Detection of Lower Albuminuria Levels and Early Development of Diabetic Kidney Disease Using an Artificial Intelligence-Based Rule Extraction Approach
Abstract
1. Introduction
1.1. Background
1.2. Importance of Microalbuminuria
1.3. Study Rationale
1.4. UACR Cut-Off Values for Diseases Associated with Diabetes
1.5. Study Motivation
1.6. Objectives and Significance
1.7. Machine Learning and Rule Extraction
1.7.1. Machine Learning and Rule Extraction
1.7.2. Objectives of Rule Extraction
2. Materials and Methods
2.1. Data Source
2.2. Variable Selection
2.3. Artificial Intelligence with Rule Extraction Technology
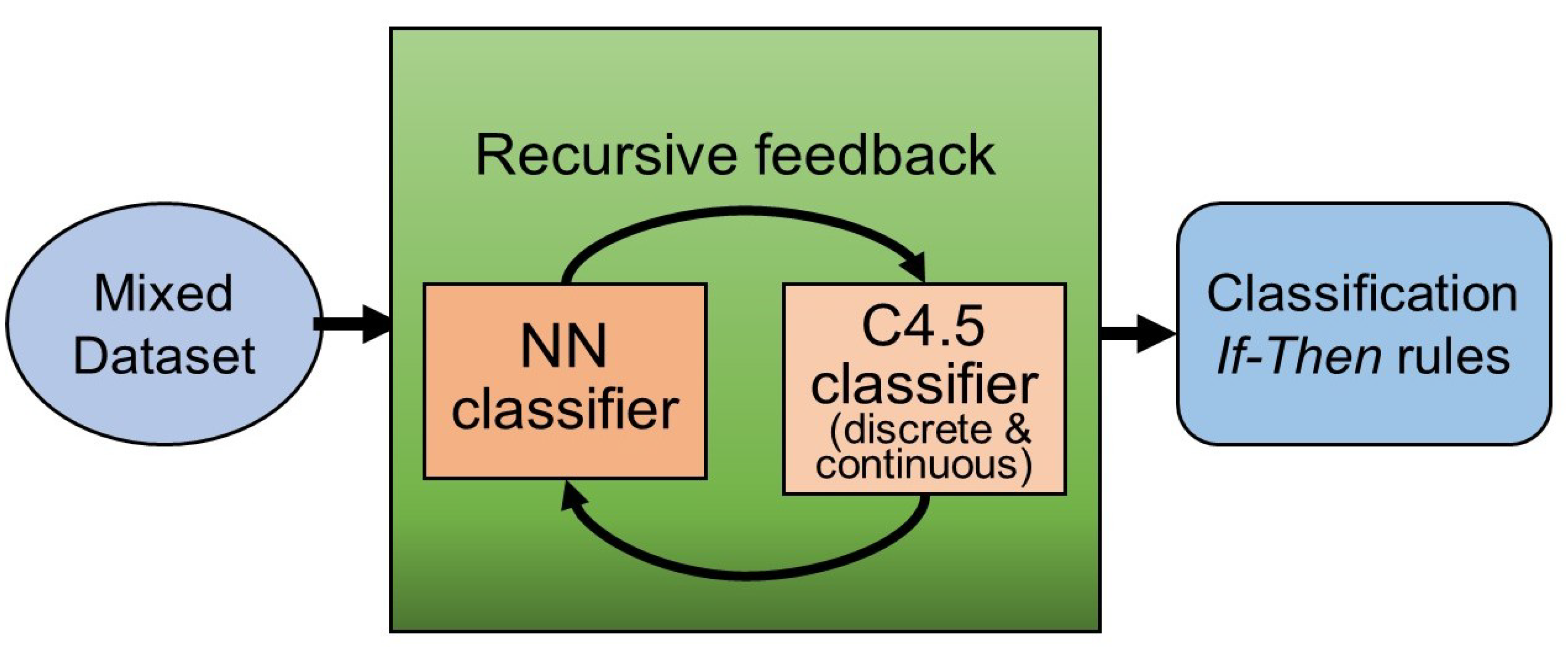
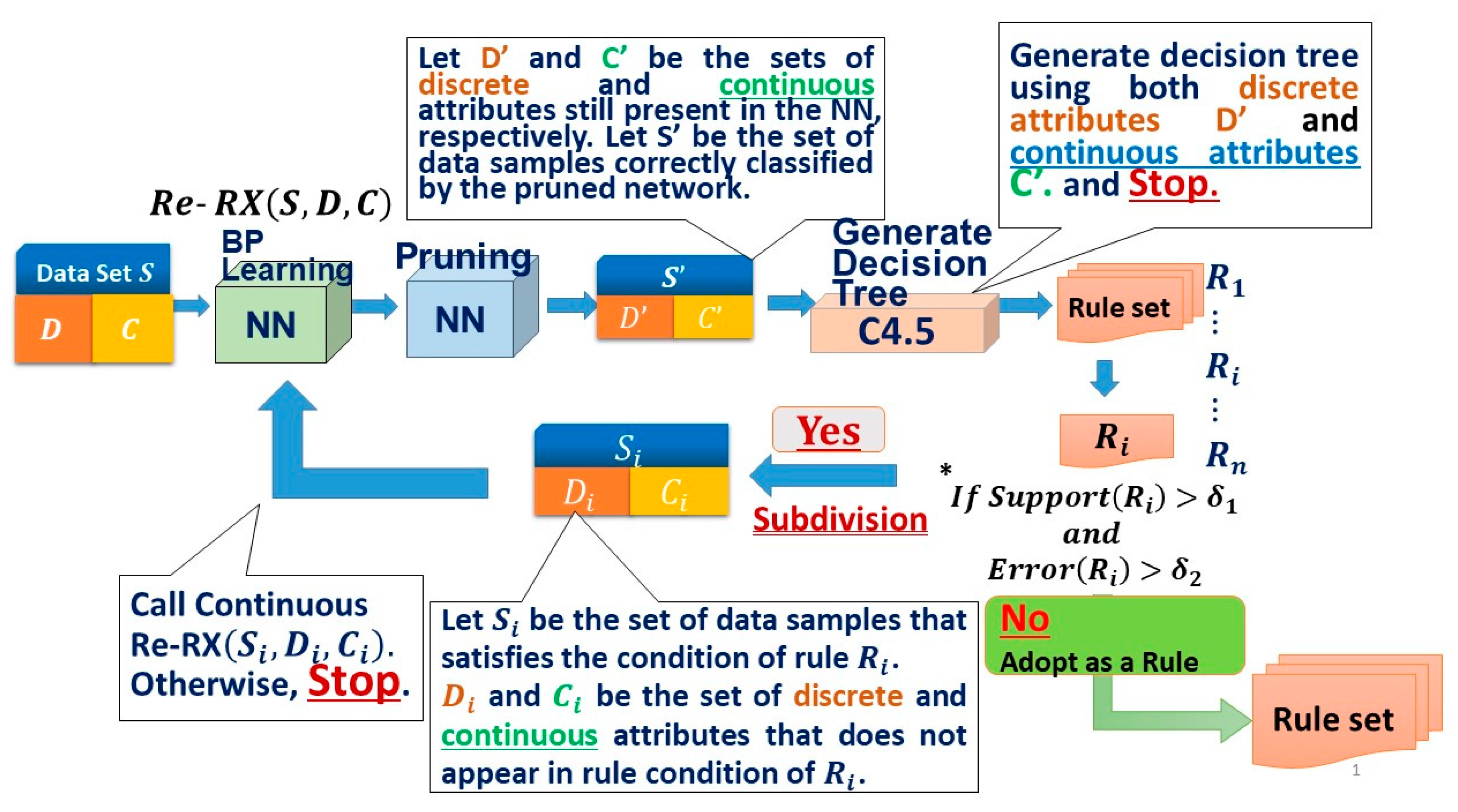
2.3.1. The Recursive-Rule eXtraction (Re-RX) Algorithm
2.3.2. Re-RX Algorithm with Continuous Attributes (Continuous Re-RX)
2.4. Performance Measures
2.5. Statistics
3. Results
3.1. Statistical Comparisons between the Prediabetes and Diabetes Patients
3.2. Performance of the Continuous Re-RX Algorithm
3.3. Rules Extracted Using Continuous Re-RX
3.3.1. Rule 1 and Rule 2
3.3.2. Rule 3, Rule 4, and Rule 5
3.3.3. Rule 6: If HbA1c ≤ 5.6 AND FPG > 122.7 AND UACR ≤ 71.00, Then Class 2 (Prediabetes)
3.3.4. Rule 8 and Rule 9
3.3.5. Rule 10 and Rule 11
3.3.6. Rule 11: If HbA1c ∈(6.1, 6.4) AND LDL ≤ 142.0 AND FPG ≤ 108.5 AND
3.3.7. Rule 13, Rule 14, and Rule 15
4. Discussion
4.1. Significance of Finding Attributes that are Closely Related to DKD
4.2. Significance and Clinical Relevance of This Study
4.3. Significance of This Study for Point-of-Care Clinical Relevance
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 7th ed.; International Diabetes Federation: Brussel, Belgium, 2015. [Google Scholar]
- Senior, P.A. Diabetes and chronic kidney disease: Concern, confusion, clarity? Can. J. Diabetes 2014, 38, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Choudhuri, S.; Mondal, S.A.; Mukherjee, S.; Chowdhury, S. Urinary albumin: creatinine ratio predicts prediabetes progression to diabetes and reversal to normoglycemia: Role of associated insulin resistance, inflammatory cytokines and low vitamin D. J. Diabetes 2014, 6, 316–322. [Google Scholar] [CrossRef]
- Newman, D.J.; Pugia, M.J.; Lott, J.A.; Wallace, J.F.; Hiar, A.M. Urinary protein and albumin excretion corrected by creatinine and specific gravity. Clin. Chim. Acta 2000, 294, 139–155. [Google Scholar] [CrossRef]
- Park, S.K.; Seo, M.H.; Ryoo, J.H.; Kim, M.G.; Choi, J.M.; Shin, H.; Choi, Y.S.; Hong, H.P. Urinary albumin excretion within the normal range predicts the development of diabetes in Korean men. Diabetes Res. Clin. Pract. 2015, 109, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Park, Y.K.; Cho, K.H.; Choi, H.J.; Han, J.H.; Han, K.D.; Han, B.D.; Yoon, Y.J.; Kim, Y.H. Normal range albuminuria and metabolic syndrome in South Korea: The 2011–2012 Korean National Health and Nutrition Examination Survey. PLoS ONE 2015, 10, e0125615. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD workgroup. KDIGO 2012 Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013, 3, 5–14. [Google Scholar]
- McTaggart, M.P.; Price, C.P.; Pinnock, R.G.; Stevens, P.E.; Newall, R.G.; Lamb, E.J. The diagnostic accuracy of a urine albumin-creatinine ratio point-of-care test for detection of albuminuria in primary care. Am. J. Kidney Dis. 2012, 60, 787–794. [Google Scholar] [CrossRef]
- Moresco, R.N.; Sangoi, M.B.; De Carvalho, J.A.; Tatsch, E.; Bochi, G.V. Diabetic nephropathy: Traditional to proteomic markers. Clin. Chim. Acta 2013, 421, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Parving, H.H. Commentary: Microalbuminuria: Past, present and glorious future. Int. J. Epidemiol. 2014, 43, 21–22. [Google Scholar] [CrossRef]
- Halimi, J.M.; Hadjadj, S.; Aboyans, V.; Allaert, F.A.; Artigou, J.Y.; Beaufils, M.; Berrut, G.; Fauvel, J.P.; Gin, H.; Nitenberg, A.; et al. Microalbuminuria and urinary albumin excretion: French clinical practice guidelines. Diabetes Metab. 2007, 33, 303–309. [Google Scholar] [CrossRef]
- Jong, P.E.; Gansevoort, R.T.; Bakker, S.J.L. Macroalbuminuria and microalbuminuria: Do both predict renal and cardiovascular events with similar strength? J. Nephrol. 2007, 20, 375–380. [Google Scholar] [PubMed]
- Forman, J.P.; Fisher, N.D.; Schopick, E.L.; Curhan, G.C. Higher levels of albuminuria within normal range predict incident hypertension. J. Am. Soc. Nephrol. 2008, 19, 1983–1988. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of Medical Care in Diabetes—2017. Diabetes Care 2017, 40 (Suppl. 1), S1–S135. [Google Scholar]
- Ritz, E.; Viberti, G.C.; Ruilope, L.M.; Rabelink, A.J.; Izzo J.L., Jr.; Katayama, S.; Ito, S.; Mimran, A.; Menne, J.; Rump, L.C.; et al. Determinants of urinary albumin excretion within the normal range in patients with type 2 diabetes: The Randomised Olmesartan and Diabetes Microalbuminuria Prevention (ROADMAP) study. Diabetologia 2010, 53, 49–57. [Google Scholar] [CrossRef][Green Version]
- He, N.J.; Ahn, J.M.; Lee, T.W.; Chin, H.J.; Na, K.Y.; Chae, D.W.; Kim, S. Very low-grade albuminuria reflects susceptibility to chronic kidney disease in combination with cardiovascular risk factors. Hypertens. Res. 2010, 33, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Roshan, B.; Stanton, R.C. A study of microalbuminuria and diabetic nephropathy. J. Nephropathol. 2013, 2, 234–240. [Google Scholar]
- Chida, S.; Fujita, Y.; Ogawa, A.; Hayashi, A.; Ichikawa, R.; Kamata, Y.; Takeuchi, A.; Takano, K.; Shichiria, M. Levels of albuminuria and risk of developing macroalbuminuria in type 2 diabetes: Historical cohort study. Sci. Rep. 2016, 6, 26380. [Google Scholar] [CrossRef] [PubMed]
- Wahab, M.A.K.A.; Saad, M.M.; Baraka, K.A.G. Microalbuminuria is a late event in patients with hypertension: Do we need a lower threshold? J. Saudi Heart Assoc. 2017, 29, 30–36. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Huang, F.; Li, D.; Wu, X.; Li, J.; Chen, X.; Liao, W. Significance of microalbuminuria in predicting the risk of diabetic retinopathy in type 2 diabetes mellitus patients. Int. J. Clin. Exp. Med. 2017, 10, 8208–8215. [Google Scholar]
- Lee, M.K.; Han, K.D.; Lee, J.H.; Sohn, S.Y.; Hong, O.K.; Jeong, J.S.; Kim, M.K.; Baek, K.H.; Song, K.H.; Kwon, H.S. Normal-to-mildly increased albuminuria predicts the risk for diabetic retinopathy in patients with type 2 diabetes. Sci. Rep. 2017, 7, 11757. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zheng, Z.; Huang, Y.; Guo, K.; Lu, J.; Zhang, L.; Yu, H.; Bao, Y.; Jia, W. A microalbuminuria threshold to predict the risk for the development of diabetic retinopathy in type 2 diabetes mellitus patients. PLoS ONE 2012, 7, e36718. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Poncelas, A.; Mundet-Tuduri, X.; Miravert-Jimenez, S.; Casellas, A.; Barrot-De la Puente, J.F.; Franch-Nadal, J.; Coll-de Tuero, G. Chronic kidney disease and diabetic retinopathy in patients with type 2 diabetes. PLoS ONE 2016, 11, e0149448. [Google Scholar] [CrossRef] [PubMed]
- MacIsaac, R.J.; Ekinci, E.I.; Jerums, G. Progressive diabetic nephropathy. How useful is microalbuminuria? Contra. Kidney Int. 2014, 86, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Echouffo-Tcheugui, J.B.; Narayan, K.M.; Weisman, D.; Golden, S.H.; Jaar, B.G. Systematic review or meta-analysis association between prediabetes and risk of chronic kidney disease: A systematic review and meta-analysis. Diabet. Med. 2016, 33, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Markus, M.R.P.; Ittermann, T.; Baumeister, S.E.; Huth, C.; Thorand, B.; Herder, C.; Roden, M.; Siewert-Markus, U.; Rathmann, W.; Koenig, W.; et al. Prediabetes is associated with microalbuminuria, reduced kidney function and chronic kidney disease in the general population The KORA (Cooperative Health Research in the Augsburg Region) F4-Study. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Chartrand, G.; Cheng, P.M.; Vorontsov, E.; Drozdzal, M.; Turcotte, S.; Pal, C.J.; Kadoury, S.; Tang, A. Deep learning: A primer for radiologists. Radiographics 2017, 37, 2113–2131. [Google Scholar] [CrossRef]
- Tickle, A.B.; Andrews, R.; Golea, M.; Diederich, J. The truth will come to light: Directions and challenges in extracting the knowledge embedded within trained artificial neural networks. IEEE Trans. Neural Netw. 1998, 9, 1057–1068. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. CDC Prediabetes Screening Test. 2016. Available online: http://www.cdc.gov/diabetes/prevention/pdf/prediabetestest.pdf (accessed on 30 May 2019).
- American Diabetes Association. Are You at Risk? Type 2 Diabetes Risk Test. 2017. Available online: http://www.diabetes.org/are-you-at-risk/diabetes-risk-test/ (accessed on 30 May 2019).
- Hayashi, Y.; Nakano, S.; Fujisawa, S. Use of the recursive-rule extraction algorithm with continuous attributes to improve diagnostic accuracy in thyroid disease. Inform. Med. Unlocked 2015, 1, 1–8. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Hayashi, Y. Application of a rule extraction algorithm family based on the Re-RX algorithm to financial credit risk assessment from a Pareto optimal perspective. Oper. Res. Perspect. 2016, 3, 32–42. [Google Scholar] [CrossRef]
- Quinlan, J.R. C4.5: Programs for Machine Learning; Morgan Kaufman Publishers, Inc.: San Mateo, CA, USA, 1993. [Google Scholar]
- National Health and Nutrition Examination Survey 1999–2014. Available online: https://www.cdc.gov/nchs/nhanes/ (accessed on 1 August 2019).
- Takagi, M.; Babazono, T.; Uchigata, Y. Differences in risk factors for the onset of albuminuria and decrease in glomerular filtration rate in people with Type 2 diabetes mellitus: Implications for the pathogenesis of diabetic kidney disease. Diabet. Med. 2015, 32, 1354–1360. [Google Scholar] [CrossRef]
- Radcliffe, N.J.; Seah, J.-M.; Clarke, M.; MacIsaac, R.J.; Jerums, G.; Ekinci, E.I. Clinical predictive factors in diabetic kidney disease progression. J. Diabetes Investig. 2017, 8, 6–18. [Google Scholar] [CrossRef]
- Yun, K.-J.; Kim, H.J.; Kim, M.K.; Kwon, H.-S.; Baek, K.-H.; Roh, Y.J.; Song, K.H. Risk Factors for the Development and Progression of Diabetic Kidney Disease in Patients with Type 2 Diabetes Mellitus and Advanced Diabetic Retinopathy. Diabetes Metab. J. 2016, 40, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Setiono, R.; Baesens, B.; Mues, C. Recursive neural network rule extraction for data with mixed attributes. IEEE Trans. Neural Netw. 2008, 19, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Tatsuhiro, O. High accuracy-priority rule extraction for reconciling accuracy an interpretability in credit scoring. New Gener. Comput. 2018, 36, 393–418. [Google Scholar] [CrossRef]
- Marqués, A.I.; García, V.; Sánchez, J.S. On the suitability of resampling techniques for the class imbalance problem in credit scoring. J. Oper. Res. Soc. 2013, 64, 1060–1070. [Google Scholar] [CrossRef]
- Salzberg, S.L. On comparing classifiers: Pitfalls to avoid and a recommended approach. Data Min. Knowl. Discov. 1997, 1, 317–328. [Google Scholar] [CrossRef]
- Aviles-Santa, M.L.; Schneiderman, N.; Savage, P.J.; Kaplan, R.C.; Teng, Y.; Pérez, C.M.; Suárez, E.L.; Cai, J.; Giachello, A.L.; Talavera, G.A.; et al. Identifying probable diabetes mellitus among Hispanic/Latinos from four U.S. cities: Findings from the Hispanic Community Health Study/Study of Latinos. Endocr. Pract. 2016, 22, 1151–1160. [Google Scholar] [CrossRef]
- American Diabetes Association. Statistics about Diabetes; 2016. Available online: https://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf (accessed on 1 August 2019).
- Lee, D.-Y.; Park, S.K.; Kim, B.; Moon, K.H. Risk of diabetes increased according to the level of urinary albumin excretion even normal range. Nephrol. Dial. Transplant. 2015, 30 (Suppl. 3), 531–536. [Google Scholar] [CrossRef][Green Version]
- Zamora, C.R.; Cubeddu, L.X. Microalbuminuria: Do we need a new threshold? J. Hum. Hypertens. 2009, 23, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R. Urine dipstick readings ≥1+ had limited sensitivity but high specificity for detecting albuminuria in adults. Ann. Intern. Med. 2011, 155, JC4-10. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Yamamoto, H.; Yoshida, K.; Niwa, K.; Nishi, Y.; Mizuno, A.; Kuwabara, M.; Asano, T.; Sakoda, K.; Niinuma, H.; et al. The total urine protein-to-creatinine ratio can predict the presence of microalbuminuria. PLoS ONE 2014, 9, e91067. [Google Scholar] [CrossRef] [PubMed]
- Laurence, C.O.; Moss, J.R.; Briggs, N.E.; Beilbyet, J.J. PoCT Trial Management Group. The cost-effectiveness of point of care testing in a general practice setting: Results from a randomised controlled trial. BMC Health Serv. Res. 2010, 10, 165. [Google Scholar] [CrossRef]
- Shiwa, T.; Nishimura, M.; Kato, M. The effectiveness of the semi-quantitative assessment of microalbuminuria using routine urine dipstick screening in patients with diabetes. Intern. Med. 2018, 57, 503–506. [Google Scholar] [CrossRef]
- Nagrebetsky, A.; Jin, J.; Stevens, R.; James, T.; Adler, A.; Park, P.; Craven, A.; Shine, B.; Farmer, A. Diagnostic accuracy of urine dipstick testing in screening for microalbuminuria in type 2 diabetes: A cohort study in primary care. Fam. Pract. 2013, 30, 142–152. [Google Scholar] [CrossRef] [PubMed]
- McTaggart, M.P.; Newall, R.G.; Hirst, J.A.; Bankhead, C.R.; Lamb, E.J.; Roberts, N.W.; Price, C.P. Diagnostic accuracy of point-of-care tests for detecting albuminuria: A systematic review and meta-analysis. Ann. Intern. Med. 2014, 160, 550–557. [Google Scholar] [CrossRef] [PubMed]
| Attribute | Possible Values or Ranges | |
|---|---|---|
| Age (years) | 20–80+ | Continuous |
| Sex | Male, Female | Binary |
| Race/ethnicity |
| Nominal |
| Systolic blood pressure (mmHg) | 62–228 | Continuous |
| Diastolic blood pressure (mmHg) | 0–118 | Continuous |
| Waist circumference (cm) | 40.2–177.9 | Continuous |
| Body mass index (kg/m2) | 12.1–82.9 | Continuous |
| Total cholesterol (mg/dL) | 69–813 | Continuous |
| Urine albumin-to-creatinine ratio (mg/g Cr) | 0.21–9600 | Continuous |
| Glycohemoglobin (%) | 3.5–17.5 | Continuous |
| Tobacco use | Every day; some days; not at all | Nominal |
| Alcohol (average no. alcoholic drinks/day) | 1–25 | Continuous |
| Exercise to lose weight | No, Yes | Binary |
| Triglycerides (mg/dL) | 13–4233 | Continuous |
| LDL-cholesterol (mg/dL) | 14–375 | Continuous |
| Direct HDL-cholesterol (mg/dL) | 8–138 | Continuous |
| Fasting plasma glucose (mg/dL) | 51–421 | Continuous |
| Insulin (μU/mL) | 0.14–682.48 | Continuous |
| Total bilirubin (mg/dL) | 0.1–7.1 | Continuous |
| Attribute | Prediabetes (SD) | Diabetes (SD) | p-Value |
|---|---|---|---|
| Age (years) | 55.04 (15.79) | 60.01 (13.54) | <0.0001 |
| Sex | Male (1): 45.80% Female (0): 54.19% | Male (1): 50.74% Female (0): 49.25% | 0.0069 |
| Race/ethnicity | Mexican-American: 13.74% Other Hispanic: 6.87% Hispanic: 9.235% Non-Hispanic White: 51.33% Non-Hispanic Black: 18.51% Other race, including multiracial: 9.54% | Mexican-American: 21.54% Other Hispanic: 9.235% Non-Hispanic White: 38.85% Non-Hispanic Black: 23.03% Other race, including multiracial: 7.32% | <0.0001 |
| Systolic blood pressure (mmHg) | 125.47 (18.10) | 130.57 (20.15) | <0.0001 |
| Diastolic blood pressure (mmHg) | 69.18 (13.14) | 68.15 (14.99) | 0.0086 |
| Waist circumference (cm) | 104.66 (15.75) | 107.73 (15.73) | <0.0001 |
| Body mass index (kg/m2) | 30.95 (7.05) | 31.74 (6.93) | 0.0018 |
| Total cholesterol (mg/dL) | 198.19 (40.73) | 183.31 (40.37) | <0.0001 |
| Urine albumin-to-creatinine ratio (mg/g Cr) | 8.85 | 14.85 | 0.337 |
| Glycohemoglobin (%) HbA1c | 5.92 (1.05) | 7.38 (1.81) | <0.0001 |
| Tobacco use | Every day: 14.88% Some days: 1.90% Not at all: 83.20% | Every day: 14.33% Some days: 2.22% Not at all: 83.43% | 0.88 |
| Alcohol (average # alcoholic drinks/day) median | 1 | 1 | 0.002 |
| Exercise to lose weight | Yes: 30.34% No: 69.65% | Yes: 26.64% No: 73.35% | 0.13 |
| Triglycerides (mg/dL) | 134.76 (68.22) | 145.92(71.38) | 0.0001 |
| LDL-cholesterol (mg/dL) | 118.79 (36.29) | 104.18 (34.82) | <0.0001 |
| Direct HDL-cholesterol (mg/dL) | 52.47 (14.88) | 49.93 (13.63) | <0.0001 |
| Fasting plasma glucose (mg/dL) median | 108.0 | 139.8 | <0.0001 |
| Insulin (mU/mL) median | 12.1 | 12.74 | 0.21 |
| Total bilirubin (mg/dL) | 0.730 (0.28) | 0.72 (0.28) | 0.20 |
| NHANES Diabetes Dataset | TR ACC (%) | TS ACC (%) | # Rules | AUC-ROC (%) |
|---|---|---|---|---|
| Continuous Re-RX [10 × 5 CV] | 79.65 ± 0.62 | 77.56 ± 2.19 | 15.82 | 75 |
| IF Part | Condition 1 | Condition 2 | Condition 3 | Condition 4 | Condition 5 | THEN |
|---|---|---|---|---|---|---|
| R1 | HbA1c ≤ 5.8 | FPG ≤ 122.7 | LDL ≤ 101.0 | Mexican-American = 0 (No) | Class 2 (Prediabetes) | |
| R2 | HbA1c ≤ 5.8 | FPG ≤ 122.7 | LDL ≤ 101.0 | Mexican American = 1: (Yes) | AGE ≤ 40 | Class 2 (Prediabetes) |
| R3 | HbA1c ≤ 5.8 | FPG ≤ 122.7 | LDL ≤ 101.0 | Mexican-American =1: (Yes) | AGE > 40 | Class 1 (Diabetes) |
| R4 | HbA1 ∈ (5.8, 6.1) | FPG ≤ 122.7 | LDL ≤ 101.0 | Class 1 (Diabetes) | ||
| R5 | HbA1c ≤ 6.1 | FPG ≤ 122.7 | LDL > 101.0 | Class 1 (Diabetes) | ||
| R6 | HbA1c ≤ 5.6 | FPG > 122.7 | UACR ≤ 71.00 | Class 2 (Prediabetes) | ||
| R7 | HbA1c ≤ 5.6 | FPG > 122.7 | UACR > 71.00 | Class 1 (Diabetes) | ||
| R8 | HbA1c ∈ (5.6, 6.1) | FPG > 122.7 | LDL ≤ 151.0 | Class 1 (Diabetes) | ||
| R9 | HbA1c ∈ (5.6, 6.1) | FPG > 122.7 | LDL > 151.0 | Class 2 (Prediabetes) | ||
| R10 | HbA1c ∈ (6.1, 6.4) | LDL ≤ 142.0 | FPG ≤ 108.5 | Non-Hispanic Black = 0: (No) | Class 1 (Diabetes) | |
| R11 | HbA1c ∈ (6.1, 6.4) | LDL ≤ 142.0 | FPG ≤ 108.5 | Non-Hispanic Black = 1: (Yes) | UACR ≤ 6.1 | Class 2 (Prediabetes) |
| R12 | HbA1c ∈ (6.1, 6.4) | LDL ≤ 142.0 | FPG ≤ 108.5 | Non-Hispanic Black = 1: (Yes) | UACR > 6.1 | Class 1 (Diabetes) |
| R13 | HbA1c ∈ (6.1, 6.4) | LDL ≤ 142.0 | FPG > 108.5 | Class 1 (Diabetes) | ||
| R14 | HbA1c ∈ (6.1, 6.4) | LDL > 142.0 | Class 2 (Prediabetes) | |||
| R15 | HbA1c > 6.4 | Class 1 (Diabetes) |
| Japan | UK | The US | |
|---|---|---|---|
| Cost of laboratory test for UACR | JPY 1080 [50] | ₤7.4 [51] | $16 [48] |
| Cost of a POC testing for a semi-quantitative UACR | JPY 230 [50] | ₤2.31 [51] | --- |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, Y. Detection of Lower Albuminuria Levels and Early Development of Diabetic Kidney Disease Using an Artificial Intelligence-Based Rule Extraction Approach. Diagnostics 2019, 9, 133. https://doi.org/10.3390/diagnostics9040133
Hayashi Y. Detection of Lower Albuminuria Levels and Early Development of Diabetic Kidney Disease Using an Artificial Intelligence-Based Rule Extraction Approach. Diagnostics. 2019; 9(4):133. https://doi.org/10.3390/diagnostics9040133
Chicago/Turabian StyleHayashi, Yoichi. 2019. "Detection of Lower Albuminuria Levels and Early Development of Diabetic Kidney Disease Using an Artificial Intelligence-Based Rule Extraction Approach" Diagnostics 9, no. 4: 133. https://doi.org/10.3390/diagnostics9040133
APA StyleHayashi, Y. (2019). Detection of Lower Albuminuria Levels and Early Development of Diabetic Kidney Disease Using an Artificial Intelligence-Based Rule Extraction Approach. Diagnostics, 9(4), 133. https://doi.org/10.3390/diagnostics9040133





