Abstract
Like several neurodegenerative disorders, such as Prion and Parkinson diseases, Alzheimer’s disease (AD) is characterized by spreading mechanism of aggregated proteins in the brain in a typical “prion-like” manner. Recent genetic studies have identified in four genes associated with inherited AD (amyloid precursor protein-APP, Presenilin-1, Presenilin-2 and Apolipoprotein E), rare mutations which cause dysregulation of APP processing and alterations of folding of the derived amyloid beta peptide (Aβ). Accumulation and aggregation of Aβ in the brain can trigger a series of intracellular events, including hyperphosphorylation of tau protein, leading to the pathological features of AD. However, mutations in these four genes account for a small of the total genetic risk for familial AD (FAD). Genome-wide association studies have recently led to the identification of additional AD candidate genes. Here, we review an update of well-established, highly penetrant FAD-causing genes with correlation to the protein misfolding pathway, and novel emerging candidate FAD genes, as well as inherited risk factors. Knowledge of these genes and of their correlated biochemical cascade will provide several potential targets for treatment of AD and aging-related disorders.
1. Introduction
Alzheimer’s disease (AD) is responsible for about 60–70% of cases of dementia, equivalent to an estimated population of 40–50 million persons worldwide. This more than doubled from 1990 to 2016 [1]. Typically, AD is featured by a progressive neurodegeneration that lead to a gradual loss of memory and alterations affecting other cognitive functions, such as spatial cognition, reasoning, word-finding, judgment and problem-solving [2]. Aging is the most important AD risk factor and, even if it may occur at any age, more often AD appears in older individuals (later than 65 years of age) [3,4]. However, early onset AD cases, characterized by the occurrence of clinical signs between 30 and 65 years old, have been also reported [3,4]. Even if atypical phenotypes have been described, clinical and pathological features seem to be the same between early and late onset AD, so that it may be difficult to distinguish these 2 groups [4]. Interestingly, while late onset AD is usually sporadic and doesn’t show any segregation within the families, it has been highlighted that early onset AD is featured by a high recurrence rate within the affected families, thus suggesting the presence of inherited forms of AD [3].
In particular, it has been estimated that 15–25% of total AD accounts for familial Alzheimer’s disease (FAD) (Figure 1) [5].
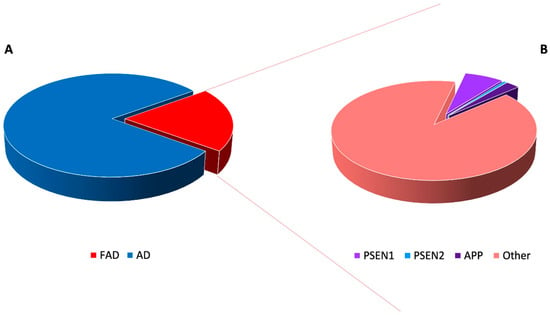
Figure 1.
Prevalence and genetic causes of familial Alzheimer’s disease (FAD). FAD represents only a small fraction (about 20%) of all Alzheimer’s disease (AD) cases (A). In addition, within FAD, mutations the amyloid precursor protein (APP), presenilin 1 (PSEN1) or presenilin 2 (PSEN2) genes account for a small proportion of cases, underlying that the molecular bases of the larger fraction still remain to be unveiled (B).
In this category, it is included the autosomal dominant Alzheimer’s disease (ADAD), associated to the presence of known causative gene mutations, mainly in the amyloid precursor protein (APP), presenilin 1 (PSEN1) or presenilin 2 (PSEN2) genes, that have been described as highly penetrant, disease-causing FAD genes [3,4,5]. However, mutations in these genes are able to explain just a small percentage of all FAD cases, suggesting the existence of other, inherited, disease-predisposing genes [4]. Since FAD typically shows a sequential, clinical progression from pre-dementia to dementia stage, thus it is crucial to recognize and diagnose it before symptoms onset in order to begin treatments as soon as possible [6,7]. As a consequence, a better understanding of FAD molecular bases, i.e., the identification of the causative genes, may ameliorate the management and the clinical outcome of these patients and of their families.
Recent advances in genomics, i.e., the availability of highly performing next generation sequencing (NGS)-based methods to accurately analyze single genes [8,9] or a subset of genes of interest [10,11,12], up to the whole exome [13,14] or the whole genome [13,14], has prompted the study of the molecular bases of human diseases and is a promising tool to discover novel FAD-related genes [4].
Here, we will review the current knowledge regarding the genetic etiology of FAD. In particular, we will focus first on well-established, highly penetrant, FAD-causing genes. In this context, the relationship between mutations affecting AD-related genes and proteins’ trafficking, folding and aggregation properties will be highlighted, with special attention to APP. Next, novel, emerging and candidate FAD genes, as well as inherited risk factors will be also discussed, suggesting that enlarged genetic testing may be useful in FAD families in order to improve the identification and management of the at-risk subjects.
2. Methods
Indexed articles in English were searched in PubMed using the following keywords: “Alzheimer’s disease molecular bases”, “Alzheimer’s disease mutations”, “Alzheimer’s disease germline mutations”, “Alzheimer’s disease genes”, “inherited Alzheimer’s disease”, “familial Alzheimer’s disease”, “APP mutations”, “PSEN1 mutations”, “PSEN2 mutations” and “novel Alzheimer’s disease genes”. In the attempt to focus on the most recent and updated papers on these topics, we fixed the time within 2010 and 2020 as temporal window; however, a manual search for oldest references mentioned in the found articles was also carried out. Papers in the search results reporting somatic mutations or describing genetic risk factors related to sporadic, late onset AD were not included since are out of the topic of the present review (Figure 2).
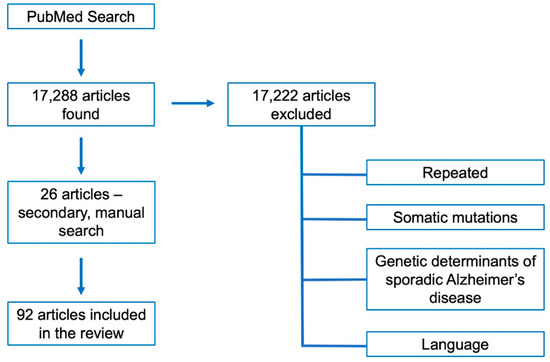
Figure 2.
Flow-diagram summarizing the steps for the selection of the articles reviewed herein.
3. Highly Penetrant Familial Alzheimer Disease-Causing Genes
Genetic analysis of large FAD families allowed the discovery of the three well established and high-penetrant genes related to this disease, namely, APP, PSEN1 and PSEN2 (Figure 3).
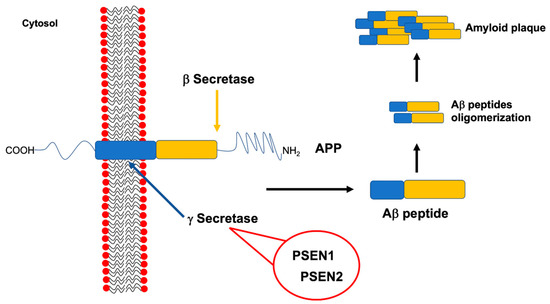
Figure 3.
Amyloid precursor protein (APP) structure and amyloid beta (Aβ) peptide production. The cleavage of APP by specific secretases is required to produce the Aβ peptide. One of these secretases, namely the γ secretase, is a multimeric complex involving also presenilin 1 (PSEN1) and presenilin 2 (PSEN2). Mutations affecting APP, as well as PSEN1 or PSEN2 genes, have been associated to familial Alzheimer’s disease (FAD) because of their ability to increase Aβ peptides production and, consequently, their aggregation up to amyloid plaques formation.
The amyloid precursor protein gene (APP, OMIM #104760, chromosome 21q21.3) encodes for an integral type 1 membrane glycoprotein that is almost ubiquitously expressed. APP is sequentially processed for proteolytic cleavage to produce amyloid β (Aβ), by β- and γ-secretases (the latter composed by PSEN1, PSEN2, Nicastrin and Aph-1). Because APP processing by γ-secretase is not restricted to a single site, it gives rise to different Aβ species, Aβ42 being more prone to aggregate [15].
Recently it has become evident that the AD-typical Aβ assemblies are able to adopt alternative conformations and become self-propagating, like prions [16,17,18,19].
To date, 32 pathogenic mutations in APP were reported within or flanking the Aβ sequence (https://www.alzforum.org/mutations/app), being located mostly near the β- and γ-secretase sites [20]. As a consequence, APP mutations seem to be able to increase Aβ aggregation rate, thus causing FAD (Figure 3) [21].
The p.K670N and p.M671L Swedish AD-related pathogenic mutations are localized near to the γ-secretase site and lead to increased absolute levels of Aβ42 (without changing Aβ42 to Aβ40 ratio) [22]. Interestingly, Del Prete et al., found that in a Swedish cell culture model of AD, APP and its catabolites are present in mitochondrial-ER associated membranes (MAMs) and β- and γ-secretases harbor APP processing activities in MAMs [23]. This finding is extremely interesting, considering the fact that the localization of APP and its enzymes plays a critical role in the Aβ generation and its signaling inside the cell [24]. The p.T714I, p.V715M, p.V715A, p.I716V, p.V717I and p.V717L APP mutations are all located in proximity of the γ-secretase site, affect the cleavage and, contrary to the double Swedish mutations, cause an increase of the Aβ42 to Aβ40 ratio and are able to influence the stability of APP C-terminal fragments [20,25]. Very recently, the p.I716T APP mutation [25], as well as the p.L723P featured by the local unfolding of the C-terminal turn [26], was described to affect the efficiency of the γ-secretase ε-cleavage and to induce a major Aβ42 to Aβ40 ratio. This effect is due to the additional H-bond between the T716 side chain and the transmembrane backbone, which can affect the cleavage domain dynamic [25].
Mutations within the Aβ sequence have been predicted not only to affect APP processing, thus regulating the amount of total Aβ production, but were also thought to affect the aggregation properties of the resulting Aβ peptide [25]. Indeed, the APP p.A692G, p.E693Q and p.D694N mutations, all located inside the Aβ sequence, have been described to have an increased aggregative ability and neurotoxicity respect to the wild type Aβ [21].
Although a common effect of these mutations is the dysregulation of the production of different Aβ forms of APP, a recent paper form Lumsden et al. proposed also a dysregulation of iron homeostasis as a common effect of mutations related to early onset AD [27].
Table 1 summarizes APP mutations, affecting protein stability, folding, processing, from the N- to the C-terminal.

Table 1.
Amyloid precursor protein (APP) pathogenic mutations and their effects at protein level.
Despite the above, it has been reported that APP dominant mutations account for about 16% of all ADAD [3]. Some years ago, 2 APP mutations, namely p.A673V and p.E693del, have been found to be able to cause FAD only in the homozygous status supporting the existence also of a recessive pattern of inheritance [28,29]. More recently, Conidi et al., described an Italian family carrying the APP p.A713T in homozygous status; surprisingly the clinical phenotype was not more severe respect to the heterozygous carriers [30]. This finding not only highlighted that the homozygosity for APP dominant mutations is not lethal, but also suggested that other, independently inherited genetic factors, may exert a protective effect and modify the clinical presentation of the disease.
Finally, in addition to single nucleotide variants and small insertion/deletions, dominantly inherited duplications of the APP locus have been also described and associated to AD [31,32,33,34,35]. In particular, small chromosomal duplications with different genomic coordinates, but all including the APP locus, have been described in some French FAD families [31,32]. Next, APP duplications were reported also in Finnish and Dutch FAD cases [33,34,35]. The clinical consequences of these duplications are not yet clearly defined and also their frequency as FAD cause is variable in the different studies [31,32,33,34,35]. Totally, 25 duplications have been identified so far and, respect to missense mutations, these duplications seem to have a reduced penetrance and a variable age of onset [36].
Interestingly, Jonsson et al., identified a rare APP variant in the Icelandic population showing a protective effect [37]. Finally, a number of variants of unknown clinical significance have been also detected and further functional tests are required to establish their pathogenicity.
The presenilin 1 (PSEN1, OMIM #104311, chromosome 14q24.3) encodes for a protein that is a subunit of γ-secretase, i.e., one of the 2 enzymes responsible for APP proteolytic cleavage. As a consequence, mutations in PSEN1, impairing the activity of γ-secretase complex, may lead to the production of more aggregation-prone forms of the Aβ peptide, that is a typical hallmark of AD, thus inducing the disease development (Figure 3) [3,4].
PSEN1 is the most common gene related to FAD. To date, 221 PSEN1 pathogenic mutations have been described, accounting for up to 70% of ADAD cases (http://www.alzforum.org/mutations). These mutations can be both single nucleotide variants and small insertions/deletions; in addition, a deletion able to cause PSEN1 exon 9 skipping, has been also described [38]. Typically, FAD onset in PSEN1 mutations carriers ranges from 30 to 50 years, the mutations showing an autosomal dominant inheritance and almost always a complete penetrance [39]. Interestingly, de novo mutations, featured by a very early age of onset (before 30 years) have been also described [40,41,42].
As in the case of APP, Kosik et al., described a Colombian family carrying the PSEN1 mutation p.E280A in homozygous status; also, in this case, the severity of the disease was not influenced by the homozygosity of the mutation [43].
The presenilin 2 (PSEN2, OMIM #600759, chromosome 1q31-q42) gene has a genetic structure very similar to PSEN1, sharing a sequence homology of 67% [3]. It encodes for another component of the γ-secretase complex; thus, its impairment, due to pathogenic mutations, is able to increase the Aβ42/Aβ40 ratio too (Figure 3).
To date, 19 different PSEN2 pathogenic mutations have been reported (http://www.alzforum.org/mutations). As for PSEN1, these pathogenic mutations are scattered along the entire gene sequence with a higher frequency in the transmembrane domains [44,45]. The ADAD age of onset ranges from 40 to 70 years in PSEN2 mutations carriers, the penetrance of these mutations being still controversial to assess, due to the few numbers of families reported to date and showing also a so wide age-range of onset [39].
It has been estimated that totally, APP, PSEN1 and PSEN2 mutations account only for about 5–10% of all FAD (Figure 1) [46,47]. Within the FAD, considering only the ADAD forms the contribution of these genes is highly heterogeneous based on the population studied, 23% up to 88% of patients remaining without a genetic diagnosis [47,48]. Since the clinical features of FAD can be variable, the diagnosis is often difficult and delayed, underlying the importance of identifying other molecular alterations responsible for the currently unexplained FAD cases.
4. Apolipoprotein E ε4 Risk Allele and Familial Alzheimer Disease
The apolipoprotein E gene (APOE, OMIM #107741, chromosome 19q13.2) encodes a glycoprotein involved in the mobilization of peripheral cholesterol, also during neuronal growth and regeneration [49,50]. Three APOE isoforms are known, namely ApoE2, ApoE3 and ApoE4, differing at level of 2 aminoacidic residues (the 112 and 158) and coded by 3 alleles, i.e., ε2, ε3 and ε4, whose frequency varies among different populations [3].
To date, an association between the ε4 allele and the sporadic, late-onset AD has been reported [3,4]. In particular, it has been assessed an increased risk up to 3-fold in the heterozygous carriers and up to 15-fold in the homozygous [51]. Interestingly, the ε2 allele has been reported as a protective factor, reducing the AD risk and also positively impacting longevity [52]. These different features have been related to ta different binding affinity of the encoded proteins for the Aβ peptide; in particular, ApoE4 shows the highest affinity leading to the creation of monofibrils that are able to produce dense precipitates [3]. However, it is important to underline that the ε4 allele is not a cause but an AD risk factor; thus, other genetic or environmental factors are required for disease development. A working model, attempting to explain the relationship between ApoE and Alzheimer’s disease, has been proposed (Figure 4) [53].
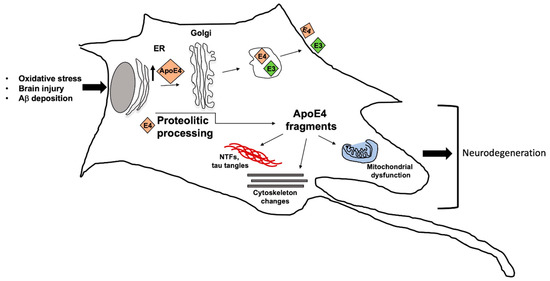
Figure 4.
Working model for Apolipoprotein E4 (ApoE4) contribution to Alzheimer’s disease (AD) development. Different stimuli can induce ApoE4 and ApoE3 overexpression, with E4 contribution to neurodegeneration in AD. ApoE4, undergoing to proteolytic cleavage, can generate different fragments which can contribute to mitochondrial dysfunction, cytoskeletal disorganization, neurofibrillary tangles and, consequently to neurodegeneration.
In particular, ApoE4, unlike ApoE3, contributes to AD by interacting with different factors through various pathways. In response to oxidative stress, aging, brain damage or Aβ deposition, neurons synthesize increasing amount of ApoE, that in turn undergoes proteolytic processing generating fragments which cause mitochondrial dysfunction, cytoskeletal changes, NFT (neurofibrillary tangles) formation, leading to neurodegeneration.
Interestingly, it has been shown that the APOE ε4 allele is able to increase also the risk for early onset AD in presence of familiarity for the disease [54]. In particular, in the ε4 homozygous carriers the risk was independent from other genetic factors, while in the heterozygous no, suggesting that it may act as disease phenotype-modifier in presence of other genetic mutations [4]. However, Genin et al., reported for APOE ε4 allele an AD risk comparable to that of other genetic factors [55]. Some studies, evaluating the effects of the APOE genotype on AD clinical features in families carrying pathogenic mutations in APP, PSEN1 or PSEN2 genes, showed that the ε4 allele is associated to an earlier age of onset in the mutations’ carriers, while the carriers of the ε2 allele had a later onset [56,57,58]. Nevertheless, it is important to underline that the significance of APOE testing in clinical practice is still under debate and it has been recently reviewed to not significantly impact diagnostic and prognostic evaluations [59]. Indeed, being a risk factor, the APOE ε4 allele is common in the general population, i.e., also in healthy individual without a positive family history of AD. Further large longitudinal studies are required to assess the contribution of APOE to AD risk and its possible use in clinical routine settings.
5. Novel, Emerging and Candidate Genes Associated to Familial Alzheimer Disease
Mutations in APP, PSEN1 and PSEN2 genes, as well as the APOE ε4 risk allele, explain only a small percentage of all FAD cases, suggesting that other genes may play a role. In the last years, NGS based studies, through the analysis of large pedigrees, are allowing the detection of novel genes potentially related to FAD.
Guerreiro et al., analyzing a Turkish FAD family, identified a pathogenic mutation in the NOTCH3 gene [60]. Interestingly, the same mutation was previously associated with a dementia disorder similar to AD and the proband belongs to a consanguineous family with a complex history of neurological disorders [60]. NOTCH3 (OMIM# 600276, chromosome 19p13.12) encodes a transmembrane receptor involved in cellular signaling and embryonic development. More than 130 mutations have been reported in this gene and related to the rare syndrome cerebral arteriopathy autosomal dominant with subcortical infarcts and leukoencephalopathy; a role also in FAD has been recently proposed [61].
The finding of a shared gene between degenerative and vascular dementias suggests the presence of a similar neurovascular unit dysfunction. Accordingly, a consensus paper by Bordet et al., based on the observation that most of patients currently seem to be affected by mixed forms, proposed that also therapeutic strategies should be common [62]. Indeed, therapeutic approaches should be oriented towards an integrated strategy, including antioxidants, anti-inflammatory, modulation of proteins aggregation and neuronal plasticity. Since in older patients, vascular cognitive impairment (VCI) leading to vascular dementia is often mixed to AD and VCI is rarely “pure”, a disease modifying strategy seems to be justified [62]. It is noteworthy that a mitochondrial dysfunction may play a role in this context as an additional cause of cognitive impairment, either of vascular, degenerative or both natures [63]. In particular, a reduction of respiratory chain complex I activity, related to mitochondrial dysfunction, has been reported in a group of patients with vascular dementia and several mitochondrial mechanisms have been invoked in Aβ-related cerebrovascular degeneration [63].
Pottier et al., carried out the WES of 29 probands from FAD families resulted negative for mutations in the 3 main FAD genes and found 7 mutations in the SORL1 gene [64]. In particular, one of these mutations, i.e., the p.G511R, has been shown to be able to reduce the ability of the protein to bind the Aβ peptide, thus inducing its accumulation [65]. The sortilin-related receptor (SORL1, OMIM# 602005, chromosome 11q24.1) encodes for a mosaic protein that is the receptor of neuronal ApoE. Accordingly, SORL-1 mutations have been described in 2 families with early onset AD. In particular, the SORL-1 variants where shown to be able to weaken the interaction with APP, interfering with APP trafficking and altering the Aβ levels [66]. Other studies have confirmed the role of SORL1 mutations in FAD and also in late onset AD [67,68]. Li et al., have recently reported the case of a patient with early onset AD and cognitive impairment, carrying a heterozygous mutation in the SORL1 gene [69]. Taken together, these items of evidence suggest that SORL1 mutations may be involved in FAD, its contribution being probably underestimated, and that this gene should be tested in the affected families in addition to APP, PSEN1 and PSEN2 genes. Indeed, the analysis of SORL1 in larger cohorts of patients may allow to better clarify its contribution to FAD.
Interestingly, genome-wide association studies identified about 30 additional risk factors/alleles for late onset AD [70,71]. Among these, variants affecting CLU (APOJ) or CR1 (complement component 3b/4b receptor 1), being involved in the clearance of Aβ have been associated to AD [72] and heterozygous missense mutations in TREM2 (triggering receptor myeloid 2 cells) have been described to increase by 3-fold the risk of AD [73]. It has been proposed that these genes may be responsible also for FAD cases.
In particular, an increased frequency of CLU gene rare coding mutations has been highlighted in AD patients, predominantly affecting the β chain of the protein [74]. Clusterin (CLU, OMIM# 185430, chromosome 8p21.1) encodes a protein involved in synapsis turnover. Most of the CLU variants described so far are able to promote CLU degradation, thus reducing its activity [75].
Two NGS-based studies identified a rare variant (p.Arg47His) in TREM2 gene [73,76] TREM2 (OMIM# 605086, chromosome 6p21.1) encodes a type I transmembrane protein belonging to the immunoglobulin receptor superfamily and involved in immune responses activation. Interestingly, TREM2 has been found to be able to bind ApoE, thus increasing the phagocytosis of ApoE-bound apoptotic neurons [77]. Some TREM2 variants may increase AD risk by reducing the affinity for ApoE, and thus decreasing Aβ peptide clearance. Additionally, it has been showed that TREM2 mutations in its extracellular domain impair protein maturation and its phagocytic activity [78,79]. The TREM2 p.Arg47His variant has been reported in different population as associated to AD, including some cases of FAD [73,76,80,81,82]. The role of other TREM2 variants is still poorly understood.
Three independent studies identified loss-of-function mutations in the ABCA7 gene in AD patients [83,84,85]. The ATP-binding cassette, subfamily A, member 7 (ABCA7, OMIM# 605414, chromosome 19p13.3) encodes a transporter protein able to move lipids across the membranes. It has been reported that the inhibition of ABCA7 expression is able to increase β secretase cleavage of APP, thus increasing the production of Aβ peptide [86]. In particular, Cuyvers et al., identified an ABCA7 frameshift mutation as a founder mutation in a Belgian population, since it was detected in several FAD families showing a dominant pattern of inheritance [83].
Vardarajan et al., by sequencing 76 AD-related loci, identified a rare missense mutation in the EPHA1 gene (p.P460L) segregating within a large Caribbean FAD family [85]. The Ephrin receptor (EPHA1, OMIM # 179610, chromosome 7q34-q35) encodes a tyrosine kinase receptor implicated in neuronal development.
The main features of novel FAD candidate genes are summarized in Table 2.

Table 2.
Novel candidate genes and inherited risk factors associated to familial Alzheimer’s disease (FAD).
It is noticeable that the same genes identified as risk factors for sporadic and late onset AD, harbor also rare variants segregating with FAD. Even if data in this field are still inconclusive since they are often based on isolated findings, however they suggest that some familial cases may be due the combination of rare variants and other risk factors. Recent NGS-based screening including FAD cases, identified established risk alleles with moderate penetrance and one or more variants of uncertain significance, thus suggesting the hypothesis, in presence of no mutations in the 3 main FAD genes, of a polygenic inheritance [87,88]. The use of NGS-based methods for the analysis of large genomic regions in several patients simultaneously may provide further insights to improve the diagnosis of disorders featured by high genetic and phenotypic variability, such as AD. However, the interpretation of NGS data, due to the large number of variants of unknown significance, is often challenging and inconclusive in clinical settings [14,89].
6. Conclusions
AD incidence is showing an increasing trend worldwide, so its early and accurate diagnosis has become mandatory. Indeed, the earlier the diagnosis is made, the sooner the treatments may begin, the latter being an important prognostic factor to ameliorate patients’ clinical outcome. It is becoming evident that within the “AD” definition are included different entities, whose correct identification may be important to drive treatments choices. While most of AD cases are sporadic, featured by late onset and by the presence of polygenic risk factors, a small percentage of AD is familial, often featured by early age of onset and related to the presence of rare, pathogenic mutations segregating within the affected families. To date, 3 main genes (APP, PSEN1 and PSEN2) have been related to autosomal dominant FAD, accounting just for a small percentage of cases. Novel candidate genes are being identified. Larger studies on large group of patients are required to better address their contribution to the disease and discover other potential candidates. This will allow a better prognostic classification of the patients and a better management of the probands and of their families, by the identification of the -at risk individuals. These genetic data, together with novel clues coming from other “omics” [90,91,92], will allow also the development of novel and even more personalized therapies for AD and FAD treatment.
Author Contributions
V.D. and D.S.: conceptualization; writing—original draft preparation; writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Collaborators GBDD. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 181, 88–106. [Google Scholar]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef]
- Guerreiro, R.J.; Gustafson, D.R.; Hardy, J. The genetic architecture of Alzheimer’s disease: Beyond APP, PSENs and APOE. Neurobiol. Aging 2012, 33, 437–456. [Google Scholar] [CrossRef]
- Cacace, R.; Sleegers, K.; Van Broeckhoven, C. Molecular genetics of early-onset Alzheimer’s disease revisited. Alzheimers Dement. 2016, 12, 733–748. [Google Scholar] [CrossRef]
- Goldman, J.S.; Hahn, S.E.; Catania, J.W.; LaRusse-Eckert, S.; Butson, M.B.; Rumbaugh, M.; Strecker, M.N.; Roberts, J.S.; Burke, W.; Mayeux, R.; et al. American College of Medical Genetics and the National Society of Genetic Counselors. Genetic counseling and testing for Alzheimer disease: Joint practice guidelines of the American College of Medical Genetics and the National Society of Genetic Counselors. Genet. Med. 2011, 136, 597–605. [Google Scholar] [CrossRef]
- Reiman, E.M.; Langbaum, J.B.; Tariot, P.N.; Lopera, F.; Bateman, R.J.; Morris, J.C.; Sperling, R.A.; Aisen, P.S.; Roses, A.D.; Welsh-Bohmer, K.A.; et al. CAP-advancing the evaluation of preclinical Alzheimer disease treatments. Nat. Rev. Neurol. 2016, 121, 56–61. [Google Scholar] [CrossRef]
- Tariot, P.N.; Lopera, F.; Langbaum, J.B.; Thomas, R.G.; Hendrix, S.; Schneider, L.S.; Rios-Romenets, S.; Giraldo, M.; Acosta, N.; Tobon, C.; et al. The Alzheimer’s Prevention Initiative Autosomal-Dominant Alzheimer’s Disease Trial: A study of crenezumab versus placebo in preclinical PSEN1 E280A mutation carriers to evaluate efficacy and safety in the treatment of autosomal-dominant Alzheimer’s disease, including a placebo-treated noncarrier cohort. Alzheimers Dement. (N. Y.) 2018, 4, 150–160. [Google Scholar]
- Bergougnoux, A.; D’Argenio, V.; Sollfrank, S.; Verneau, F.; Telese, A.; Postiglione, I.; Lackner, K.J.; Claustres, M.; Castaldo, G.; Rossman, H.; et al. Multicenter validation study for the certification of a CFTR gene scanning method using next generation sequencing technology. Clin. Chem. Lab. Med. 2018, 56, 1046–1053. [Google Scholar] [CrossRef]
- D’Argenio, V.; Esposito, M.V.; Telese, A.; Precone, V.; Starnone, F.; Nunziato, M.; Cantiello, P.; Iorio, M.; Evangelista, E.; D’Aiuto, M.; et al. The molecular analysis of BRCA1 and BRCA2: Next-generation sequencing supersedes conventional approaches. Clin. Chim. Acta 2015, 446, 221–225. [Google Scholar] [CrossRef]
- D’Argenio, V.; Frisso, G.; Precone, V.; Boccia, A.; Fienga, A.; Pacileo, G.; Limongelli, G.; Paolella, G.; Calabrò, R.; Salvatore, F. DNA sequence capture and next-generation sequencing for the molecular diagnosis of genetic cardiomyopathies. J. Mol. Diagn. 2014, 16, 32–44. [Google Scholar] [CrossRef]
- Cariati, F.; D’Argenio, V.; Tomaiuolo, R. The evolving role of genetic tests in reproductive medicine. J. Transl. Med. 2019, 17, 267. [Google Scholar] [CrossRef]
- Nunziato, M.; Esposito, M.V.; Starnone, F.; Diroma, M.A.; Calabrese, A.; Del Monaco, V.; Buono, P.; Frasci, G.; Botti, G.; D’Aiuto, M.; et al. A multi-gene panel beyond BRCA1/BRCA2 to identify new breast cancer-predisposing mutations by a picodroplet PCR followed by a next-generation sequencing strategy: A pilot study. Anal. Chim. Acta 2019, 1046, 154–162. [Google Scholar] [CrossRef]
- Precone, V.; Del Monaco, V.; Esposito, M.V.; De Palma, F.D.; Ruocco, A.; Salvatore, F.; D’Argenio, V. Cracking the code of human diseases using next-generation sequencing: Applications, challenges, and perspectives. Biomed. Res. Int. 2015, 161648. [Google Scholar] [CrossRef]
- D’Argenio, V. The high-throughput analyses era: are we ready for the data struggle? High. Throughput 2018, 7, 8. [Google Scholar] [CrossRef]
- Belinova, I.; Karran, E.; De Strooper, B. The toxic Ab oligomer and Alzheimer’s disease: An emperor in need of clothes. Nat. Neurosci. 2012, 15, 349–357. [Google Scholar]
- Goedert, M.; Clavaguera, F.; Tolnay, M. The propagation of prion-like protein inclusions in neurodegenerative diseases. Trends Neurosci. 2010, 33, 317–325. [Google Scholar] [CrossRef]
- Jucker, M.; Walker, L.C. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature 2013, 501, 45–51. [Google Scholar] [CrossRef]
- Sarnataro, D.; Pepe, A.; Zurzolo, C. Cell biology of prion protein. Prog. Mol. Biol. Transl. Sci. 2017, 150, 57–82. [Google Scholar]
- Sarnataro, D. Attempt to untangle the prion-like misfolding mechanism for neurodegenerative diseases. Int. J. Mol. Sci. 2018, 19, 3081. [Google Scholar] [CrossRef]
- De Jonghe, C.; Esselens, C.; Kumar-Singh, S.; Craessaerts, K.; Serneela, S.; Checler, F.; Annaert, W.; Van Broeckhoven, C.; De Strooper, B. Pathogenic APP mutations near the γ-secretase cleavage site differentially affect Aβ secretion and APP C-terminal fragment stability. Hum. Mol. Gen. 2001, 16, 1665–1671. [Google Scholar] [CrossRef]
- Murakami, K.; Irie, K.; Morimoto, A.; Ohigashi, H.; Shindo, M.; Nagao, M.; Shimizu, T.; Shirasawa, T. Synthesis, aggregation, neurotoxicity, and secondary structure of various Aβ1-42 mutants of familial Alzheimer’s disease at positions 21–23. Biochem. Biophys. Res. Commun. 2002, 294, 5–10. [Google Scholar] [CrossRef]
- Kumar-Singh, S. Hereditary and sporadic forms of Aβ-cerebrovascular amyloidosis and relevant transgenic mouse models. Int. J. Mol. Sci. 2009, 10, 1872–1895. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, D.; Suski, J.M.; Oulès, B.; Debayle, D.; Gay, A.S.; Lacas-Gervais, S.; Bussiere, R.; Bauer, C.; Pinton, P.; Paterlini-Bréchot, P.; et al. Localization and processing of the Amyloid-β protein precursor in mitochondria-associated membranes. J. Alzheimers Dis. 2017, 55, 1549–1570. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Limone, A.; Napolitano, F.; Cerchia, C.; Parisi, S.; Minopoli, G.; Montuori, N.; Lavecchia, A.; Sarnataro, D. APP maturation and intracellular localization are controlled by a specific inhibitor of 37/67 kDa laminin-1 receptor in neuronal cells. Int. J. Mol. Sci. 2020, 21, 1738. [Google Scholar] [CrossRef] [PubMed]
- Götz, A.; Hogel, P.; Silber, M.; Chaitoglou, I.; Luy, B.; Muhle-Goll, C.; Scharnagl, C.; Langosch, D. Increased H-bond stability relates to altered e-cleavage efficiency and Ab levels in the I45T familial Alzheimer’s disease mutant of APP. Sci. Rep. 2019, 9, 5321. [Google Scholar] [CrossRef]
- Bocharov, E.V.; Nadezhdin, K.D.; Urban, A.S.; Volynsky, P.E.; Pavlov, K.V.; Efremov, R.G.; Arseniev, A.S.; Bocharova, O.V. Familial L723P mutation can shift the distribution between the alternative APP transmembrane domain cleavage cascades by local unfolding of the E-cleavage site suggesting a straightforward mechanism of Alzheimer’s disease pathogenesis. ACS Chem. Biol. 2019, 4, 1573–1582. [Google Scholar] [CrossRef]
- Lumsden, A.L.; Rogers, J.T.; Majd, S.; Newman, M.; Sutherland, G.T.; Verdile, G.; Lardelli, M. Dysregulation of neuronal iron homeostasis as an alternative unifying effect of mutations causing familial Alzheimer’s disease. Front. Neurosci. 2018, 12, 533. [Google Scholar] [CrossRef]
- Di Fede, G.; Catania, M.; Morbin, M.; Rossi, G.; Suardi, S.; Mazzoleni, G.; Merlin, M.; Giovagnoli, A.R.; Prioni, S.; Erbetta, A.; et al. A recessive mutation in the APP gene with dominant-negative effect on amyloidogenesis. Science 2009, 323, 1473–1477. [Google Scholar] [CrossRef]
- Tomiyama, T.; Nagata, T.; Shimada, H.; Teraoka, R.; Fukushima, A.; Kanemitsu, H.; Takuma, H.; Kuwano, R.; Imagawa, M.; Ataka, S.; et al. A new amyloid beta variant favoring oligomerization in Alzheimer’stype dementia. Ann. Neurol. 2008, 63, 377–387. [Google Scholar] [CrossRef]
- Conidi, M.E.; Bernardi, L.; Puccio, G.; Smirne, N.; Muraca, M.G.; Curcio, S.A.; Colao, R.; Piscopo, P.; Gallo, M.; Anfossi, M.; et al. Homozygous carriers of APP A713T mutation in an autosomal dominant Alzheimer disease family. Neurology 2015, 84, 2266–2273. [Google Scholar] [CrossRef]
- Cabrejo, L.; Guyant-Marechal, L.; Laquerriere, A.; Vercelletto, M.; De la Fourniere, F.; Thomas-Anterion, C.; Verny, C.; Letournel, F.; Pasquier, F.; Vital, A.; et al. Phenotype associated with APP duplication in five families. Brain 2006, 129, 2966–2976. [Google Scholar] [CrossRef] [PubMed]
- Rovelet-Lecrux, A.; Hannequin, D.; Raux, G.; Le Meur, N.; Laquerriere, A.; Vital, A.; Dumanchin, C.; Feuillette, S.; Brice, A.; Vercelletto, M.; et al. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat. Genet. 2006, 38, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Remes, A.M.; Finnila, S.; Mononen, H.; Tuominen, H.; Takalo, R.; Herva, R.; Majamaa, K. Hereditary dementia with intracerebral hemorrhages and cerebral amyloid angiopathy. Neurology 2004, 63, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Rovelet-Lecrux, A.; Frebourg, T.; Tuominen, H.; Majamaa, K.; Campion, D.; Remes, A.M. APP locus duplication in a Finnish family with dementia and intracerebral haemorrhage. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1158–1159. [Google Scholar] [CrossRef] [PubMed]
- Sleegers, K.; Brouwers, N.; Gijselinck, I.; Theuns, J.; Goossens, D.; Wauters, J.; Del-Favero, J.; Cruts, M.; van Duijn, C.M.; Van Broeckhoven, C. APP duplication is sufficient to cause early onset Alzheimer’s dementia with cerebral amyloid angiopathy. Brain 2006, 129, 2977–2983. [Google Scholar] [CrossRef] [PubMed]
- Hooli, B.V.; Mohapatra, G.; Mattheisen, M.; Parrado, A.R.; Roehr, J.T.; Shen, Y.; Gusella, J.F.; Moir, R.; Saunders, A.J.; Lange, C.; et al. Role of common and rare APP DNA sequence variants in Alzheimer disease. Neurology 2012, 78, 1250–1257. [Google Scholar] [CrossRef]
- Jonsson, T.; Atwal, J.K.; Steinberg, S.; Snaedal, J.; Jonsson, P.V.; Bjornsson, S.; Stefansson, H.; Sulem, P.; Gudbjartsson, D.; Maloney, J.; et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature 2012, 488, 96–99. [Google Scholar] [CrossRef]
- Cruts, M.; Theuns, J.; Van Broeckhoven, C. Locus-specific mutation databases for neurodegenerative brain diseases. Hum. Mutat. 2012, 33, 1340–1344. [Google Scholar] [CrossRef]
- Pilotto, A.; Padovani, A.; Borroni, B. Clinical, biological, and imaging features of monogenic Alzheimer’s Disease. Biomed. Res. Int. 2013, 689591. [Google Scholar] [CrossRef]
- Portet, F.; Dauvilliers, Y.; Campion, D.; Raux, G.; Hauw, J.J.; Lyon-Caen, O.; Camu, W.; Touchon, J. Very early onset AD with a de novo mutation in the presenilin 1 gene (Met 233 Leu). Neurology 2003, 61, 1136–1137. [Google Scholar] [CrossRef]
- Golan, M.P.; Styczynska, M.; Jozwiak, K.; Walecki, J.; Maruszak, A.; Pniewski, J.; Lugiewicz, R.; Filipek, S.; Zekanowski, C.; Barcikowska, M. Early-onset Alzheimer’s disease with a de novo mutation in the presenilin 1 gene. Exp. Neurol. 2007, 208, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Lanoiselèe, H.M.; Nicolas, G.; Wallon, D.; Rovelet-Lecrux, A.; Lacour, M.; Rousseau, S.; Richard, A.C.; Pasquier, F.; Rollin-Sillaire, A.; Martinaud, O.; et al. Collaborators of the CNR-MAJ project. APP, PSEN1, and PSEN2 mutations in early-onset Alzheimer disease: A genetic screening study of familial and sporadic cases. PLoS Med. 2017, 14, e1002270. [Google Scholar] [CrossRef] [PubMed]
- Kosik, K.S.; Munoz, C.; Lopez, L.; Arcila, M.L.; Garcia, G.; Madrigal, L.; Moreno, S.; Ríos Romenets, S.; Lopez, H.; Gutierrez, M.; et al. Homozygosity of the autosomal dominant Alzheimer disease presenilin 1 E280A mutation. Neurology 2015, 84, 206–208. [Google Scholar] [CrossRef]
- Hardy, J.; Crook, R. Presenilin mutations line up along transmembrane alpha-helices. Neurosci. Lett. 2001, 306, 203–205. [Google Scholar] [CrossRef]
- Guerreiro, R.J.; Beck, J.; Gibbs, J.R.; Santana, I.; Rossor, M.N.; Schott, J.M.; Nalls, M.A.; Ribeiro, H.; Santiago, B.; Fox, N.C.; et al. Genetic Variability in CLU and Its Association with Alzheimer’s Disease. PLoS ONE 2010, 5, e9510. [Google Scholar] [CrossRef]
- Brouwers, N.; Sleegers, K.; Van Broeckhoven, C. Molecular genetics of Alzheimer’s disease: An update. Ann. Med. 2008, 40, 562–583. [Google Scholar] [CrossRef]
- Wingo, T.S.; Lah, J.J.; Levey, A.I.; Cutler, D.J. Autosomal recessive causes likely in early-onset Alzheimer disease. Arch. Neurol. 2012, 69, 59–64. [Google Scholar] [CrossRef]
- Jarmolowicz, A.I.; Chen, H.Y.; Panegyres, P.K. The Patterns of Inheritance in Early-Onset Dementia: Alzheimer’s Disease and Frontotemporal Dementia. Am. J. Alzheimers Dis. Other Dement. 2014, 30, 299–306. [Google Scholar] [CrossRef]
- Mahley, R.W. Apolipoprotein E: Cholesterol transport protein with expanding role in cell biology. Science 1988, 240, 622–630. [Google Scholar] [CrossRef]
- Mahley, R.W.; Rall, S.C., Jr. Apolipoprotein E: Far more than a lipid transport protein. Annu Rev. Genomics Hum. Genet. 2000, 1, 507–537. [Google Scholar] [CrossRef]
- Ashford, J.W. APOE genotype effects on Alzheimer’s disease onset and epidemiology. J. Mol. Neurosci. 2004, 23, 157–165. [Google Scholar] [CrossRef]
- Corder, E.H.; Saunders, A.M.; Risch, N.J.; Strittmatter, W.J.; Schmechel, D.E.; Gaskell, P.C., Jr.; Rimmler, J.B.; Locke, P.A.; Conneally, P.M.; Schmader, K.E. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat. Genet. 1994, 7, 180–184. [Google Scholar] [CrossRef]
- Huang, Y. Apolipoprotein E and Alzheimer disease. Neurology 2006, 66, S79–S85. [Google Scholar] [CrossRef] [PubMed]
- Van Duijn, C.M.; de Knijff, P.; Cruts, M.; Wehnert, A.; Havekes, L.M.; Hofman, A.; Van Broeckhoven, C. Apolipoprotein E4 allele in a population-based study of early-onset Alzheimer’s disease. Nat. Genet. 1994, 7, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Genin, E.; Hannequin, D.; Wallon, D.; Sleegers, K.; Hiltunen, M.; Combarros, O.; Bullido, M.J.; Engelborghs, S.; De Deyn, P.; Berr, C.; et al. APOE and Alzheimer disease: A major gene with semi-dominant inheritance. Mol. Psychiatry 2011, 16, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Sorbi, S.; Nacmias, B.; Forleo, P.; Piacentini, S.; Latorraca, S.; Amaducci, L. Epistatic effect of APP717 mutation and apolipoprotein E genotype in familial Alzheimer’s disease. Ann. Neurol. 1995, 38, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Pastor, P.; Roe, C.M.; Villegas, A.; Bedoya, G.; Chakraverty, S.; Garcia, G.; Bedoya, G.; Chakraverty, S.; García, G.; Tirado, V.; et al. Apolipoprotein Eepsilon4 modifies Alzheimer’s disease onset in an E280A PS1 kindred. Ann. Neurol. 2003, 54, 163–169. [Google Scholar] [CrossRef]
- Wijsman, E.M.; Daw, E.W.; Yu, X.; Steinbart, E.J.; Nochlin, D.; Bird, T.D.; Schellenberg, G.D. APOE and other loci affect age-at-onset in Alzheimer’s disease families with PS2 mutation. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2005, 132, 14–20. [Google Scholar] [CrossRef]
- Petersen, R.C. Mild Cognitive Impairment. Continuum (Minneap Minn) 2016, 22, 404–418. [Google Scholar] [CrossRef]
- Guerreiro, R.J.; Lohmann, E.; Kinsella, E.; Bras, J.M.; Luu, N.; Gurunlian, N.; Dursun, B.; Bilgic, B.; Santana, I.; Hanagasi, H.; et al. Exome sequencing reveals an unexpected genetic cause of disease: NOTCH3 mutation in a Turkish family with Alzheimer’s disease. Neurobiol. Aging 2012, 33, 1008–1023. [Google Scholar] [CrossRef]
- Patel, D.; Mez, J.; Vardarajan, B.N.; Staley, L.; Chung, J.; Zhang, X.; Farrell, J.J.; Rynkiewicz, M.J.; Cannon-Albright, L.A.; Teerlink, C.C.; et al. Alzheimer’s Disease Sequencing Project. Association of Rare Coding Mutations with Alzheimer Disease and Other Dementias Among Adults of European Ancestry. JAMA Netw. Open 2019, 2, 191350. [Google Scholar] [CrossRef] [PubMed]
- Bordet, R.; Ihl, R.; Korczyn, A.D.; Lanza, G.; Jansa, J.; Hoerr, R.; Guekht, A. Towards the concept of disease-modifier in post-stroke or vascular cognitive impairment: A consensus report. BMC Med. 2017, 15, 107. [Google Scholar] [CrossRef] [PubMed]
- Lanza, G.; Cantone, M.; Musso, S.; Borgione, E.; Scuderi, C.; Ferri, R. Early-onset subcortical ischemic vascular dementia in an adult with mtDNA mutation 3316G>A. J. Neurol. 2018, 265, 968–969. [Google Scholar] [CrossRef] [PubMed]
- Pottier, C.; Hannequin, D.; Coutant, S.; Rovelet-Lecrux, A.; Wallon, D.; Rousseau, S.; Legallic, S.; Paquet, C.; Bombois, S.; Pariente, J.; et al. PHRC GMAJ Collaborators. High frequency of potentially pathogenic SORL1 mutations in autosomal dominant early-onset Alzheimer disease. Mol. Psychiatry 2012, 17, 875–879. [Google Scholar] [CrossRef]
- Caglayan, S.; Takagi-Niidome, S.; Liao, F.; Carlo, A.S.; Schmidt, V.; Burgert, T.; Kitago, Y.; Füchtbauer, E.M.; Füchtbauer, A.; Holtzman, D.M.; et al. Lysosomal sorting of amyloid-beta by the SORLA receptor is impaired by a familial Alzheimer’s disease mutation. Sci. Transl. Med. 2014, 6, 223. [Google Scholar] [CrossRef]
- Cuccaro, M.L.; Camey, R.M.; Zhang, Y.; Bohm, C.; Kunkle, B.W.; Vardarajan, B.N.; Whitehead, P.L.; Cukier, H.N.; Mayeux, R.; George-Hyslop, P.; et al. SORL1 mutations in early- and late-onset Alzheimer disease. Neurol. Genet. 2016, 2, e116. [Google Scholar]
- Nicolas, G.; Charbonnier, C.; Wallon, D.; Quenez, O.; Bellenguez, C.; Grenier-Boley, B.; Rousseau, S.; Richard, A.C.; Rovelet-Lecrux, A.; Le Guennec, K.; et al. CNR-MAJ collaborators. SORL1 rare variants: A major risk factor for familial early-onset Alzheimer’s disease. Mol. Psychiatry 2016, 21, 831–836. [Google Scholar] [CrossRef]
- Vardarajan, B.N.; Zhang, Y.; Lee, J.H.; Cheng, R.; Bohm, C.; Ghani, M.; Cheng, R.; Bohm, C.; Ghani, M.; Reitz, C.; et al. Coding mutations in SORL1 and Alzheimer disease. Ann. Neurol. 2015, 77, 215–227. [Google Scholar] [CrossRef]
- Li, X.; Xiong, Z.; Liu, Y.; Yuan, Y.; Deng, J.; Xiang, W.; Li, Z. Case report of first—Episode psychotic symptoms in a patient with early-onset Alzheimer’s disease. BMC Psychiatry 2020, 20, 128. [Google Scholar] [CrossRef]
- Cuyvers, E.; Sleegers, K. Genetic variations underlying Alzheimer’s disease: Evidence from genome-wide association studies and beyond. Lancet Neurol. 2016, 15, 857–868. [Google Scholar] [CrossRef]
- Shen, L.; Jia, J. An overview of Genome-Wide Association Studies in Alzheimer’s Disease. Neurosci. Bull. 2016, 32, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.C.; Heath, S.; Even, G.; Campion, D.; Sleegers, K.; Hiltunen, M.; Combarros, O.; Zelenika, D.; Bullido, M.J.; Tavernier, B.; et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat. Gen. 2009, 41, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, T.; Stefansson, H.; Steinberg, S.; Jonsdottir, I.; Jonsson, P.V.; Snaedal, J.; Bjornsson, S.; Huttenlocher, J.; Levey, A.I.; Lah, J.J.; et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N. Engl. J. Med. 2013, 368, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Bettens, K.; Brouwers, N.; Engelborghs, S.; Lambert, J.C.; Rogaeva, E.; Vandenberghe, R.; Le Bastard, N.; Pasquier, F.; Vermeulen, S.; Van Dongen, J.; et al. Both common variations and rare non-synonymous substitutions and small insertion/deletions in CLU are associated with increased Alzheimer risk. Mol. Neurodegener. 2012, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Bettens, K.; Vermeulen, S.; Van Cauwenberghe, C.; Heeman, B.; Asselbergh, B.; Robberecht, C.; Engelborghs, S.; Vandenbulcke, M.; Vandenberghe, R.; De Deyn, P.P.; et al. Reduced secreted clusterin as a mechanism for Alzheimer-associated CLU mutations. Mol. Neurodegener. 2015, 10, 30. [Google Scholar] [CrossRef]
- Guerreiro, R.; Wojtas, A.; Bras, J.; Carrasquillo, M.; Rogaeva, E.; Majounie, E.; Cruchaga, C.; Sassi, C.; Kauwe, J.S.; Younkin, S.; et al. Alzheimer Genetic Analysis Group. TREM2 variants in Alzheimer’s disease. Engl. J. Med. 2013, 368, 117–127. [Google Scholar] [CrossRef]
- Bailey, C.C.; DeVaux, L.B.; Farzan, M. The triggering receptor expressed on myeloid cells 2 binds apolipoprotein E. J. Biol. Chem. 2015, 290, 26033–26042. [Google Scholar] [CrossRef]
- Lue, L.F.; Schmitz, C.; Walker, D.G. What happens to microglial TREM2 in Alzheimer’s disease: Immunoregulatory turned into immunopathogenic? Neuroscience 2015, 302, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Kleinberger, G.; Yamanishi, Y.; Suarez-Calvet, M.; Czirr, E.; Lohmann, E.; Cuyvers, E.; Struyfs, H.; Pettkus, N.; Wenninger-Weinzierl, A.; Mazaheri, F.; et al. TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Sci. Transl. Med. 2014, 6, 243ra86. [Google Scholar] [CrossRef] [PubMed]
- Cuyvers, E.; Bettens, K.; Philtjens, S.; Van Langenhove, T.; Gijselinck, I.; van der Zee, J.; Engelborghs, S.; Vandenbulcke, M.; Van Dongen, J.; Geerts, N.; et al. BELNEU consortium. Investigating the role of rare heterozygous TREM2 variants in Alzheimer’s disease and frontotemporal dementia. Neurobiol. Aging 2014, 35, 726–729. [Google Scholar] [CrossRef] [PubMed]
- Pottier, C.; Wallon, D.; Rousseau, S.; Rovelet-Lecrux, A.; Richard, A.C.; Rollin-Sillaire, A.; Frebourg, T.; Campion, D.; Hannequin, D. TREM2 R47H variant as a risk factor for early-onset Alzheimer’s disease. J. Alzheimers Dis. 2013, 35, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Cruchaga, C.; Karch, C.M.; Jin, S.C.; Benitez, B.A.; Cai, Y.; Guerreiro, R.; Harari, O.; Norton, J.; Budde, J.; Bertelsen, S.; et al. Rare coding variants in the phospholipase D3 gene confer risk for Alzheimer’s disease. Nature 2014, 505, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Cuyvers, E.; De Roeck, A.; Van den Bossche, T.; Van Cauwenberghe, C.; Bettens, K.; Vermeulen, S.; Mattheijssens, M.; Peeters, K.; Engelborghs, S.; Vandenbulcke, M.; et al. Mutations in 74 ABCA7 in a Belgian cohort of Alzheimer’s disease patients: A targeted resequencing study. Lancet Neurol. 2015, 14, 814–822. [Google Scholar] [CrossRef]
- Steinberg, S.; Stefansson, H.; Jonsson, T.; Johannsdottir, H.; Ingason, A.; Helgason, H.; Sulem, P.; Magnusson, O.T.; Gudjonsson, S.A.; Unnsteinsdottir, U.; et al. Loss-of-function variants in ABCA7 confer risk of Alzheimer’s disease. Nat. Genet. 2015, 47, 445–447. [Google Scholar] [CrossRef]
- Vardarajan, B.N.; Ghani, M.; Kahn, A.; Sheikh, S.; Sato, C.; Barral, S.; Lee, J.H.; Cheng, R.; Reitz, C.; Lantigua, R.; et al. Rare coding mutations identified by sequencing of Alzheimer disease genome-wide 76 association studies loci. Ann. Neurol. 2015, 78, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Abe-Dohmae, S.; Yokoyama, S.; St George-Hyslop, P.; Fraser, P.E. ATP-binding cassette transporter A7 (ABCA7) loss of function alters Alzheimer amyloid processing. J. Biol. Chem. 2015, 290, 24152–24165. [Google Scholar] [CrossRef]
- Bonvicini, C.; Scassellati, C.; Benussi, L.; Di Maria, E.; Maj, C.; Ciani, M.; Fostinelli, S.; Mega, A.; Bocchetta, M.; Lanzi, G.; et al. Next Generation Sequencing Analysis in Early Onset Dementia Patients. J. Alzheimers Dis. 2019, 67, 243–256. [Google Scholar] [CrossRef]
- Cochran, J.N.; McKinley, E.C.; Cochran, M.; Amaral, M.D.; Moyers, B.A.; Lasseigne, B.N.; Gray, D.E.; Lawlor, J.M.J.; Prokop, J.W.; Geier, E.G.; et al. Genome sequencing for early-onset or atypical dementia: High diagnostic yield and frequent observation of multiple contributory alleles. Cold Spring Harb. Mol. Case Stud. 2019, 5, a003491. [Google Scholar] [CrossRef] [PubMed]
- Cali, F.; Cantone, M.; Cosentino, F., II; Lanza, G.; Ruggeri, G.; Chiavetta, V.; Salluzzo, R.; Ragalmuto, A.; Vinci, M.; Ferri, R. Interpreting Genetic Variants: Hints from a Family Cluster of Parkinson’s Disease. J. Parkinsons Dis. 2019, 9, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Qazi, T.J.; Quan, Z.; Mir, A.; Qing, H. Epigenetics in Alzheimer’s Disease: Perspective of DNA Methylation. Mol. Neurobiol. 2018, 55, 1026–1044. [Google Scholar] [CrossRef]
- D’Argenio, V.; Sarnataro, D. Microbiome influence in the pathogenesis of prion and Alzheimer’s Diseases. Int. J. Mol. Sci. 2019, 20, 4704. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Song, M.Y.; Kim, D.; Park, C.; Park, D.K.; Kim, D.G.; Yoo, J.S.; Kim, Y.H. A Proteotranscriptomic-based computational drug-repositioning method for Alzheimer’s Disease. Front. Pharmacol. 2020, 10, 1653. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).