Abstract
We aimed to determine the oncological outcomes of patients with clinical T1 renal cell carcinoma (RCC) upstaged to pathological T3a and to identify the preoperative predictive factors for upstaging. We retrospectively reviewed 272 patients with clinical T1 RCC who underwent surgical treatment. Thirty-three patients (12%) were upstaged to pathological T3a. These patients had a significantly larger tumor size on computed tomography (p < 0.0001), a higher aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio (p = 0.037), and an elevated c-reactive protein (CRP) level (p = 0.014) preoperatively compared with those with pathological T1 RCC. On multivariate analysis, tumor diameter was the only significant preoperative predictive factor for upstaging [hazard ratio (HR), 3.61; 95% confidence interval (CI), 1.32–9.84; p = 0.01]. The AST/ALT ratio tended to be a preoperative predictive factor for upstaging, although it was not significant (HR, 2.14; 95% CI, 0.97–4.73; p = 0.06). Pathological T3a upstaging occurred in 25% of those with a tumor diameter ≥30 mm and a preoperative AST/ALT ratio ≥1.1. There was a significant correlation between pathological T3a upstaging and the number of preoperative risk factors (p = 0.0002). The preoperative tumor diameter and serum AST/ALT ratio can be predictive factors for pathological T3a upstaging in patients with clinical T1 RCC.
1. Introduction
Clinical staging is essential for treatment decision-making in patients with renal cell carcinoma (RCC). The selection of the surgical technique (partial or radical nephrectomy) often depends on the clinical T stage of the tumor; T1 and T2 tumors are classified according to the tumor size, whereas T3a tumors are defined based on the presence of peripheral fat invasion, renal sinus fat infiltration, or renal vein extension, regardless of the tumor size. However, microscopic perirenal invasion, renal sinus fat infiltration, and renal vein extension can be missed during contrast-enhanced computed tomography (CT) imaging, occasionally leading to the pathological upstaging of a clinical T1 tumor to pathological T3a. Several previous studies have reported a poor prognosis of patients with RCC with cT1 tumors upstaged to pT3a when compared with a prognosis of patients with pathological T1 tumors [1,2,3,4]. This study investigates the oncological outcomes of pathological T3a upstaging and identifies the preoperative predictive factors for upstaging in patients with cT1 RCC.
2. Materials and Methods
We retrospectively reviewed the records of all patients with non-metastatic clinical T1 renal tumors who underwent partial nephrectomy (PN) or radical nephrectomy (RN) at Nara Medical University and Nara Prefecture General Medical Center between January 2000 and December 2017. Institutional review board approval was obtained for this study. Two hundred seventy-two patients (196 men and 76 women) diagnosed with RCC who had complete records of preoperative demographics and postoperative outcomes were included for further analysis. Patients who underwent renal replacement therapy, such as hemodialysis or peritoneal dialysis, and those with liver disease during treatment were excluded. We also excluded patients with non-RCC pathology, bilateral or multiple renal tumors, and von Hippel–Lindau disease. The clinical T stage was assessed with contrast-enhanced CT.
We evaluated the following preoperative characteristics: Age at the time of operation, gender, body mass index (BMI), tumor laterality, clinical T stage, RENAL nephrometry score, and tumor diameter upon contrast-enhanced CT, as well as serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), and c-reactive protein(CRP) levels. The AST/ALT ratio was also calculated. Surgical and histopathological data included surgical technique (PN, RN, laparoscopic surgery, or open surgery), tumor histology, Fuhrman grade, and the presence of microscopic venous invasion and/or lymphatic invasion. The surgical technique was determined by discussion among experienced urologists after taking into account patient comorbidities, tumor size, tumor location, and intraoperative findings. Lastly, outcomes were measured using overall survival (OS), cancer-specific survival (CSS), and recurrence-free survival (RFS).
Data were analyzed using JMP® 13 software (SAS Institute Inc., Cary, NC, USA). The Mann–Whitney, chi-square, and Fisher’s exact tests were used to compare the pT3a upstage and pT1 groups. OS, CSS and RFS was estimated using Kaplan-Meier method. Cox proportional hazards regression was used to examine variables associated with pT3a upstaging. A p-value < 0.05 was considered to be significant.
3. Results
Patient characteristics are presented in Table 1.

Table 1.
Characteristics of the patients (n = 272).
The median patient age at time of operation was 65 years, and the median BMI was 23.6. One hundred thirty-three (49%) patients had a right-sided tumor, whereas 139 (51%) patients had a left-sided tumor. The clinical T stage was cT1a in 193 (71%) patients and cT1b in 79 (29%) patients. The mean RENAL nephrometry score was 7. Median tumor diameter was 30 mm. RN was performed in 170 (63%) patients, whereas PN was performed in 102 (37%) patients. Overall, the median operative time was 197 min. During the median follow-up period of 35 months, five (2%) patients died. Sixteen (6%) showed recurrence (four local and 12 distant metastasis).
Pathological T3a upstaging (pT3a group) occurred in 33 (12%) patients. The remaining 239 (88%) were diagnosed as pathological T1 (pT1 group). The preoperative characteristics of the pT3a group and the pT1 group are presented in Table 2.

Table 2.
The differences of the preoperative parameters between pT3a and pT1 groups.
There was no significant difference between the groups with respect to age at operation, gender, tumor laterality, BMI, and RENAL nephrometry score.
The clinical T1b stage was significantly more prevalent in the pT3a group than the pT1 group (64% vs. 24%, p < 0.0001). The tumor diameter was significantly larger in the pT3a group than in the pT1 group (50 mm vs. 30 mm, p < 0.0001). The preoperative AST/ALT ratio was significantly higher in the pT3a group than in the pT1 group (1.13 vs. 1.09, p = 0.037). The CRP levels were also significantly higher in the pT3a group than in the pT1 group (0.1 vs. 0.09, p = 0.014). There was no significant difference in terms of preoperative AST and ALT levels. The results of multivariate regression analyses of preoperative predictive factors for pathological T3a upstaging of clinical T1 RCC are shown in Table 3.

Table 3.
Multivariate analysis for pathological T3a upstaging.
Upon multivariate analyses, tumor diameter (<30 mm vs. ≥30 mm) was the only significant preoperative predictor of pT3a upstaging [hazard ratio (HR), 3.61; 95% confidence interval (CI), 1.32–9.84; p = 0.01]. The AST/ALT ratio (<1.1 vs. ≥1.1) tended to be a preoperative predictor of pT3a upstaging, although it was not significant (HR 2.14; 95% CI: 0.97–4.73; p = 0.06).
Pathological T3a upstaging occurred in 25% of patients with both a preoperative tumor diameter ≥30 mm and a preoperative AST/ALT ratio ≥1.1. There was a significant correlation between pathological T3a upstaging and the number of preoperative risk factors (chi-square, p = 0.0002) (Table 4).

Table 4.
The number of preoperative risk factors and the incidence of pathological T3a upstaging.
The surgical and histopathological data of the pT3a and pT1 groups are presented in Table 5.

Table 5.
The differences of the tumor characteristics and postoperative parameters between the pT3a and pT1 groups.
The pT3a group had a higher proportion of RN compared to the pT1 group (100% vs. 57%, p < 0.0001). High grade tumors (Fuhrman grade 3–4) were significantly more common in the pT3a group compared to the pT1 group (24% vs. 4%, p < 0.0001). Microscopic venous invasion (v+) and microscopic lymphatic invasion (ly+) were also significantly more prevalent in the pT3a group compared to the pT1 group (64% vs. 5%, p < 0.001; 15% vs. 0%, p < 0.0001, respectively). There was no significant difference in tumor histology between the groups (p = 0.22).
With respect to outcomes, there were no significant differences between the groups in terms of OS (log rank, p = 0.96), CSS (log rank, p = 0.49), and RFS (log rank, p = 0.36). There was a difference in terms of two-year RFS rate between the groups, although it was not significant (82.5% vs. 97.3%, p = 0.09) (Figure 1).
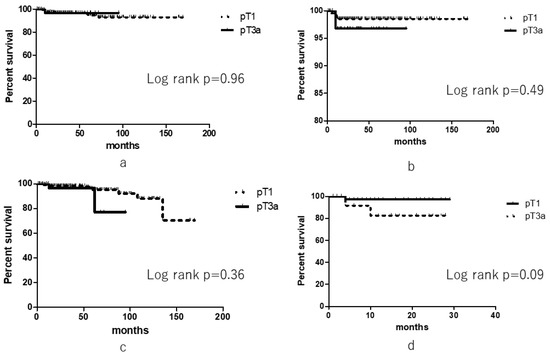
Figure 1.
Oncological outcomes of pathological T3 upstaging and pathological T1 patients. (a) Overall survival, (b) Cancer-specific survival, (c) Recurrence-free survival, (d) Two-year recurrence-free survival).
4. Discussion
There has been an increase in the detection of incidental small renal masses due to advances in diagnostic imaging technologies. PN for organ-confined small renal masses has been the standard treatment option because it provides an extension of OS, preserves renal function, and reduces cardiovascular risk compared to RN [5,6]; PN also provides similar oncological outcomes as RN [5,6,7]. However, in locally advanced RCC, the resection of Gerota’s fascia and the dissection of the perirenal fat during PN may increase the risk of recurrence [8].
The TNM classification of a tumor is essential in order to determine its appropriate treatment, surgical technique, follow-up protocol, and oncological prognosis [9,10]. Clinical T1 and T2 tumors are classified according to tumor size. Tumors with diameter of ≤4 cm are defined as clinical T1a, ones diameters from 4< to ≤7 cm are defined as clinical T1b, and diameters more than 7 cm with organ confined tumors are defined as clinical T2 tumors. However, clinical T3a tumors are defined by the presence of peripheral fat invasion, renal sinus fat infiltration, or renal vein extension regardless of tumor size. Though contrast-enhanced CT is commonly used diagnostically for segmental or main renal vein invasion, its reported sensitivity is only 59–69% [11]. Sokhi et al. reported the sensitivity of CT for identifying the tumor invasion of the renal sinus fat (88%), perirenal fat (83%), and renal vein (69%) [12]. Microscopic perirenal invasion, renal sinus fat infiltration, or renal vein extension can be missed with contrast-enhanced CT imaging, and pathological T3a upstaging occasionally occurs in patients with cT1 tumors.
Previous studies have reported a poor prognosis in RCC patients with cT1 tumors upstaged to pT3a when compared to patients with pT1 tumors. Gorin et al. reported the incidence and outcomes of pathological T3a upstaging in patients with clinical T1 RCC who underwent robotic PN [1]. Pathological T3a tumors were observed in 4.8%, and the 24-month RFS rates for pT1-2 and pT3a tumors were 99.2% and 91.8%, respectively. Jeong et al. reported that pT3a upstaging occurred in 9.2% of cT1 RCCs, and upstaging was associated with a significantly poorer prognosis compared to pT1 disease; the two-year RFS rates in the pT3a upstaged and pT1 groups were 87.3% and 98.7%, respectively (p < 0.001) [2]. Ghanie et al. [3] also analyzed the pathological upstaging of clinical T1 RCC using the National Cancer Data Base Participant User File. They reported that pT3a upstaging was observed in 5.4% patients with cT1 RCC and that upstaging led to a 40% increase in risk of death compared to patients with pT1 tumors. Russel et al. also reported significantly reduced CSS and RFS rates in patients with pT3a upstaging following PN compared to pT1 tumors [4]. In our study, however, there was no significant difference between the pT3a and pT1 groups in terms of OS, CSS, and RFS.
Previous reports have analyzed predictive factors for pT3a upstaging. Gorin et al. reported that preoperative predictive factors for pT3a upstaging were tumor diameter and location [1]. Similarly, Jeong et al. noted older age, cT1b stage, clinical symptoms, and a high Fuhrman grade as such [2]. Ghanie et al. reported that the predictive factors for pT3a upstaging were patients who were older, male, had comorbidities, had cT1b tumors, underwent RN, and had a high Fuhrman grade [3]. Ramaswamy et al. [13] demonstrated that T3a upstaging was associated with a clear cell histology, tumor size >4 cm, and a positive surgical margin on pathological examination. Tay et al. [14] found that a high RENAL nephrometry score was a predictive factor for pT3a upstaging, but age and Fuhrman grade were not.
As noted above, the previous reports tended to describe the relationship between histopathological findings and pT3a upstaging. We wanted to investigate preoperative predictive factors for pT3a upstaging, because they may affect the surgical approach or technique in patients with clinical T1 RCC.
In our study, the preoperative predictive factors for pathological T3a upstaging were tumor diameter, CRP level, and AST/ALT ratio in univariate analysis. A multivariate analysis showed that tumor diameter was the only significant preoperative predictor of pathological T3a upstaging; the AST/ALT ratio tended to be a predictor but it was not significant. When the preoperative tumor size and AST/ALT ratio were further considered in detail, pathological T3a upstaging occurred in 25% of patients with both tumor diameter ≥3.0 mm and an AST/ALT ratio ≥1.1.
Aminotransaminases, including AST and ALT, are well-known liver enzymes produced by both malignant and non-malignant cells, and they are blood-based circulating biomarkers. ALT is only existent in the hepatocellular cytoplasm and mitochondria. AST, on the other hand, is widely spread in several organs, including the heart, kidney, brain, skeleton, muscle, and liver [15]. The functions of AST and ALT represent crucial metabolic interactions between protein and carbohydrate metabolism. AST and ALT are also important in all cells that have a high metabolic activity; AST is particularly vital for aerobic glycolysis [15]. Malignant cells show a higher rate of glycolysis than non-malignant cells [16]. Pathological processes that can lead to a higher proliferative state, tissue damage, and high tumor cell turnover tend to increase AST but not ALT, making the AST/ALT ratio an attractive potential biomarker.
Previous reports have demonstrated that these enzymes could be a significant prognostic biomarker in several malignancies such as those of the lung, colon, pancreatic, and breast cancer [17,18,19,20].
The AST/ALT ratio has been widely used in the evaluation of liver disease. Moreover, it could also be a crucial prognostic factor in various malignancies [21,22,23]. Bezan et al. [24] reported that the preoperative AST/ALT ratio is an independent prognostic factor in patients with non-metastatic RCC; a high AST/ALT ratio was significantly associated with poor outcomes regarding progression-free survival (PFS) and OS. Lee et al. [25] also reported that an elevated AST/ALT ratio was significantly associated with worse postoperative survival in patients surgically treated for non-metastatic RCC; a high AST/ALT ratio was significantly associated with poor PFS, CSS, and OS. Canat et al. reported [11] a significant correlation between the AST/ALT ratio and tumor histopathological variables and demonstrated that RCC patients with a high preoperative AST/ALT ratio were more likely to have renal vein invasion observed on histopathological examination. Venous invasion is said to be a poor prognostic factor and associated with poor survival rates. Though CT is commonly used as diagnostic imaging for segmental or main renal vein invasion, the reported sensitivity of this modality is reportedly only 59–69%; the AST/ALT ratio may be considered a sensitive biomarker for the prediction of renal vein invasion [11].
Jeong et al. [2] found that surgical technique (PN or RN) did not affect the recurrence rate for cT1 RCC upstaged to pT3a. Capitanio et al. [26] also reported no difference in terms of metastatic PFS and CSS between PN and RN cohorts in patients upstaged to pathological T3a RCC disease. They concluded that cancer control is similar between patients treated with an extirpation of the entire kidney and those with partial resection, even if the final histopathological examination demonstrated a tumor that was unexpectedly upstaged from clinical T1 to pathological T3a.
On the other hand, Shah et al. [27] reported that those who undergo PN appear to have inferior RFS relative to those who undergo RN. They reviewed the records of 1250 patients with clinical T1 RCC who underwent partial or radical nephrectomies and showed that pathological T3a upstaging was noted in 140 patients (11%); among the pathological T3a upstaged patients, PN was associated with shorter RFS compared to RN.
Controversy remains regarding whether surgical approach (PN or RN) affects the recurrence rate in RCC. We believe that attention should be paid to the resection of perirenal fat and renal parenchyma during surgery in patients with clinical T1 RCC who have a large tumor and a high preoperative AST/ALT ratio, because these tumors may have a higher possibility to be upstaged to pT3a.
There are some limitations to this study. It is retrospective in nature with a limited follow-up period and examined a relatively small number of patients. However, to the best of our knowledge, this is the first study showing the correlation between the preoperative AST/ALT ratio and pathological T3a upstaging in patients with clinical T1 RCC. Further prospective studies are needed to validate our findings.
5. Conclusions
In conclusion, this study showed that larger tumors and probably preoperative high AST/ALT ratio were significantly associated with pathological T3a upstaging in patients with clinical T1 RCC, suggesting that they are potential preoperative predictive factors.
Author Contributions
Conceptualization—S.F., M.M.; methodology—S.F.; formal analysis—S.F., M.M.; investigation—S.F., K.I., S.H., K.O., Y.M., Y.K.; supervision—K.F.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gorin, M.A.; Ball, M.W.; Pierorazio, P.M.; Tanagho, Y.S.; Bhayani, S.B.; Kaouk, J.H.; Rogers, C.G.; Stifelman, M.D.; Khalifeh, A.; Kumar, R.; et al. Outcomes and predictors of clinical T1 to pathological T3a tumor up-staging after robotic partial nephrectomy: A multi-institutional analysis. J. Urol. 2013, 190, 1907–1911. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Kim, J.K.; Park, J.; Jeon, H.J.; Yoon, M.Y.; Jeong, C.W.; Ku, J.H.; Kim, H.H.; Kwak, C. Pathological T3a Upstaging of Clinical T1 Renal Cell Carcinoma: Outcomes According to Surgical Technique and Predictors of Upstaging. PLoS ONE 2016, 11, e0166183. [Google Scholar] [CrossRef] [PubMed]
- Ghanie, A.; Formica, M.K.; Wang, D.; Bratslavsky, G.; Stewart, T. Pathological upstaging of clinical T1 renal cell carcinoma: An analysis of 115,835 patients from National Cancer Data Base, 2004–2013. Int. Urol. Nephrol. 2018, 50, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.M.; Lebastchi, A.H.; Chipollini, J.; Niemann, A.; Mehra, R.; Morgan, T.M.; Miller, D.C.; Palapattu, G.S.; Hafez, K.S.; Sexton, W.J.; et al. Multi-institutional Survival Analysis of Incidental Pathologic T3a Upstaging in Clinical T1 Renal Cell Carcinoma Following Partial Nephrectomy. Urology 2018, 117, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Porpiglia, F.; Mari, A.; Bertolo, R.; Antonelli, A.; Bianchi, G.; Fidanza, F.; Fiori, C.; Furlan, M.; Morgia, G.; Novara, G.; et al. Partial Nephrectomy in Clinical T1b Renal Tumors: Multicenter Comparative Study of Open, Laparoscopic and Robot-assisted Approach (the RECORd Project). Urology 2016, 89, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Kreshover, J.E.; Richstone, L.; Kavoussi, L.R. Renal cell recurrence for T1 tumors after laparoscopic partial nephrectomy. J. Endourol. 2013, 27, 1468–1470. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.H.; Siddiqui, S.; Lohse, C.M.; Leibovich, B.C.; Russo, P.; Blute, M.L. Partial versus radical nephrectomy for 4 to 7 cm renal cortical tumors. J. Urol. 2009, 182, 2601–2606. [Google Scholar] [CrossRef]
- Breau, R.H.; Crispen, P.L.; Jimenez, R.E.; Lohse, C.M.; Blute, M.L.; Leibovich, B.C. Outcome of stage T2 or greater renal cell cancer treated with partial nephrectomy. J. Urol. 2010, 183, 903–908. [Google Scholar] [CrossRef]
- Baccos, A.; Brunocilla, E.; Schiavina, R.; Borghesi, M.; Rocca, G.C.; Chessa, F.; Saraceni, G.; Fiorentino, M.; Martorana, G. Differing risk of cancer death among patients with pathologic T3a renal cell carcinoma: Identification of risk categories according to fat infiltration and renal vein thrombosis. Clin. Genitourin Cancer 2013, 11, 451–457. [Google Scholar] [CrossRef]
- Van Oostenbrugge, T.J.; Kroeze, S.G.; Bosch, J.L.; van Melick, H.H. The blind spots in follow-up after nephrectomy or nephron-sparing surgery for localized renal cell carcinoma. World J. Urol. 2015, 33, 881–887. [Google Scholar] [CrossRef]
- Canat, L.; Ataly, H.A.; Agalarov, S.; Alkan, I.; Altunrende, F. The effect of AST/ALT (De Ritis) ratio on survival and its relation to tumor histopathological variables in patients with localized renal cell carcinoma. Int. Braz. J. Urol. 2018, 44, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Sokhi, H.K.; Mok, W.Y.; Patel, U. Stage T3a renal cell carcinoma: Staging accuracy of CT for sinus fat, perinephric fat or renal vein invasion. Br. J. Radiol. 2015, 88, 20140504. [Google Scholar] [CrossRef]
- Ramaswamy, K.; Kheterpal, E.; Pham, H.; Mohan, S.; Stifelman, M.; Taneja, S.; Huang, W.C. Significance of Pathologic T3a Upstaging in Clinical T1 Renal Masses Undergoing Nephrectomy. Clin. Genitourin Cancer 2015, 13, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Tay, M.H.; Thamboo, T.P.; Wu, F.M.; Zhaojin, C.; Choo, T.B.; Ramaan, L.; Tiong, H.Y. High R.E.N.A.L. Nephrometry scores are associated with pathologic upstaging of clinical T1 renal-cell carcinomas in radical nephrectomy specimens: Implications for nephron-sparing surgery. J. Endourol. 2014, 28, 1138–1142. [Google Scholar] [CrossRef] [PubMed]
- Botros, M.; Sikaris, K.A. The de ritis ratio: The test of time. Clin. Biochem. Rev. 2013, 34, 117–130. [Google Scholar] [PubMed]
- Hsu, P.P.; Sabatini, D.M. Cancer cell metabolism: Warburg and beyond. Cell 2008, 134, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Lindmark, G.; Gerdin, B.; Påhlman, L.; Bergström, R.; Glimelius, B. Prognostic predictors in colorectal cancer. Dis. Colon Rectum. 1994, 37, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Rawson, N.S.; Peto, J. An overview of prognostic factors in small cell lung cancer. A report from the Subcommittee for the Management of Lung Cancer of the United Kingdom Coordinating Committee on Cancer Research. Br. J. Cancer 1990, 61, 597–604. [Google Scholar] [CrossRef]
- Stocken, D.D.; Hassan, A.B.; Altman, D.G.; Billingham, L.J.; Bramhall, S.R.; Johnson, P.J.; Freemantle, N. Modelling prognostic factors in advanced pancreatic cancer. Br. J. Cancer 2008, 99, 883–893. [Google Scholar] [CrossRef]
- Thornburg, J.M.; Nelson, K.K.; Clem, B.F.; Lane, A.N.; Arumugam, S.; Simmons, A.; Eaton, J.W.; Telang, S.; Chesney, J. Targeting aspartate aminotransferase in breast cancer. Breast Cancer Res. 2008, 10, R84. [Google Scholar] [CrossRef]
- Takenaka, Y.; Takemoto, N.; Yasui, T.; Yamamoto, Y.; Uno, A.; Miyabe, H.; Ashida, N.; Shimizu, K.; Nakahara, S.; Hanamoto, A.; et al. Transaminase Activity Predicts Survival in Patients with Head and Neck Cancer. PLoS ONE 2016, 11, e0164057. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Choi, Y.H.; Sung, H.H.; Han, D.H.; Jeon, H.G.; Chang Jeong, B.; Seo, S.I.; Jeon, S.S.; Lee, H.M.; Choi, H.Y. De Ritis Ratio (AST/ALT) as a Significant Prognostic Factor in Patients with Upper Tract Urothelial Cancer Treated with Surgery. Clin. Genitourin Cancer 2017, 15, e379–e385. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.S.; Chang, C.S.; Yang, S.S.; Yeh, H.Z.; Lin, C.W. Retrospective evaluation of serum markers APRI and AST/ALT for assessing liver fibrosis and cirrhosis in chronic hepatitis B and C patients with hepatocellular carcinoma. Intern. Med. 2008, 47, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Bezan, A.; Mrsic, E.; Krieger, D.; Stojakovic, T.; Pummer, K.; Zigeuner, R.; Hutterer, G.C.; Pichler, M. The Preoperative AST/ALT (De Ritis) Ratio Represents a Poor Prognostic Factor in a Cohort of Patients with Nonmetastatic Renal Cell Carcinoma. J. Urol. 2015, 194, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, S.E.; Byun, S.S.; Kim, H.H.; Kwak, C.; Hong, S.K. De Ritis ratio (aspartate transaminase/alanine transaminase ratio) as a significant prognostic factor after surgical treatment in patients with clear-cell localized renal cell carcinoma: A propensity score-matched study. BJU Int. 2017, 119, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Capitanio, U.; Stewart, G.D.; Klatte, T.; Akdogan, B.; Roscigno, M.; Marszalek, M.; Dell’Oglio, P.; Zaffuto, E.; Rodriguez Faba, O.; Salagierski, M.; et al. Does the Unexpected Presence of Non-organ-confined Disease at Final Pathology Undermine Cancer Control in Patients with Clinical T1N0M0 Renal Cell Carcinoma Who Underwent Partial Nephrectomy? Eur. Urol. Focus 2018, 4, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.H.; Moreira, D.M.; Patel, V.R.; Gaunay, G.; George, A.K.; Alom, M.; Kozel, Z.; Yaskiv, O.; Hall, S.J.; Schwartz, M.J.; et al. Partial Nephrectomy is Associated with Higher Risk of Relapse Compared with Radical Nephrectomy for Clinical Stage T1 Renal Cell Carcinoma Pathologically Up Staged to T3a. J. Urol. 2017, 198, 289–296. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).