Prevalence of Oral Lesions and Correlation with Intestinal Symptoms of Inflammatory Bowel Disease: A Systematic Review
Abstract
1. Introduction
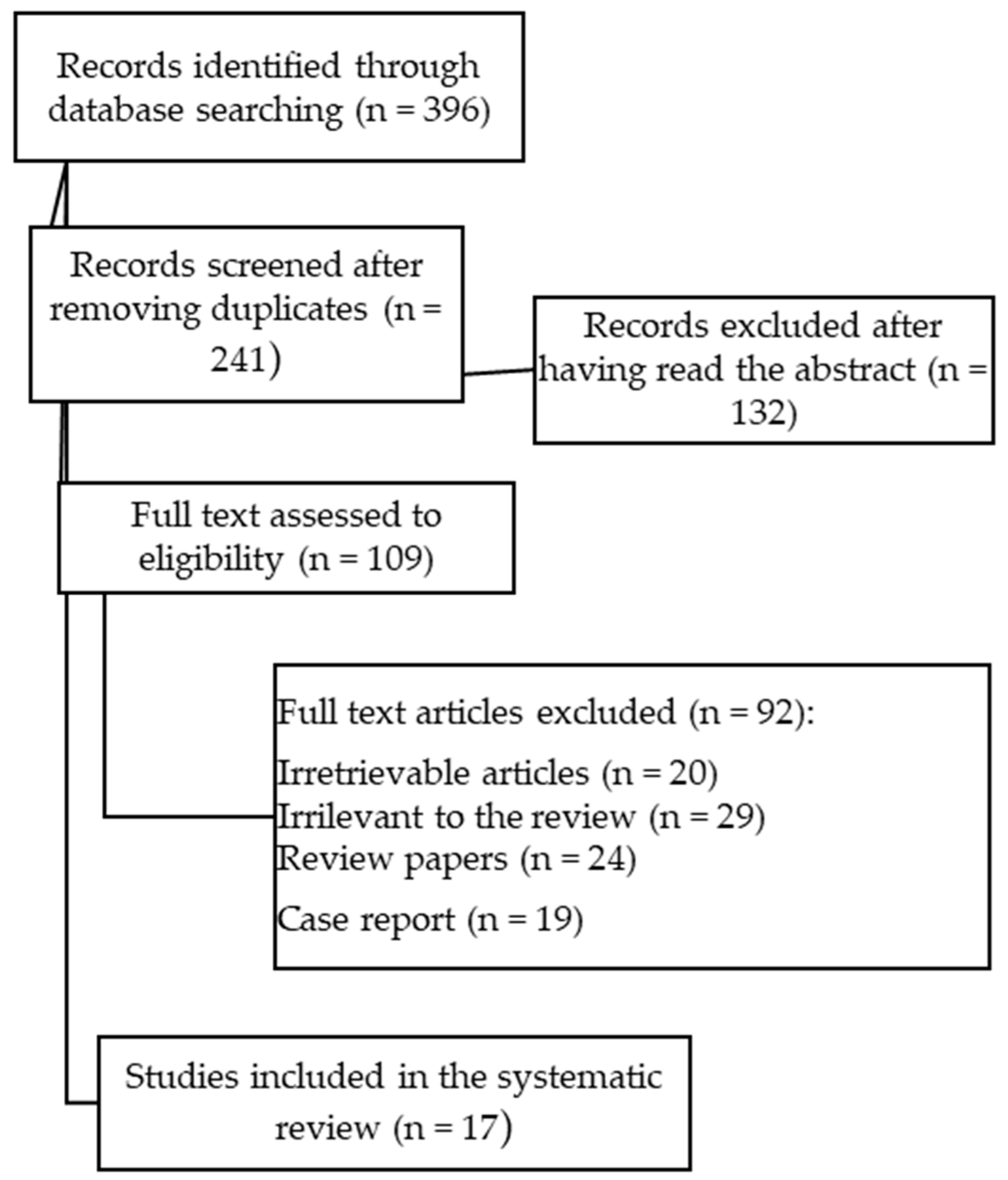
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Habashneh, R.A.; Khader, Y.S.; Alhumouz, M.K.; Jadallah, K.; Ajlouni, Y. The association between inflammatory bowel disease and periodontitis among Jordanians: A case-control study. J. Periodontal. Res. 2012, 47, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Greuter, T.; Bertoldo, F.; Rechner, R.; Straumann, A.; Biedermann, L.; Zeitz, J.; Misselwitz, B.; Scharl, M.; Rogler, G.; Safroneeva, E.; et al. Extraintestinal manifestations of pediatric inflammatory bowel disease: Prevalence, presentation and anti-TNF treatment. J. Pediatric. Gastroenterol. 2017, 65, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Zippi, M.; Corrado, C.; Pica, R.; Avallone, E.V.; Cassieri, C.; De Nitto, D.; Paoluzi, P.; Vernia, P. Extraintestinal manifestations in a large series of Italian inflammatory bowel disease patients. World. J. Gastroenterol. 2014, 20, 17463–17467. [Google Scholar] [CrossRef] [PubMed]
- Khouri, J.M.; Bohane, T.D.; Day, A.S. Is orofacial granulomatosis in children a feature of Crohn’s disease? Acta. Paediatr. 2005, 94, 501–504. [Google Scholar] [CrossRef]
- Jose, F.A.; Garnett, E.A.; Vittinghoff, E.; Ferry, G.D.; Winter, H.S.; Baldassano, R.N.; Kirschner, B.S.; Cohen, S.A.; Gold, B.D.; Abramson, O.; et al. Development of Extraintestinal Manifestations in Pediatric Patients with Inflammatory Bowel Disease. Inflamm. Bowel. Dis. 2009, 151, 63–68. [Google Scholar] [CrossRef]
- Brito, F.; de Barros, F.C.; Zaltman, C.; Carvalho, A.T.P.; Carneiro, A.J.V.; Fischer, R.G.; Gustafsson, A.; Figueredo, C.M.S. Prevalence of periodontitis and DMFT index in patients with Crohn’s disease and ulcerative colitis. J. Clin. Periodontol. 2008, 35, 555–560. [Google Scholar] [CrossRef]
- Grössner-Schreiber, B.; Fetter, T.; Hedderich, J.; Kocher, T.; Schreiber, S.; Jepsen, S. Prevalence of dental caries and periodontal disease in patients with inflammatory bowel disease: A case-control study. J. Clin. Periodontol. 2006, 33, 478–484. [Google Scholar] [CrossRef]
- Szymanska, S.; Lördal, M.; Rathnayake, N.; Gustafsson, A.; Johannsen, A. Dental Caries, Prevalence and Risk Factors in Patients with Crohn’s Disease. PLoS ONE 2014, 9, e91059. [Google Scholar] [CrossRef]
- Koutsochristou, V.; Zellos, A.; Dimakou, K.; Panayotou, I.; Siahanidou, S.; Roma-Giannikou, E.; Tsami, A. Dental Caries and Periodontal Disease in Children and Adolescents with Inflammatory Bowel Disease: A Case–Control Study. Inflamm. Bowel. Dis. 2015, 21, 1839–1846. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 4 July 2019).
- Harty, S.; Fleming, P.; Rowland, M.; Crushell, E.; McDermott, M.; Drumm, B.; Bourke, B. A prospective study of the oral manifestations of Crohn’s disease. Clin. Gastroenterol. Hepatol. 2005, 3, 886–891. [Google Scholar] [CrossRef]
- Elahi, M.; Telkabadi, M.; Samadi, V.; Vakili, H. Association of oral manifestations with ulcerative colitis. Gastroenterol. Hepatol. Bed. Bench. 2012, 5, 155–160. [Google Scholar] [CrossRef]
- Katz, J.; Shenkman, A.; Stavropoulos, F.; Melzer, E. Oral signs and symptoms in relation to disease activity and site of involvement in patients with inflammatory bowel disease. Oral Dis. 2003, 9, 34–40. [Google Scholar] [CrossRef]
- Laranjeira, N.; Fonseca, J.; Meira, T.; Freitas, J.; Leitão, J. Oral Mucosa Lesions And Oral Symptoms In Inflammatory Bowel Disease Patients. Arq. Gastroenterol. 2015, 52, 105–110. [Google Scholar] [CrossRef]
- Mohan Kumar, K.P.; Nachiammai, N.; Madhushankari, G.S. Association of oral manifestations in ulcerative colitis: A pilot study. J. Oral Maxillofac. Pathol. 2018, 22, 199–203. [Google Scholar] [CrossRef]
- Rikardsson, S.; Jönsson, J.; Hultin, M.; Gustafsson, A.; Johannsen, A. Perceived oral health in patients with Crohn’s disease. Oral Health. Prev. Dent. 2009, 7, 277–282. [Google Scholar]
- Zervou, F.; Gikas, A.; Merikas, E.; Peros, G.; Sklavaina, M.; Loukopoulos, J.; Triantafillidis, J.K. Oral manifestations of patients with inflammatory bowel disease. Ann. Gastroenterol. 2004, 17, 395–401. [Google Scholar]
- Oviedo, C.; Yañez, M.; Pennacchiotti, V. Frequency of oral manifestation in patients with inflammatory bowel disease in Chile. Int. J. Odontostomat. 2017, 11, 267–271. [Google Scholar] [CrossRef][Green Version]
- Scully, C.; Hodgson, T. Recurrent oral ulceration: Aphthous-like ulcers in periodic syndromes. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2008, 106, 845–852. [Google Scholar] [CrossRef]
- Rubesin, S.E.; Scotiniotis, I.; Birnbaum, B.A.; Ginsberg, G.G. Radiologic and Endoscopic Diagnosis of Crohn’s Disease. Surg. Clin. North Am. 2001, 81, 39–70. [Google Scholar] [CrossRef]
- Wiesenfeld, D.; Ferguson, M.M.; Mitchell, D.N.; MacDonald, D.G.; Scully, C.; Cochran, K.; Russel, R.I. Oro-facial granulomatosis-a clinical and pathological analysis. QJ. Med. 1985, 54, 101–113. [Google Scholar]
- Kabbani, T.A.; Koutroubakis, I.E.; Schoen, R.E.; Ramos-Rivers, C.; Shah, N.; Swoger, J.; Regueiro, M.; Barrie, A.; Schwartz, M.; Hashash, J.G.; et al. Association of Vitamin D Level with Clinical Status in Inflammatory Bowel Disease: A 5-Year Longitudinal Study. Am. J. Gastroenterol. 2016, 111, 712–719. [Google Scholar] [CrossRef]
- Schroth, R.J.; Rabbani, R.; Loewen, G.; Moffatt, M.E. Vitamin D and Dental Caries in Children. J. Dent. Res. 2016, 95, 173–179. [Google Scholar] [CrossRef]
- Andrade, P.; Lopes, S.; Albuquerque, A.; Osório, F.; Pardal, J.; Macedo, G. Oral Lichen Planus in IBD Patients: A Paradoxical Adverse Effect of Anti-TNF-α Therapy. Dig. Dis. Sci. 2015, 60, 2746–2749. [Google Scholar] [CrossRef]
- Sánchez, A.R.; Rogers, R.S., III; Sheridan, P.J. Oral ulcerations are associated with the loss of response to infliximab in Crohn’s disease. J. Oral. Pathol. Med. 2005, 34, 53–55. [Google Scholar] [CrossRef]
- Docktor, M.J.; Paster, B.J.; Abramowicz, S.; Ingram, J.; Wang, Y.E.; Correll, M.; Jiang, H.; Cotton, S.L.; Kokaras, A.S.; Bousvaros, A. Alterations in diversity of the oral microbiome in pediatric inflammatory bowel disease. Inflamm. Bowel. Dis. 2012, 18, 935–942. [Google Scholar] [CrossRef]
- Russell, R.K.; Ip, B.; Aldhous, M.C.; MacDougall, M.; Drummond, H.E.; Arnott, I.D.R.; Gillet, P.M.; McGrogan, P.; Weaver, L.T.; Bisset, W.M.; et al. Anti-Saccharomyces cerevisiae antibodies status is associated with oral involvement and disease severity in Crohn disease. J. Pediatr. Gastroenterol. Nutr. 2009, 48, 161–167. [Google Scholar] [CrossRef]

| Parameter | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Patients | Patients from 0 to 99 years with IBD | Patients with another bowel disease not universally recognized as IBD |
| Intervention | Not applicable | |
| Comparator | Not applicable | |
| Outcomes | Diagnostic accuracy Connections between oral pathology and IBD Relate IBD to caries and periodontitis | |
| Study design | Prospective, retrospective or concurrent cohort studies Cross sectional studies |
|
| Study | Selection | Comparability | Outcome | Total Score | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of Exposed Cohort | Selection of Non-Exposed Cohort | Ascertainment of Exposure | Demonstration that Outcome of Interest was not Present at Start of Study | Comparability of Cohort on the Basis of the Design or Analysis | Assessment of Outcome | Was Follow-Up Long Enough for Outcomes to Occur | Adequacy of Follow-Up of Cohorts | ||
| Greuter et al. [2] | * | / | * | / | * (age) | * | * | * | 6/9 |
| Harty et al. [12] | * | / | * | / | ** (age, sex) | * | / | / | 5/9 |
| Jose et al. [5] | * | / | * | / | ** (age at diagnosis, type of EIM) | * | * | / | 6/9 |
| Khouri et al. [4] | / | / | * | / | / | * | * | / | 3/9 |
| Zippi et al. [3] | * | / | * | / | ** (sex, age at diagnosis, clinical history, smoking habit, EIM) | * | * | * | 7/9 |
| Study | Selection | Comparability | Exposure | Total Score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case Definition Adequate | Representativeness of the Case | Selection of Controls | Definition of Controls | Main Factor | Additional Factor | Ascertainment of Exposure | Same Method of Ascertainment for Cases and Reports | Non Response Date | ||
| Brito et al. [6] | * | * | / | * | *(age) | (other factors) | * | * | / | 7/9 |
| Elahi et al. [13] | * | * | / | * | *(age) | *(sex) | * | * | / | 7/9 |
| Grössner-Schreiber et al. [7] | * | * | / | * | *(age) | *(other factors) | * | * | / | 7/9 |
| Habashneh et al. [1] | * | * | / | * | *(age) | *(other factors) | * | * | / | 7/9 |
| Katz et al. [14] | * | / | / | * | *(age) | *(sex) | * | * | / | 6/9 |
| Koutsochristou et al. [9] | * | / | / | * | *(age) | *(other factors) | * | * | / | 6/9 |
| Laranjeira et al. [15] | * | * | / | * | *(age) | *(other factors) | * | * | / | 7/9 |
| Mohan Kumar et al. [16] | * | / | / | * | *(age) | *(sex) | * | * | / | 6/9 |
| Rikardsson et al. [17] | * | * | / | * | *(age) | *(sex) | / | * | * | 7/9 |
| Szymanska et al. [8] | * | * | / | * | *(age) | *(other factors) | * | * | * | 8/9 |
| Zervou et al. [18] | * | / | / | * | *(age) | *(sex) | * | * | / | 6/9 |
| Study | Selection | Comparability | Outcome | Total Score | ||||
|---|---|---|---|---|---|---|---|---|
| Representativeness of the Sample | Sample Size | Ascertainment of Exposure | Non Respondents | Comparability of Subjects on the Basis of the Design or Analysis | Assessment of Outcome | Statistical Test | ||
| Oviedo et al. [19] | * | * | * | / | ** (age, sex) | * | / | 6/9 |
| Study | Design; Setting | Patients n (M/F); Mean Age | IBD Patients n (M/F); Mean Age | Oral Sign and Symptoms in IBD Patients | DMFTe dmft Index | Periodontal Manifestations | Pharmacological Treatments | Smoke Habits | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Brito et al. [6] | CC; Brazil | 253 (88/165); 40.8 ± 22.5 yr Cr = 74 (24/50); 40.3 ± 12.9 yr | CD=99 (31/68); 39 ± 12.9 yr UC = 80 (33/47); 43.3 ± 13.2 yr | Candidiasis = 20 (8 CD, 8 UC, 4 Cr) NS ulcerous aphtous = 3 (2 CD, 1 UC) NS lichen planus = 5 (1 CD, 3 UC, 1 Cr) NS | CD = 15.1 ± 7.2 (p = 0.018) UC = 16.4 ± 6.6 (p < 0.0001) Cr = 12.5 ± 6.8 | CD :PPD = 2.3 ± 1.3 mm (p < 0.0001) CAL = 0.9 ± 0.9 mm NS % of sites with BOP = 19.6 ± 20.5 (p = 0.038) Periodontitis = 81 (81.8%) Cr: PPD = 1.6 ± 0.4 CAL = 1.2 ± 1.0; % of sites with BOP = 24.4 ± 29.7 Cr periodontitis = 50 (67.6%). UC :PPD = 2.3 ± 0.4 (p < 0.0001) CAL = 1.3 ± 1.4 (p = 0.004) % of sites with BOP = 21.5 ± 21.9 NS periodontitis = 72 (90%) (p<0.001) | Aminosalicylates, immunomodulators, corticosteroids, antibiotics, anti TNF alpha | CD: smokers = 12 (12.1%) non-smokers = 63 (63.3%) former smokers = 24 (24.3%). UC: smokers = 7 (8.7%) non-smokers = 38 (47.5%) former smokers = 35 (43.8%) Cr: smokers = 9 (12.2%) non-smokers=57 (77%) former smokers = 8 (10.8%) |
| 2 | Elahi et al. [13] | CC; Iran | 100 (54/46); 39 ± 25,6 yr. Cr = 50 (26/24); 40 ± 20 yr | UC = 50 (28/22); 38 ± 16 yr | Oral ullerations = 20 (p = 0.028); tongue coating = 14 (p = 0.012); dry mouth = 30 (p = 0.023); halitosis = 34 (p = 0.001); acidic taste = 20 (p = 0.008), taste changes = 20 (p 0.001) | NN | NN | NO | NN |
| 3 | Greuter et al. [2] | R; Switzerland | 329 (181/148); 12 yr 55 had at least 1 EIMs (39 CD, 12 UC, 4 NS) | CD = 173 (104/69); 12 yr. UC/ND = 156 (77/79); 11 yr | aphtous stomatitis = 24 (5 CD, 18 UC,1 NS) (7.3% of the entire study population but 43.6% of 55 patients with EIMs | NN | NN | 5-ASA, antibiotics, steroids, immunomodulators, anti-TNF | NN |
| 4 | Grössner-Schreiber Et al. [7] | CC; Germany | 121 (48/73); 38.3 ± 14.3 yr. Cr = 59 (24/35); 38,2 ± 10 yr | CD = 46 UC = 16 | Mucobuccal hyperplasia or oedema = 15, swelling of the gingiva = 17, ulcera = 5, Aphthae = 6, Candidiasis = 5, lichen planus = 3, leucoplachia=2, labial rhagades = 3 | DMF-S (p 0.212 NS IBD = 54.1 ± 31.6. Cr = 46.5 ± 26.5 Dentine caries: (p = 0.033) IBD = 25 (40%); Cr = 13 (22%) | BOP (p = 0.958) NS IBD = 23.4 ± 20.1; Cr = 20.8 ± 13.5. PPD (p = 0.014) IBD = 2.22 ± 0.57; Cr = 2.29 ± 0.33. (p = 0.014) CAL > 4 mm: (p = 0.07) IBD = 50 (81%). Cr= 38. (64%) CAL > 5mm: (p = 0.07) IBD = 39 (63%) Cr = 27 (46%) | Corticosteroids, immunosuppressants, aminosalicylate, anti TNF, antibiotics | IBD nonsmokers = 34 (55%) IBD smokers = 25 IBD former smokers= 3 (5%); Cr nonsmokers = 29 (49%) Cr smokers = 24. Cr former smokers = 6 (10%) |
| 5 | Habashneh et al. [1] | CC; Jordania | 260 (156/104); 39.4 ± 0.7 yr Cr = 100 (62/38) | CD = 59 (33/26) UC = 101 (61/40) | NN | NN | CD: PPD = 1.29 ± 0.47; CAL = 1.95 ± 0.98. % of sites with BOP = 10.84 ± 16.20 gingival recession = 0.53 ± 0.55 UC: PPD = 1.51 ± 0.47; CAL = 2.36 ± 1.13 % of sites with BOP = 10.20 ± 14.25 gingival recession = 0.86 ± 0.72 Cr; PPD = 1.25 ± 0.37, CAL = 1.70 ± 0.89 % of sites with BOP = 4.70 ± 8.30 gingival recession = 0.44 ± 0.60 | NN | CD Nonsmoker = 23; Smoker = 31; ex-smoker = 5 UC nonsmoker = 55; smoker = 17; ex-smoker = 29 Cr nonsmoker = 44; smoker = 49; ex-smoker = 7 |
| 6 | Harty et al. [12] | P; Ireland | 80 | CD = 49 (25/24); 11,95 yr UC = 22 ND = 9 oral CD = 20 (12/8) 11.4 yr non oral CD = 28 | patients with oral CD compared to nonoral CD: oral symptoms = 14 (70%) (p = 0.01) mouth ulcers = 11 (55%) NS angular stomatitis = 5 (25%) NS cheek swelling=5 (25%) NS vomiting=2 (10%) NS | NN | nonspecific gingivitis = 8 (16.7%) | NN | NN |
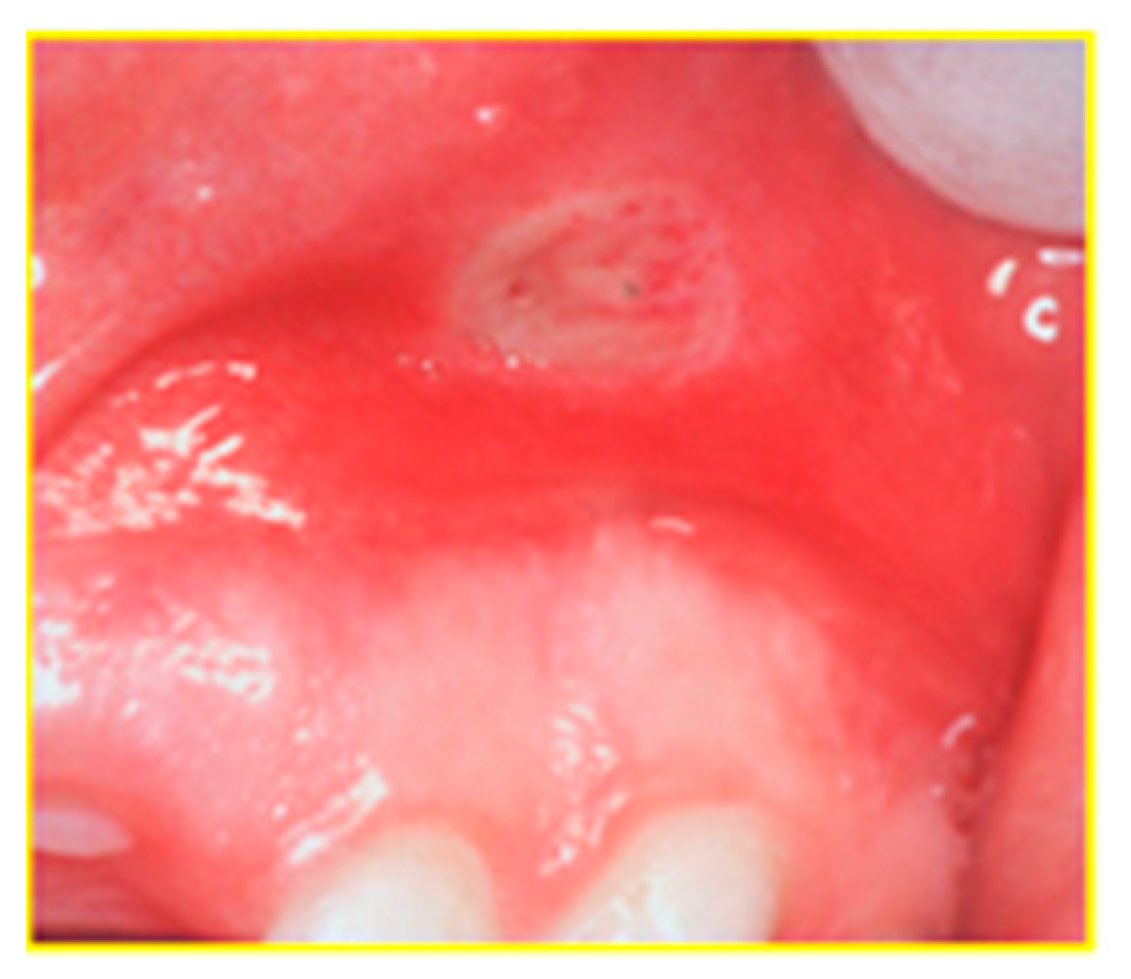
| 7 | Jose et al. [5] | R/P; USA | 1649 (893/756); 11.1 ± 4.15 | CD = 1007. UC = 471. ND = 171 | 53 patients with aphthous stomatitis (13.7% of all EIM) before diagnoses | NN | NN | NN | NN |
| 8 | Katz et al. [14] | CC; Israel | 96 (49/47); 38.5 ± 26.9 Cr:42 (22/20); 40 ± 20 yr | CD = 34 (20/14); 33 ± 16 yr UC = 20 (7/13); 44 ± 18 yr | Halitosis = 50% UC (p = 0.0008); 29% CD, (p = 0.026) dry mouth = 30% UC, (p = 0.04); 29% CD (p = 0.026) geographic tongue = 15% CD (p = 0.01), dysphagia = 15% UC, (p = 0.05); 9% CD NS nausea = 30% UC (p = 0.017), 50% CD (p = 0.001) vomiting = 15% UC, NS; 41% CD (p = 0.01) regurgitation = 45% UC,(p = 0.017); 21% CD, NS | NN | NN | NN | NN |
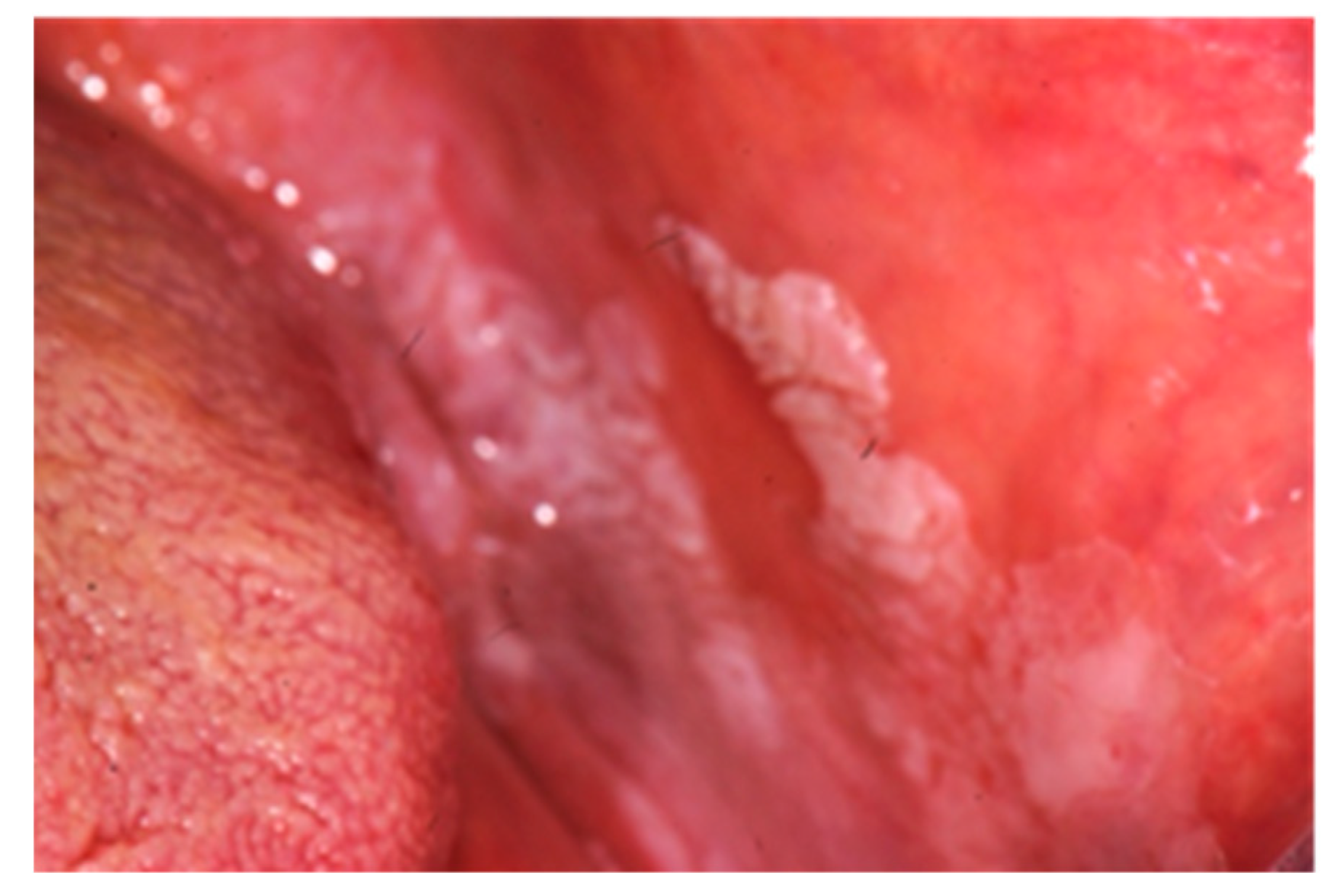
| 9 | Khouri et al. [4] | R; Australia | 6 (5/1); 6.33 yr | CD = 4 M; 6.25 yr | Lip swelling = 6, granulomatous cheilitis = 6, cobblestoning mucosa = 2 | NN | NN | NN | NN |
| 10 | Koutsochristou et al. [9] | CC; Greece | 110 (50/60); 12.26 ± 5.22 yr Cr = 55 (25/30); 12.21 ± 3,96 yr | CD = 36 (18/18); UC = 19 (7/12); 13 of 55 patients had oral lesions (23%) | Aphthae = 8, aphthae with swelling of gums or ulcers or candidiasis = 5 | DMFT IBD = 5.81 (p < 0.001) Cr = 2.04 dmft IBD = 2.95 (p < 0.001) Cr = 0.91 | CPITN index: IBD: score 0 = 0 (0%) score 1 = 20 (36%) score 2 = 30 (54%) score 3 = 5 (9%) Cr: score 0 = 22 (40%) score 1 = 25 (45%) score 2 = 8 (14%) score 3 = 0 (0%) | aminosalicylates, corticosteroids, anti-TNF, or immuno-modulators | NN |
| 11 | Laranjeira et al. [15] | CC; Portugal | 171 (85/86); 45.5 ± 16.9 yr. Cr = 58 (28/30); 47.4 ± 16.3 yr | CD = 65 (32/33); 41.1 ± 15.2 yr. UC = 48 (25/23); 49.2 ± 18.4 yr | Aphtous ulcers = 1 0 (8.80%), (p = 0.159 so NS); gingival swelling = 1 (0.90%), angular cheilitis = 1 (0.90%), halitosis (p = 0.038). | NN | NN | Corticosteroids,salicylate, immunosuppressants, | Non smokers Cr = 52 CD =52; UC = 42 Smokers Cr = 6 CD = 13; UC = 6 |
| 12 | Mohan Kumar et al. [16] | CC; India | 30 (16/14) | UC = 15 (8/7) | Aphtous ulcerations = 10 lichen planus = 3 dry mouth = 11 PSV = 1, coated tongue = 4 NS dysgeusia = 5, halitosis = 12 | NN | Periodontal status NS: Periodontal index UC = 1. Cr = 1.4 gingival index UC = 1.2 Cr = 1.3 loss of attachment (mm) = UC 0.4; Cr = 0.2 | Sulfapyridine; sulfasalazine | NN |
| 13 | Oviedo et al. [19] | T; Chile | 30 (9/21); 40 yr | CD = 7 (2/5) UC = 23 (7/16) | 11 patients (37%): Oral ulcerations = 1 Apthae = 4, angular ulcer = 1, macrocheilia of the lip, corrugated mucosa | NN | NN | NN | NN |
| 14 | Rikardsson et al. [17] | CC; Sweden | 2346 (32.5%/67.5%); 49.6 ± 20, 60 Cr = 748 (33%/67%); 49.5 yr ± 13.8 yr | CD = 1598 (32%/68%); 49.7 ± 15.3 yr | Oral ulcers = 32% (p < 0.001), halitosis = 23% (p < 0-001) mouth dryness = 38% (p < 0.001) toothaches = 21% (p < 0.001) mucosal lesions = 31% (p < 0.001) | Carious lesions = 41% (p < 0.001) | bleeding from gingiva = 41% (p < 0.001) periodontitis = 7% (p < 0.028) | NN | Current smokers CD = 23% Cr = 19% (p < 0.018) former smokers CD = 19% Cr = 15% (p < 0.070) |
| 15 | Szymanska et al. [8] | CC; Sweeden | 225 (102/123); 47.1 ± 24.08 yr Cr = 75 (29/46); 48.6 ± 13.4 yr | CD with RS = 71 (33/38); 50.7 ± 13.9 yr CD without RS = 79 (40/39); 42 ± 14.4 yr | Dry mouth (p = 0.001): 11% in Cr,29% in CD with RS and 38% in CD with NRS Bad breath (p = 0.008):12% in Cr, 21% in CD with RS and 33% in CD with NRS Ulcers (NS): 23% in Cr, 23% in RS and 27% in CD with NRS | Cr = 13.1 CD with RS = 15,5. CD with NRS = 11.2 | NN | NN | Cr = 5 CD with RS = 17 CD with NRS = 15 Former smokers Cr = 33 CD with RS = 25 CD with NRS = 30 |
| 16 | Zervou et al. [18] | CC; Greece | 74; 41,5 ± 20 yr. Cr = 47;43 ± 12 yr | CD = 15 UC = 15 | Ulcers = 3 (2 CD,13% (p = 0.011); 1 UC,7%, (p = 0.07)) cobblestoning = 3 CD,20%, (p = 0.002) polypoids tags = 3 CD,20%, (p = 0.002) lip swelling = 4 (3 CD,20%, (p = 0.002); 1 UC, 7%, (p = 0.07)) buccal swelling = 1 CD,7%, (p = 0.07) aphthous ulcers = 1 UC,7%, (p = 0.07) angular cheilitis = 9 (5 CD, 33% (p = 0.000); 4 UC,27%, (p < 0.0001)) Hairy tongue = 3 (2 CD, 13%, (p = 0.011); 1 UC (p = 0.07)) buccal trauma = 9 (6 CD, 40%, (p = 0.000); 3 UC, 20% (p < 0.00019)) | NN | Periodontitis=2 CD 13%, (p = 0.011) Gingivitis = 4 (3 CD, 20%, (p = 0.002); 1 UC, 7%, (p = 0.07)) Gingival bleeding = 4 CD 27% (p < 0.0001) | Mesalazine, aziathioprine | NN |
| 17 | Zippi et al. [3] | R; Italy | 811 (437/374); 32.5 ± 18,9 EIMs = 329 (155/174) (210 UC;119 CD) | CD = 216 (131/85); 31,9 ± 13,1 yr. UC = 595 (306/289); 33,1 ± 13,7 yr | 6 cases of aphtous stomatitis, 3 CD e 3 UC (1.4% CD e 0.5% UC) | NN | NN | NN | CD Smoker = 98. Non-smoker = 101 Ex smokers = 17. UC Smokers = 140. Ex smokers = 131. Non smokers = 324 |
| Specific manifestations | Oral manifestations | Crohn’s disease | Ulcerative colitis |
| Cobblestoning the mucosa | X | ||
| Granulomatous cheilitis | X | ||
| Mucosal tags | X | ||
| Pyostomatitis vegetans | X | ||
| Unspecific manifestations | Deep oral fissuring | X | |
| Cheilitis angularis | X | X | |
| Dental caries | X | X | |
| Mucogingivitis | X | X | |
| Periodontitis | X | X | |
| Lichen planus | X | X | |
| Dysphagia | X | X | |
| Dry mouth | X | X | |
| Halitosis | X | X | |
| Taste changes | X | X | |
| Aphthous ulcerations | X | X |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauritano, D.; Boccalari, E.; Di Stasio, D.; Della Vella, F.; Carinci, F.; Lucchese, A.; Petruzzi, M. Prevalence of Oral Lesions and Correlation with Intestinal Symptoms of Inflammatory Bowel Disease: A Systematic Review. Diagnostics 2019, 9, 77. https://doi.org/10.3390/diagnostics9030077
Lauritano D, Boccalari E, Di Stasio D, Della Vella F, Carinci F, Lucchese A, Petruzzi M. Prevalence of Oral Lesions and Correlation with Intestinal Symptoms of Inflammatory Bowel Disease: A Systematic Review. Diagnostics. 2019; 9(3):77. https://doi.org/10.3390/diagnostics9030077
Chicago/Turabian StyleLauritano, Dorina, Elisa Boccalari, Dario Di Stasio, Fedora Della Vella, Francesco Carinci, Alberta Lucchese, and Massimo Petruzzi. 2019. "Prevalence of Oral Lesions and Correlation with Intestinal Symptoms of Inflammatory Bowel Disease: A Systematic Review" Diagnostics 9, no. 3: 77. https://doi.org/10.3390/diagnostics9030077
APA StyleLauritano, D., Boccalari, E., Di Stasio, D., Della Vella, F., Carinci, F., Lucchese, A., & Petruzzi, M. (2019). Prevalence of Oral Lesions and Correlation with Intestinal Symptoms of Inflammatory Bowel Disease: A Systematic Review. Diagnostics, 9(3), 77. https://doi.org/10.3390/diagnostics9030077







