Pediatric Sarcoidosis: A Review with Emphasis on Early Onset and High-Risk Sarcoidosis and Diagnostic Challenges
Abstract
1. Introduction
2. Materials and Methods
3. Results
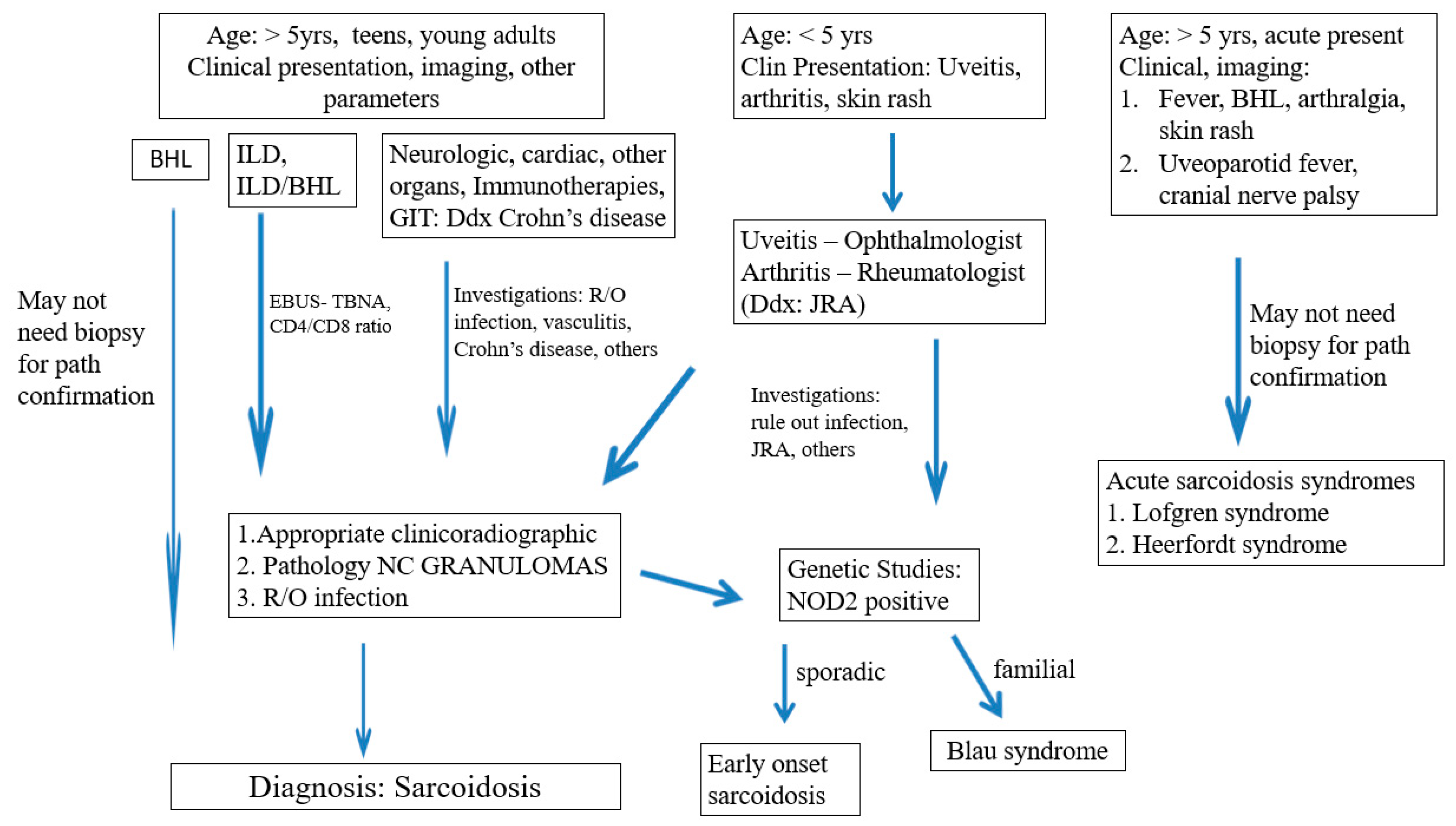
3.1. Blau Syndrome and Early Onset Sarcoidosis (BS-EOS)
3.2. High-Risk Sarcoidosis
3.3. Acute Sarcoidosis
3.4. Diagnostic Challenges and Considerations in Pediatric Sarcoidosis
4. Discussions
Author Contributions
Funding
Conflicts of Interest
References
- Maier, L.A.; Crouser, E.D.; Martin, W.J., II; Eu, J. Executive Summary of the NHLBI Workshop Report: Leveraging Current Scientific Advancements to Understand Sarcoidosis Variability and Improve Outcomes. Ann. Am. Thorac. Soc. 2017, 14, S415–S420. [Google Scholar] [CrossRef] [PubMed]
- Gedalia, A.; Khan, T.A.; Shetty, A.K.; Dimitriades, V.R.; Espinoza, L.R. Childhood sarcoidosis: Louisiana experience. Clin. Rheumatol. 2016, 35, 1879–1884. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.M.; Iannuzzi, M.C.; Montgomery, C.G.; Trudeau, S.; Datta, I.; McKeigue, P.; Fischer, A.; Nebel, A.; Rybicki, B.A. Association of ANXA11 genetic variation with sarcoidosis in African Americans and European Americans. Genes Immun. 2013, 14, 13–18. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Erdal, B.S.; Clymer, B.D.; Yildiz, V.O.; Julian, M.W.; Crouser, E.D. Unexpectedly high prevalence of sarcoidosis in a representative U.S. Metropolitan population. Respir. Med. 2012, 106, 893–899. [Google Scholar] [CrossRef]
- Gerke, A.K.; Judson, M.A.; Cozier, Y.C.; Culver, D.A.; Koth, L.L. Disease Burden and Variability in Sarcoidosis. Ann. Am. Thorac. Soc. 2017, 14, S421–S428. [Google Scholar] [CrossRef]
- Spagnolo, P.; Rossi, G.; Trisolini, R.; Sverzellati, N.; Baughman, R.P.; Wells, A.U. Pulmonary sarcoidosis. Lancet Respir. Med. 2018, 6, 389–402. [Google Scholar] [CrossRef]
- Swigris, J.J.; Oison, A.; Huie, T.J.; Fernandez-Perez, E.R.; Solomon, J.; Sprunger, D.; Brown, K.K. Sarcoidosis-related mortality in the United States from 1988 to 2007. Am. J. Respir. Crit. Care Med. 2011, 183, 1524–1530. [Google Scholar] [CrossRef]
- Shetty, A.K.; Gedalia, A. Sarcoidosis in children. Curr. Probl. Pediatr. 2000, 30, 149–176. [Google Scholar] [CrossRef]
- Sauer, W.H.; Stern, B.J.; Baughman, R.P.; Culver, D.A.; Royal, W. High-Risk Sarcoidosis. Current Concepts and Research Imperatives. Ann. Am. Thorac. Soc. 2017, 14, S437–S444. [Google Scholar] [CrossRef]
- Neville, E.; Walker, A.N.; James, D.G. Prognostic factors predicting the outcome of sarcoidosis: An analysis of 818 patients. QJM Int. J. Med. 1983, 52, 525–533. [Google Scholar]
- Hoffmann, A.L.; Milman, N.; Byg, K.E. Childhood sarcoidosis in Denmark 1979–1994: Incidence, clinical features and laboratory results at presentation in 48 children. Acta Paediatr. 2004, 93, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Hunninghake, G.W.; Costabel, U.; Ando, M.; Baughman, R.; Cordier, J.F.; du Bois, R.; Eklund, A.; Kitaichi, M.; Lynch, J.; Rizzato, G.; et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc. Diffus. Lung Dis. 1999, 16, 149–173. [Google Scholar]
- Pattishall, E.N.; Kending, E.J. Sarcoidosis in children. Pediatr. Pulmonol. 1996, 22, 195–203. [Google Scholar] [CrossRef]
- Baumann, R.J.; Robertson, W.C. Neurosarcoid presents differently in children than in adults. Pediatrics 2003, 112, e480–e486. [Google Scholar] [CrossRef] [PubMed]
- Milman, N.; Hoffmann, A.; Byg, K.E. Sarcoidosis in children. Epidemiology in Danes, clinical features, diagnosis, treatment and prognosis. Acta Paediatr. 1998, 87, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Achille, M.; Ilaria, P.; Teresa, G.; Roberto, C.; Ilir, A.; Piergiorgio, N.; Rolando, C.; Gabriele, S. Successful treatment with adalimumab for severe multifocal choroiditis and panuveitis in presumed (early-onset) ocular sarcoidosis. Int. Ophthalmol. 2016, 36, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Blau, E.B. Familial granulomatous arthritis, iritis, and rash. J. Pediatr. 1985, 107, 689–693. [Google Scholar] [CrossRef]
- Miceli-Richard, C.; Lesage, S.; Rybojad, M.; Prieur, A.M.; Manouvrier-Hanu, S.; Häfner, R.; Chamaillard, M.; Zouali, H.; Thomas, G.; Hugot, J.P. CARD15 mutations in Blau syndrome. Nat. Genet. 2001, 29, 19–20. [Google Scholar] [CrossRef]
- Rosé, C.D.; Aróstegui, J.; Martin, T.M.; Espada, G.; Scalzi, L.; Yagüe, J.; Rosenbaum, J.T.; Modesto, C.; Cristina Arnal, M.; Merino, R.; et al. NOD2-associated pediatric granulomatous arthritis, an expanding phenotype: Study of an international registry and a national cohort in Spain. Arthritis Rheum. 2009, 60, 1797–1803. [Google Scholar] [CrossRef]
- Philpott, D.J.; Sorbara, M.; Robertson, S.J.; Croitoru, K.; Girardin, S.E. NOD proteins: Regulators of inflammation in health and disease. Nat. Rev. Immunol. 2014, 14, 9–23. [Google Scholar] [CrossRef]
- Moreira, L.O.; Zamboni, D. NOD1 and NOD2 Signaling in Infection and Inflammation. Front. Immunol. 2012, 3, 328. [Google Scholar] [CrossRef] [PubMed]
- Caso, F.; Galozzi, P.; Costa, L.; Sfriso, P.; Cantarini, L.; Punzi, L. Autoinflammatory granulomatous diseases: From Blau syndrome and early-onset sarcoidosis to NOD2-mediated disease and Crohn’s disease. RMD Open 2015, 1, e000097. [Google Scholar] [CrossRef] [PubMed]
- Caso, F.; Wouters, C.; Rose, C.D.; Costa, L.; Tognon, S.; Sfriso, P.; Cantarini, L.; Rigante, D.; Punzi, L. Blau syndrome and latent tubercular infection: An unresolved partnership. Int. J. Rheum. Dis. 2014, 17, 586–587. [Google Scholar] [CrossRef] [PubMed]
- Dow, C.T.; Ellingson, J. Detection of Mycobacterium avium ss. Paratuberculosis in Blau Syndrome Tissues. Autoimmune Dis. 2010, 2011, 127692. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, S.; Kawachi, H.; Yoshida, H.; Fukao, A.; Terashita, S.; Ikeue, T.; Horikawa, S.; Sugita, T. Sarcoid-Like Granulomatosis Induced by Nivolumab Treatment in a Lung Cancer Patient. Case Rep. Oncol. 2018, 11, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Abraham, C.; Cho, J.H. Inflammatory bowel disease. N. Engl. J. Med. 2009, 36, 2066–2078. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Sandborn, W. Crohn’s disease. Lancet 2012, 380, 1590–1605. [Google Scholar] [CrossRef]
- Ogura, Y.; Bonen, D.; Inohara, N.; Nicolae, D.L.; Chen, F.F.; Ramos, R.; Britton, H.; Moran, T.; Karaliuskas, R.; Duerr, R.H.; et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 2001, 411, 603–606. [Google Scholar] [CrossRef]
- Abraham, C.; Cho, J. Functional consequences of NOD2 (CARD15) mutations. Inflamm. Bowel Dis. 2006, 12, 641–650. [Google Scholar] [CrossRef]
- Khor, B.; Gardet, A.; Xavier, R.J. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011, 474, 307–317. [Google Scholar] [CrossRef]
- Brunner, J.; Sergi, C.; Müller, T.; Gassner, I.; Prüfer, F.; Zimmerhackl, L.B. Juvenile sarcoidosis presenting as Crohn’s disease. Eur. J. Pediatr. 2006, 165, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Korsten, P.; Strohmayer, K.; Baughman, R.P.; Sweiss, N.J. Refractory pulmonary sarcoidosis—Proposal of a definition and recommendations for the diagnostic and therapeutic approach. Clin. Pulm. Med. 2016, 23, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Baughman, R.P.; Culver, D.; Judson, M.A. A concise review of pulmonary sarcoidosis. Am. J. Respir. Crit. Care Med. 2011, 183, 573–581. [Google Scholar] [CrossRef]
- Nathan, N.; Marcelo, P.; Houdouin, V.; Epaud, R.; de Blic, J.; Valeyre, D.; Houzel, A.; Busson, P.F.; Corvol, H.; Deschildre, A.; et al. Lung sarcoidosis in children: Update on disease expression and management. Thorax 2015, 70, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, P.; Bruder, D. Mechanism of granuloma formation in sarcoidosis. Curr. Opin. Hematol. 2017, 24, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Poulter, L.W.; Rossi, G.; Bjermer, L.; Costabel, U.; Israel-Biet, D.; Klech, H.; Pohl, W.; Velluti, G. The value of bronchoalveolar lavage in the diagnosis and prognosis of sarcoidosis. Eur. Respir. J. 1990, 3, 943–944. [Google Scholar]
- Gulla, K.M.; Gunathilaka, G.; Jat, K.R.; Sankar, J.; Karan, M.; Lodha, R.; Kabra, S.K. Utility and safety of endobronchial ultrasound-guided transbronchial needle aspiration and endoscopic ultrasound with an echobronchoscope-guided fine needle aspiration in children with mediastinal pathology. Pediatr. Pulmonol. 2019, 54, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Sergi, C.; Dhiman, A.; Gray, J.A. Fine Needle Aspiration Cytology for Neck Masses in Childhood. An Illustrative Approach. Diagnostics 2018, 8, 28. [Google Scholar] [CrossRef]
- Aragaki-Nakahodo, A.A.; Baughman, R.; Shipley, R.T.; Benzaquen, S. The complimentary role of transbronchial lung cryobiopsy and endobronchial ultrasound fine needle aspiration in the diagnosis of sarcoidosis. Respir. Med. 2017, 131, 65–69. [Google Scholar] [CrossRef]
- Trisolini, R.; Bauqhman, R.; Spagnolo, P.; Culver, D.A. Endobronchial ultrasound-guided transbronchial needle aspiration in sarcoidosis: Beyond the diagnostic yield. Respirology 2019, 24, 531–542. [Google Scholar] [CrossRef]
- Drent, M.; Mansour, K.; Linssen, C. Bronchoalveolar lavage in sarcoidosis. Semin. Respir. Crit. Care Med. 2007, 28, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Judson, M.; Donnino, R.; Gold, M.; Cooper, L.T., Jr.; Prystowsky, E.N.; Prystowsky, S. Cardiac sarcoidosis. Am. Heart J. 2009, 157, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Birnie, D.H.; Nery, P.; Ha, A.C.; Beanlands, R.S. Cardiac Sarcoidosis. J. Am. Coll. Cardiol. 2016, 68, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.; Vuitch, F. Causes of death in patients with sarcoidosis. A morphologic study of 38 autopsies with clinicopathologic correlations. Arch. Pathol. Lab. Med. 1995, 119, 167–172. [Google Scholar] [PubMed]
- Nowak, D.A.; Widenka, D. Neurosarcoidosis: A review of its intracranial manifestation. J. Neurol. 2001, 248, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.; Dimitriades, V.; Weimer, M.; Sandlin, C. Neurosarcoidosis in Pediatric Patients: A Case Report and Review of Isolated and Systemic Neurosarcoidosis. Pediatr. Neurol. 2016, 63, 45–52. [Google Scholar] [CrossRef]
- Kendig, E.L. The clinical picture of sarcoidosis in children. Pediatrics 1974, 54, 289–292. [Google Scholar]
- Lury, K.M.; Smith, J.; Matheus, M.G.; Castillo, M. Neurosarcoidosis--review of imaging findings. Semin. Roentgenol. 2004, 39, 495–504. [Google Scholar] [CrossRef]
- Culver, D.A. Sarcoidosis. Immunol. Allergy Clin. N. Am. 2012, 32, 487–511. [Google Scholar] [CrossRef]
- Lofgren, S.; Lundbäck, H. The bilateral hilar lymphoma syndrome; a study of the relation to tuberculosis and sarcoidosis in 212 cases. Acta Med. Scand. 1952, 142, 265–273. [Google Scholar] [CrossRef]
- Fraga, R.C.; Kakizaki, P.; Valente, N.Y.S.; Portocarrero, L.K.L.; Teixeira, M.F.S.; Senise, P.F. Do you know this syndrome? Heerfordt-Waldenström syndrome. An. Bras. Dermatol. 2017, 92, 571–572. [Google Scholar] [CrossRef] [PubMed]
- Govender, P.; Berman, J. The Diagnosis of Sarcoidosis. Clin. Chest Med. 2015, 36, 585–602. [Google Scholar] [CrossRef] [PubMed]
- Shetty, A.K.; Gedalia, A. Childhood sarcoidosis: A rare but fascinating disorder. Pediatr. Rheumatol. 2008, 23, 16. [Google Scholar] [CrossRef] [PubMed]
- Agaku, I.T.; Ayo-Yusuf, O.A.; Vardavas, C.I.; Connolly, G. Predictors and patterns of cigarette and smokeless tobacco use among adolescents in 32 countries, 2007–2011. J. Adolesc. Health 2014, 54, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Dutra, L.M.; Glantz, S. Electronic cigarettes and conventional cigarette use among U.S. adolescents: A cross-sectional study. JAMA Pediatr. 2014, 168, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Grégoire, M.C. Vaping risks for youth continue to emerge. Can. Med. Assoc. J. 2019, 191, E1113–E1114. [Google Scholar] [CrossRef] [PubMed]
- Harrell, M.B.; Weaver, S.; Loukas, A.; Creamer, M.; Marti, C.N.; Jackson, C.D.; Heath, J.W.; Navak, P.; Perry, C.L.; Pechacek, T.F.; et al. Flavored e-cigarette use: Characterizing youth, young adult, and adult users. Prev. Med. Rep. 2016, 11, 33–40. [Google Scholar] [CrossRef]
- Leigh, N.J.; Lawton, R.; Hershberger, P.A.; Goniewicz, M.L. Flavourings significantly affect inhalation toxicity of aerosol generated from electronic nicotine delivery systems (ENDS). Tob. Control 2016, 25, ii81–ii87. [Google Scholar] [CrossRef]
- Tierney, P.A.; Karpinski, C.; Brown, J.E.; Luo, W.; Pankow, J.F. Flavour chemicals in electronic cigarette fluids. Tob. Control 2016, 25, e10–e15. [Google Scholar] [CrossRef]
- Peace, M.R.; Butler, K.; Wolf, C.E.; Poklis, J.L.; Poklis, A. Evaluation of Two Commercially Available Cannabidiol Formulations for Use in Electronic Cigarettes. Front. Pharmacol. 2016, 7, 279. [Google Scholar] [CrossRef]
- Phillip, J. Could CBD oil replace drug treatments for sarcoidosis? Available online: https://marijuanastocks.com/could-cbd-oil-replace-drug-treatments-for-sarcoidosis/ (accessed on 27 February 2017).
- Cunningham, D.; Teichtahl, H.; Hunt, J.M.; Dow, C.; Valentine, R. Necrotizing pulmonary granulomata in a marijuana smoker. Chest 2000, 117, 1511–1514. [Google Scholar]
- Madsen, L.R.; Krarup, N.; Bergmann, T.K.; Baerentzen, S.; Neghabat, S.; Duval, L.; Knudsen, S.T. A Cancer that went up in smoke: Pulmonary reaction to e-cigarettes imitating metastatic cancer. Chest 2016, 149, e65–e67. [Google Scholar] [CrossRef] [PubMed]
- Crommelin, H.A.; Vorselaars, A.; van Moorsel, C.H.; Korenromp, I.H.; Deneer, V.H.; Grutters, J.C. Anti-TNF therapeutics for the treatment of sarcoidosis. Immunotherapy 2014, 6, 1127–1143. [Google Scholar] [CrossRef] [PubMed]
- Baha, A.; Hanazay, C.; Kokturk, N.; Turktas, H. A Case of Sarcoidosis Associated With Anti-Tumor Necrosis Factor Treatment. J. Investig. Med. High Impact Case Rep. 2015, 3, 2324709615571366. [Google Scholar] [CrossRef] [PubMed]
- Butnor, K.J. Pulmonary sarcoidosis induced by interferon-alpha therapy. Am. J. Surg. Pathol. 2005, 29, 976–979. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.L.; Agarwal, A.; Verma, A.R. Checkpoint inhibition in pediatric hematologic malignancies. Pediatr. Hematol. Oncol. 2017, 34, 379–394. [Google Scholar] [CrossRef]
- Lucchesi, M.; Sardi, I.; Puppo, G.; Chella, A.; Favre, C. The dawn of “immune-revolution” in children: Early experiences with checkpoint inhibitors in childhood malignancies. Cancer Chemother. Pharmacol. 2017, 80, 1047–1053. [Google Scholar] [CrossRef]
- Dunn-Pirio, A.M.; Shah, S.; Eckstein, C. Neurosarcoidosis following Immune Checkpoint Inhibitio. Case Rep. Oncol. 2018, 11, 521–526. [Google Scholar] [CrossRef]
- Casanova, N.; Zhou, T.; Knox, K.S.; Garcia, J.G.N. Identifying Novel Biomarkers in Sarcoidosis Using Genome-Based Approaches. Clin. Chest Med. 2015, 36, 621–630. [Google Scholar] [CrossRef]
- Ukae, S.; Tsutsumi, H.; Adachi, N.; Takahashi, H.; Kato, F.; Chiba, S. Preschool sarcoidosis manifesting as juvenile rheumatoid arthritis: A case report and a review of the literature of Japanese cases. Pediatr. Int. 1994, 36, 515–518. [Google Scholar] [CrossRef]
- Teirstein, A.S.; Judson, M.; Baughman, R.P.; Rossman, M.D.; Yeager, H., Jr.; Moller, D.R.; Case Control Etiologic Study of Sarcoidosis (ACCESS) Writing Group. The spectrum of biopsy sites for the diagnosis of sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. 2005, 22, 139–146. [Google Scholar]
- Hutchinson, J. Case of Livid Papillary Psoriasis; J&A Churchill: London, UK, 1877; Volume 1. [Google Scholar]
- Boeck, C. Multiple benign sarcoid of the skin. J. Cutan Genitourin. Dis. 1899, 17, 543–550. [Google Scholar]
- Moller, D.R.; Rybicki, B.; Hamzeh, N.Y.; Montgomery, C.G.; Chen, E.S.; Drake, W.; Fontenot, A.P. Genetic, Immunologic, and Environmental Basis of Sarcoidosis. Ann. Am. Thorac. Soc. 2017, 14, S429–S436. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, Y.; Lepzien, R.; Kullberg, S.; Eklund, A.; Smed-Sörensen, A.; Grunewald, J. Expanded lung T-bet+RORγT+ CD4+ T-cells in sarcoidosis patients with a favourable disease phenotype. Eur. Respir. J. 2016, 48, 484–494. [Google Scholar] [CrossRef]
- Schupp, J.C.; Tchaptchet, S.; Lützen, N.; Engelhard, P.; Müller-Quernheim, J.; Freudenberg, M.A.; Prasse, A. Immune response to Propionibacterium acnes in patients with sarcoidosis--in vivo and in vitro. BMC Pulm. Med. 2015, 15, 75. [Google Scholar] [CrossRef]
- Broos, C.E.; van Nimwegen, M.; Kleinjan, A.; ten Berge, B.; Muskens, F.; in ‘t Veen, J.C.; Annema, J.T.; Lambrecht, B.N.; Hoogsteden, H.C.; Hendriks, R.W.; et al. Impaired survival of regulatory T cells in pulmonary sarcoidosis. Respir. Res. 2015, 16, 108. [Google Scholar] [CrossRef]
- Hetherington, S. Sarcoidosis in young children. Am. J. Dis. Child. 1982, 136, 13–15. [Google Scholar] [CrossRef]
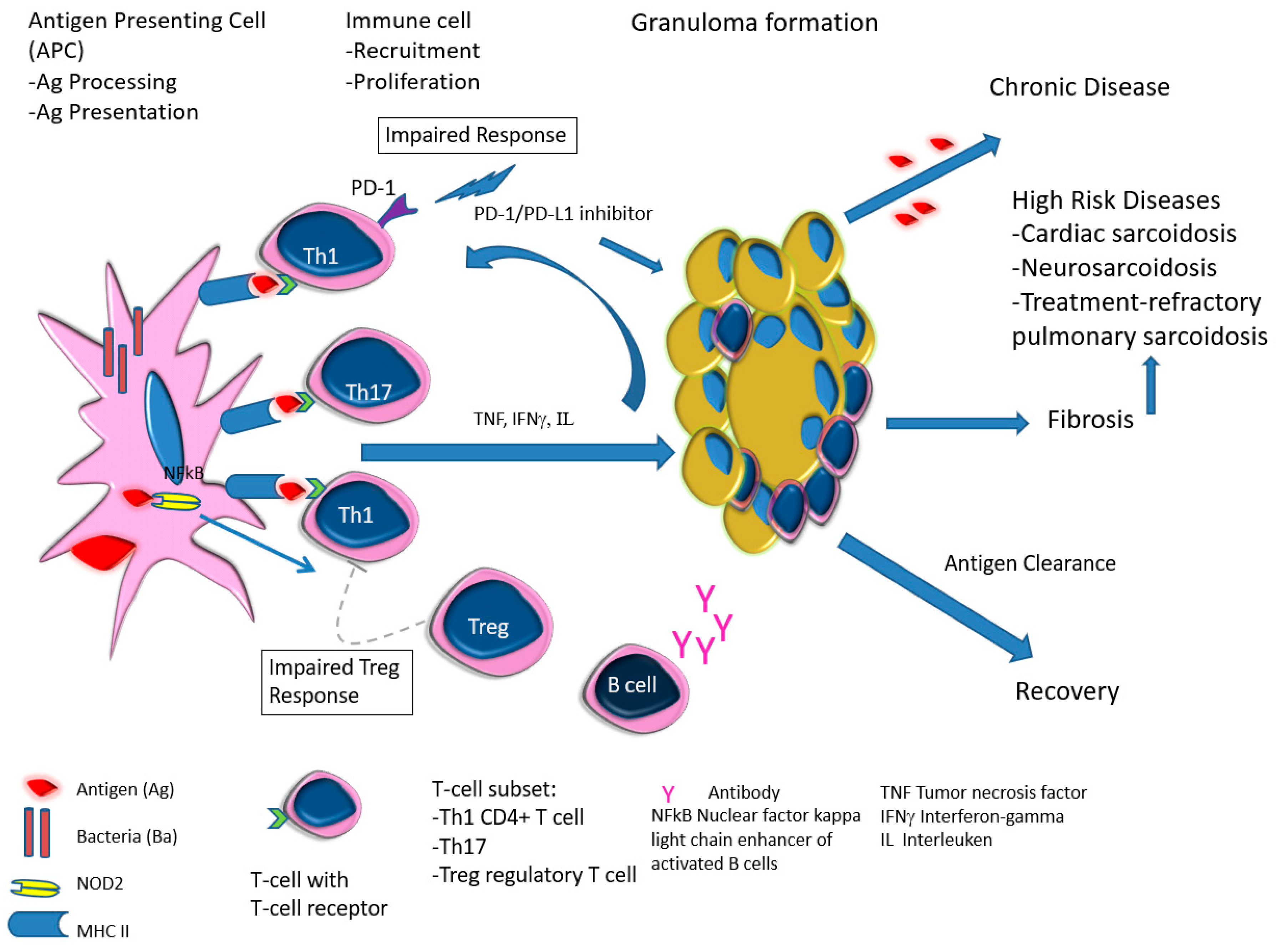
| Entity | Clinical | Laboratory | Pathology | References |
|---|---|---|---|---|
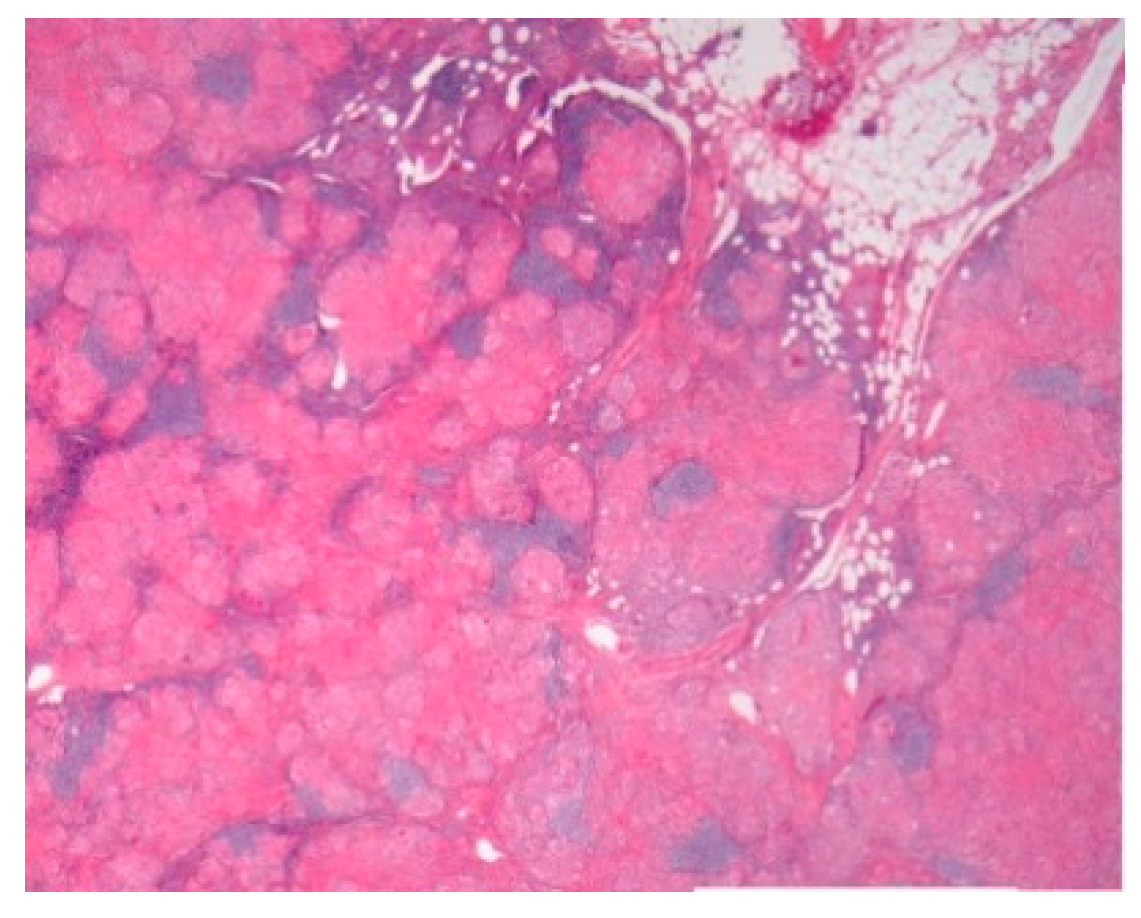
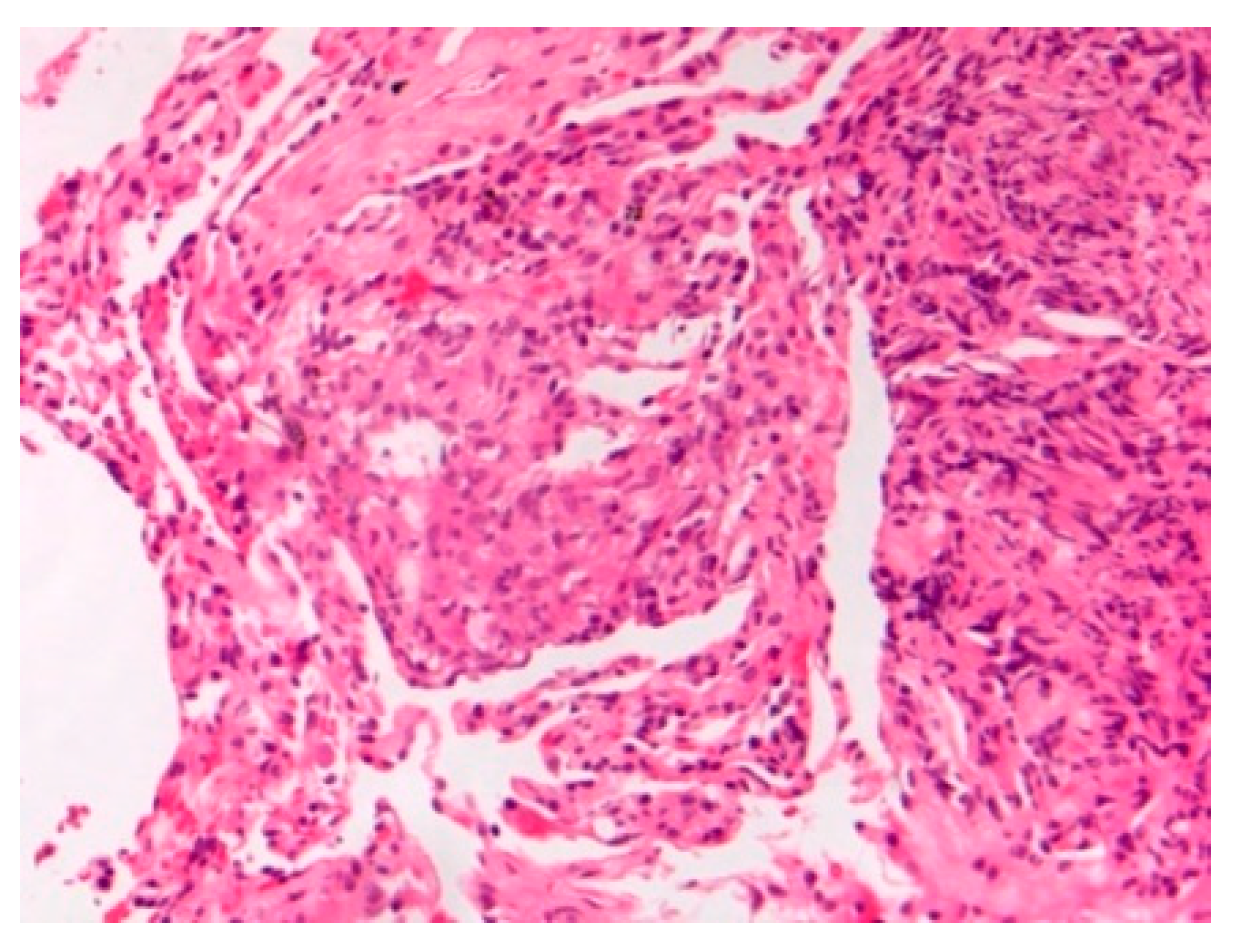
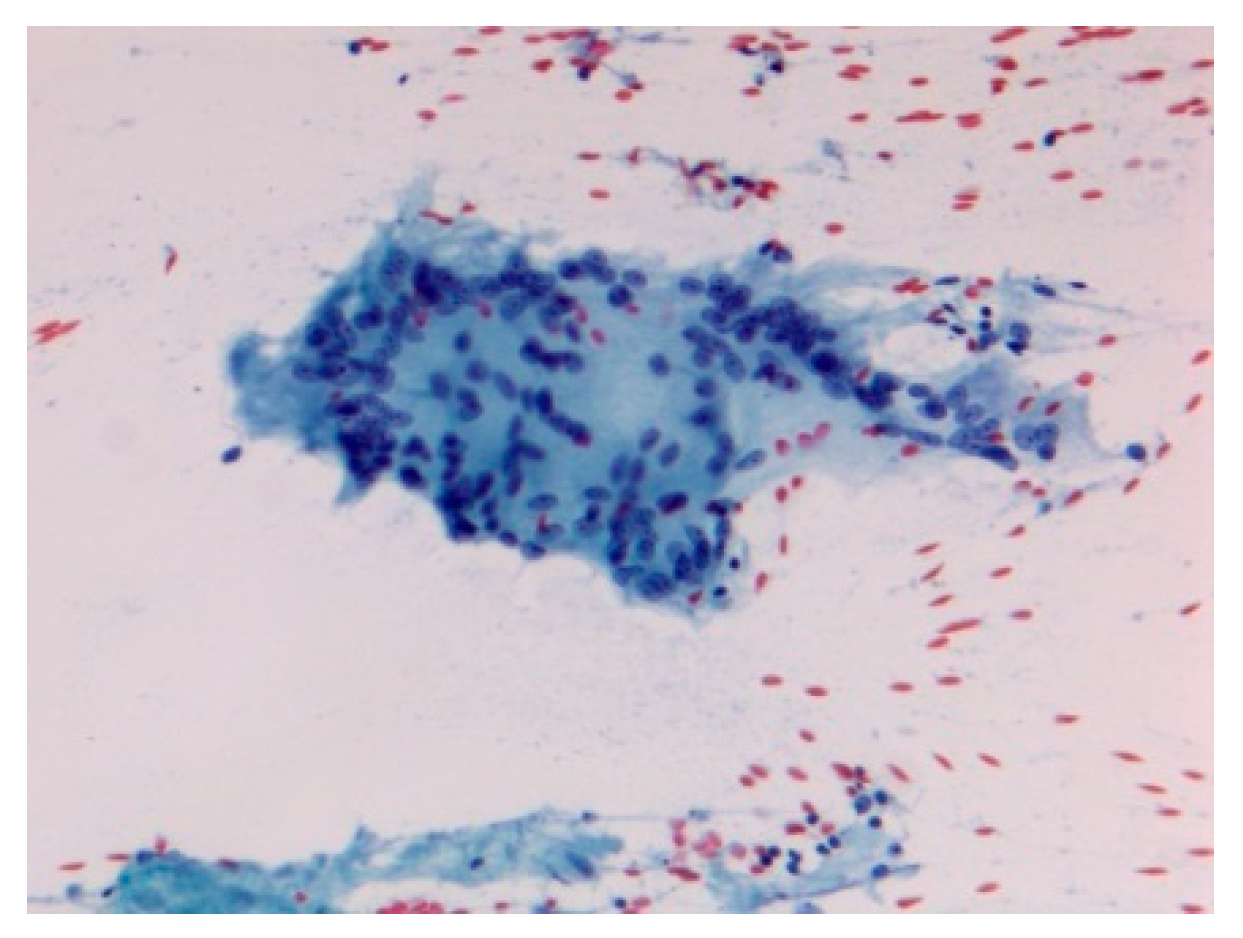
| -Prevalence -Systems involved -Prognosis | -0.22–0.29/106 -Lymph nodes/Lung -Chronic disease 12% | BAL: CD4/CD8 Biopsies | Granulomas Figure 1 | [2,8,11,13,15] |
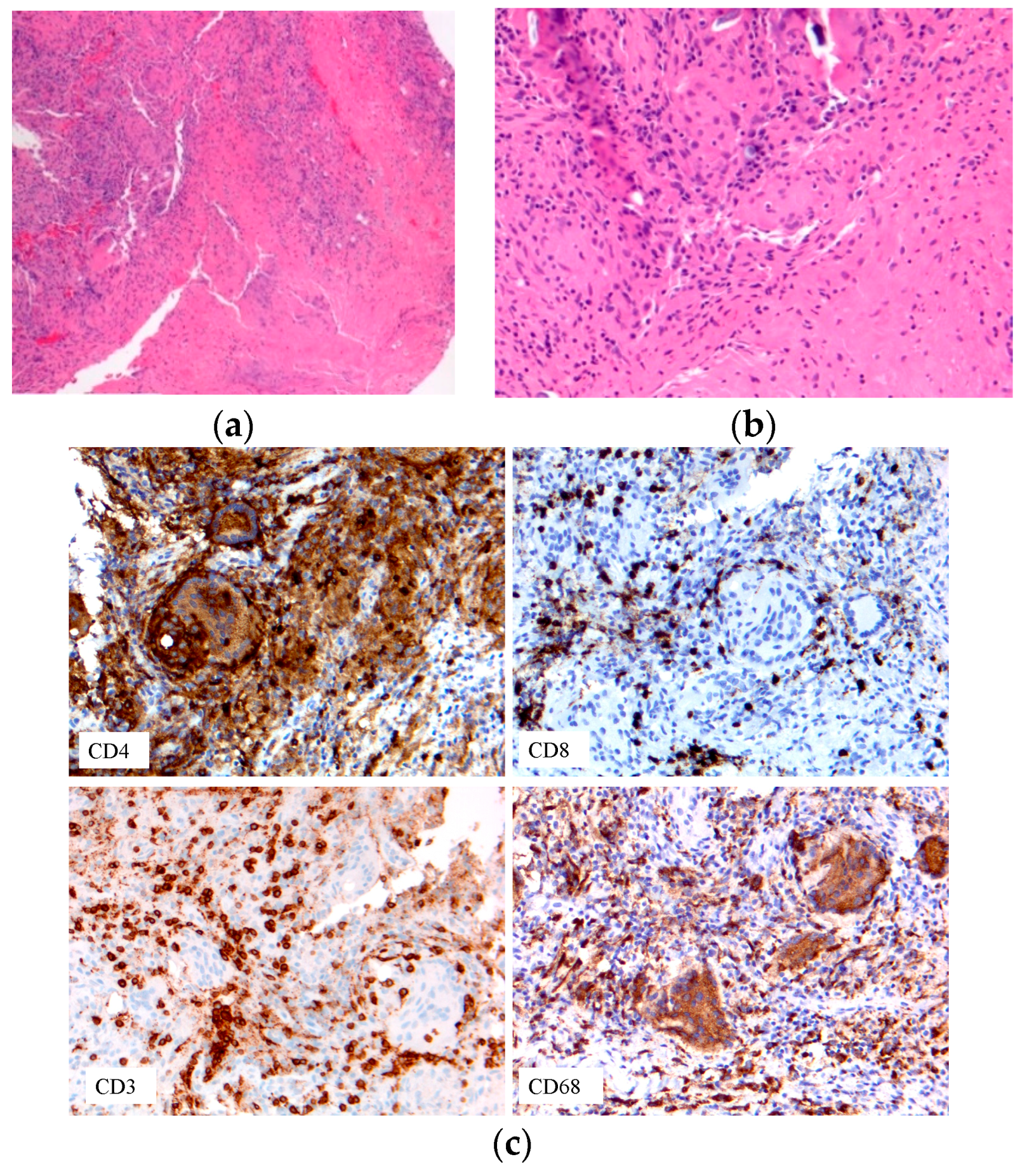
| -Early-onset sarcoidosis/ Blau syndrome | Triad: uveitis, arthritis, skin rash | Eye exam Biopsy | Granulomas Figure 7 | [22,23,79] |
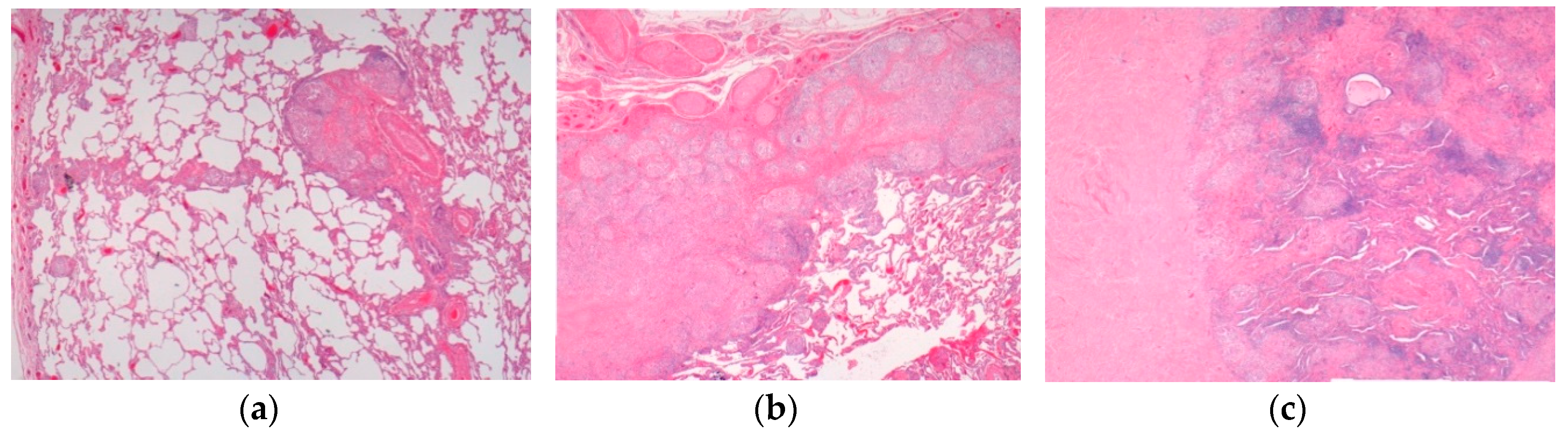
| High-risk sarcoid -Treatment-resistant Pulmonary sarcoid -Cardiac sarcoid/CS -Neurosarcoid/ NS | -Most common, progression to chronicity -CS Case reports -NS 53 cases | Chest XR, CT, EBUS/TBNA Cardiac echo CT /MRI | Figure 2 Figure 3 Figure 4 | [2,9,13]; [2,6,8,14,34,46] |
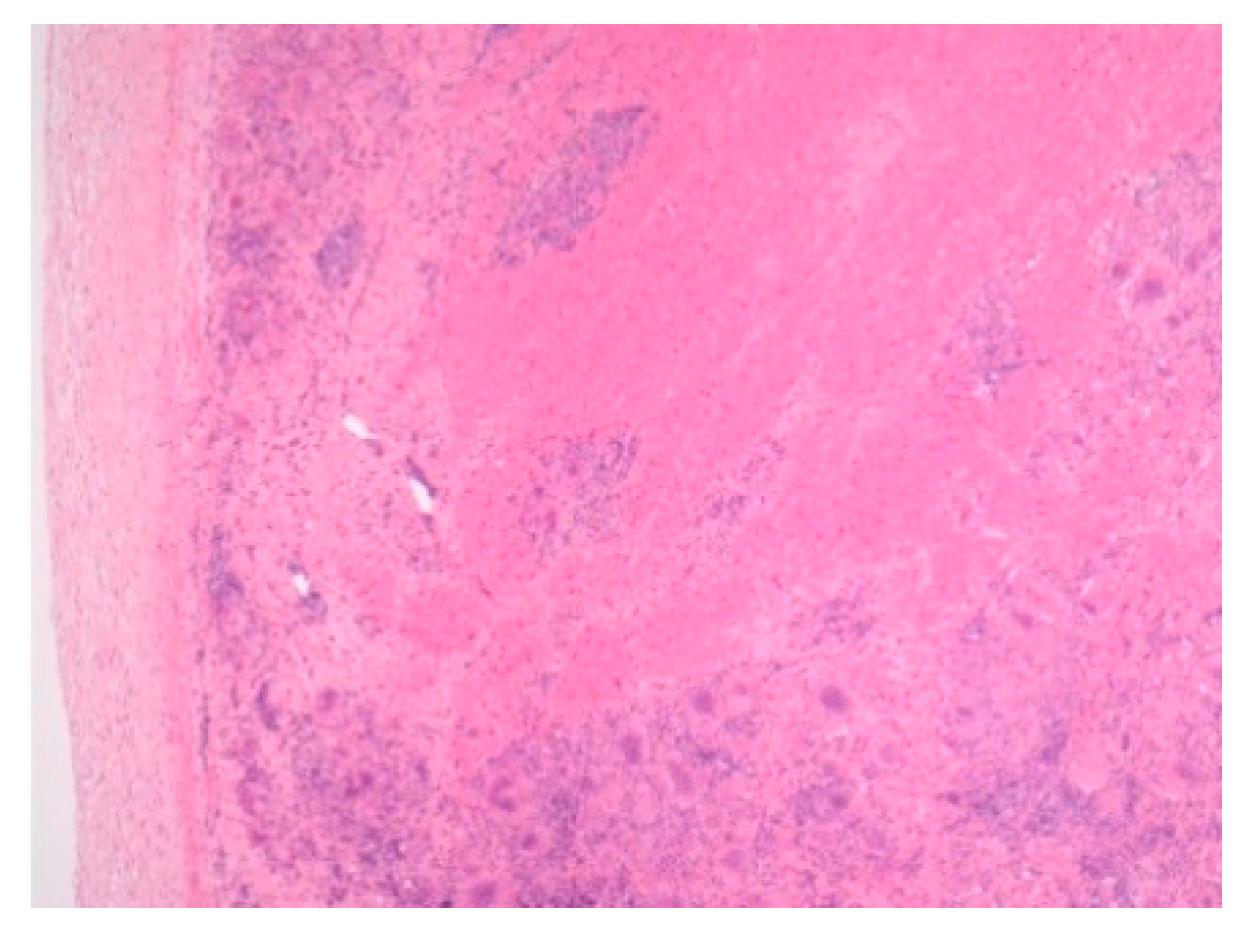
| Sarcoid-like syndrome -INF therapy -Checkpoint inhibitor -E-cig/Marijuana | -Not reported in children -Not reported in children -Teens and young adults | Imaging modalities Biopsy | Figure 5 | [66] [25,69]; [62,63] |
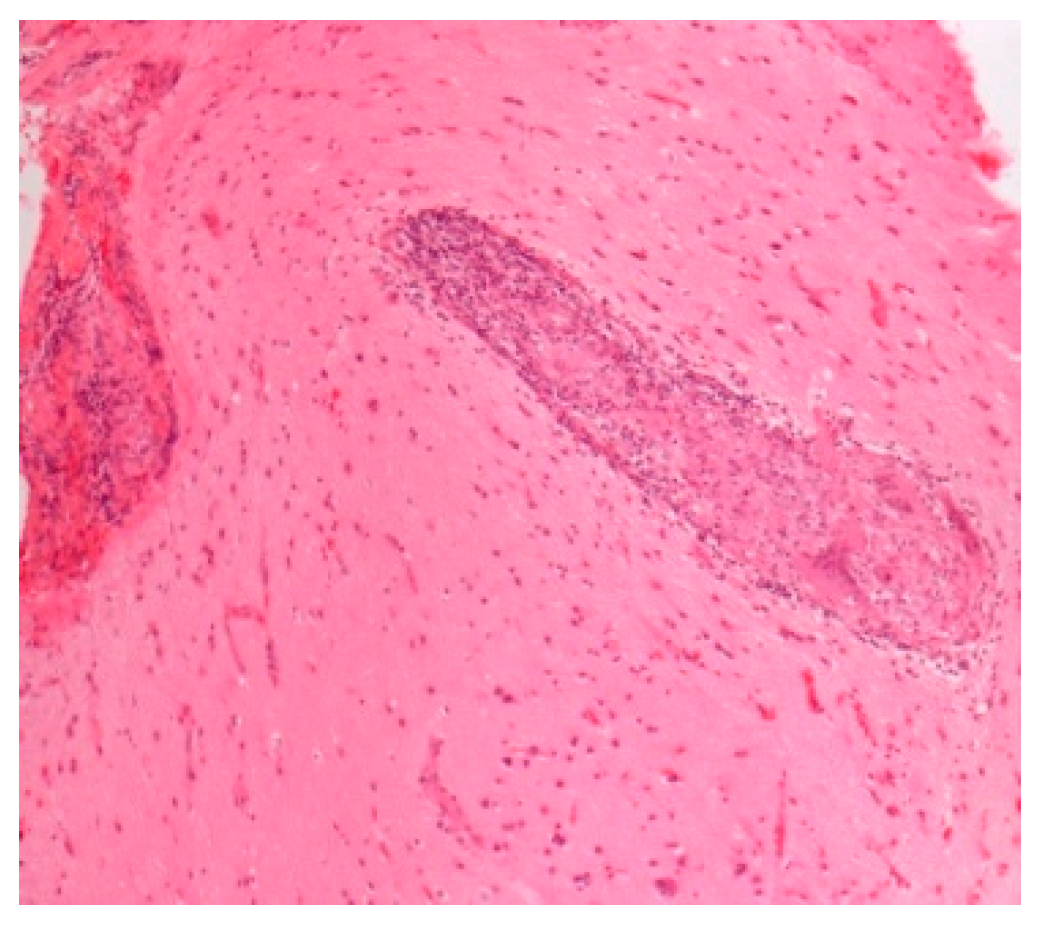
| Acute sarcoidosis -Lofgren syndrome -Heerfordt syndrome /uveoparotid fever | Triad: erythema nodosum BHL (CXR), arthritis, Resolution in two years Uveitis, parotidomegaly, facial nerve palsy, fever, Prognosis excellent | Chest XR, CT, biopsy | Both can be Diagnosed on clinical data without biopsy Figure 6 | [50]; [8,49] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, B.; Chan, J.; Das, S.; Alshamma, Z.; Sergi, C. Pediatric Sarcoidosis: A Review with Emphasis on Early Onset and High-Risk Sarcoidosis and Diagnostic Challenges. Diagnostics 2019, 9, 160. https://doi.org/10.3390/diagnostics9040160
Chiu B, Chan J, Das S, Alshamma Z, Sergi C. Pediatric Sarcoidosis: A Review with Emphasis on Early Onset and High-Risk Sarcoidosis and Diagnostic Challenges. Diagnostics. 2019; 9(4):160. https://doi.org/10.3390/diagnostics9040160
Chicago/Turabian StyleChiu, Brian, Jackie Chan, Sumit Das, Zainab Alshamma, and Consolato Sergi. 2019. "Pediatric Sarcoidosis: A Review with Emphasis on Early Onset and High-Risk Sarcoidosis and Diagnostic Challenges" Diagnostics 9, no. 4: 160. https://doi.org/10.3390/diagnostics9040160
APA StyleChiu, B., Chan, J., Das, S., Alshamma, Z., & Sergi, C. (2019). Pediatric Sarcoidosis: A Review with Emphasis on Early Onset and High-Risk Sarcoidosis and Diagnostic Challenges. Diagnostics, 9(4), 160. https://doi.org/10.3390/diagnostics9040160






