. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Williams, M.C.; Kotanidis, C.P.; Desai, M.Y.; Marwan, M.; Antonopoulos, A.S.; Thomas, K.E.; Thomas, S.; Akoumianakis, I.; Fan, L.M.; et al. A novel machine learning-derived radiotranscriptomic signature of perivascular fat improves cardiac risk prediction using coronary CT angiography. Eur. Heart J. 2019, 40, 3529–3543. [Google Scholar] [CrossRef] [PubMed]
- Elshafeey, N.; Kotrotsou, A.; Hassan, A.; Elshafei, N.; Hassan, I.; Ahmed, S.; Abrol, S.; Agarwal, A.; El Salek, K.; Bergamaschi, S.; et al. Multicenter study demonstrates radiomic features derived from magnetic resonance perfusion images identify pseudoprogression in glioblastoma. Nat. Commun. 2019, 10, 3170. [Google Scholar] [CrossRef] [Green Version]
- Noble, W.S. What is a support vector machine? Nat. Biotechnol. 2006, 24, 1565–1567. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Orrù, G.; Pettersson-Yeo, W.; Marquand, A.F.; Sartori, G.; Mechelli, A. Using Support Vector Machine to identify imaging biomarkers of neurological and psychiatric disease: A critical review. Neurosci. Biobehav. Rev. 2012, 36, 1140–1152. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.T.M.; King, J.; Symons, R.C.A.; Stylli, S.S.; Daniell, M.D.; Savino, P.J.; Kaye, A.H.; Danesh-Meyer, H.V. Temporal patterns of visual recovery following pituitary tumor resection: A prospective cohort study. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2021, 86, 252–259. [Google Scholar]
| Characteristics | Recovery Group | Non-Recovery Group | p Value |
|---|---|---|---|
| Number | 79 | 52 | |
| Age (year) | 44.81 ± 11.20 | 49.04 ± 14.46 | 0.078 |
| Gender, N (%) | 0.725 | ||
| Male | 34 (43.04) | 24 (46.15) | |
| Female | 45 (56.96) | 28 (53.85) | |
| Preoperative MD (dB) | −8.42 ± 6.10 | −10.42 ± 6.77 | 0.080 |
| Tumor diameter (mm) | 25.29 ± 5.11 | 26.78 ± 7.89 | 0.605 |
| Surgical approach | 0.513 | ||
| Transsphenoidal | 75 (94.94) | 47 (90.38) | |
| Transcranial | 4 (5.06) | 5 (9.62) |
| AUC | Accuracy | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|---|
| SVM | 0.824 | 0.747 | 0.675 | 0.798 | 0.696 | 0.795 |
| LDA | 0.801 | 0.718 | 0.598 | 0.797 | 0.674 | 0.750 |
| RF | 0.751 | 0.734 | 0.540 | 0.862 | 0.749 | 0.740 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Chen, C.; Huang, W.; Cheng, Y.; Teng, Y.; Zhang, L.; Xu, J. Machine Learning-Based Radiomics of the Optic Chiasm Predict Visual Outcome Following Pituitary Adenoma Surgery. J. Pers. Med. 2021, 11, 991. https://doi.org/10.3390/jpm11100991
Zhang Y, Chen C, Huang W, Cheng Y, Teng Y, Zhang L, Xu J. Machine Learning-Based Radiomics of the Optic Chiasm Predict Visual Outcome Following Pituitary Adenoma Surgery. Journal of Personalized Medicine. 2021; 11(10):991. https://doi.org/10.3390/jpm11100991
Chicago/Turabian StyleZhang, Yang, Chaoyue Chen, Wei Huang, Yangfan Cheng, Yuen Teng, Lei Zhang, and Jianguo Xu. 2021. "Machine Learning-Based Radiomics of the Optic Chiasm Predict Visual Outcome Following Pituitary Adenoma Surgery" Journal of Personalized Medicine 11, no. 10: 991. https://doi.org/10.3390/jpm11100991
APA StyleZhang, Y., Chen, C., Huang, W., Cheng, Y., Teng, Y., Zhang, L., & Xu, J. (2021). Machine Learning-Based Radiomics of the Optic Chiasm Predict Visual Outcome Following Pituitary Adenoma Surgery. Journal of Personalized Medicine, 11(10), 991. https://doi.org/10.3390/jpm11100991






