Perspective of Molecular Diagnosis in Healthcare: From Barcode to Pattern Recognition
Abstract
1. Introduction
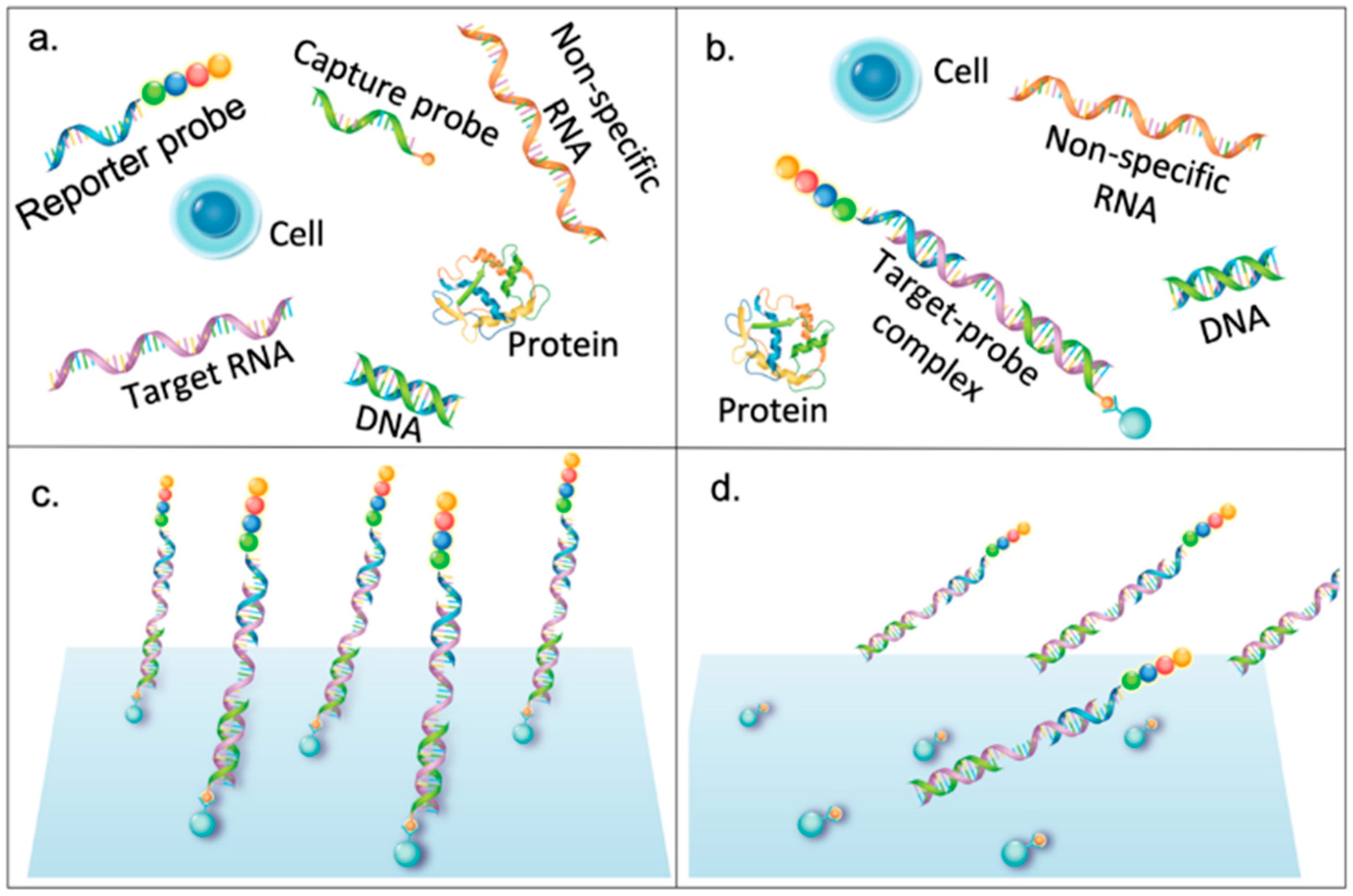
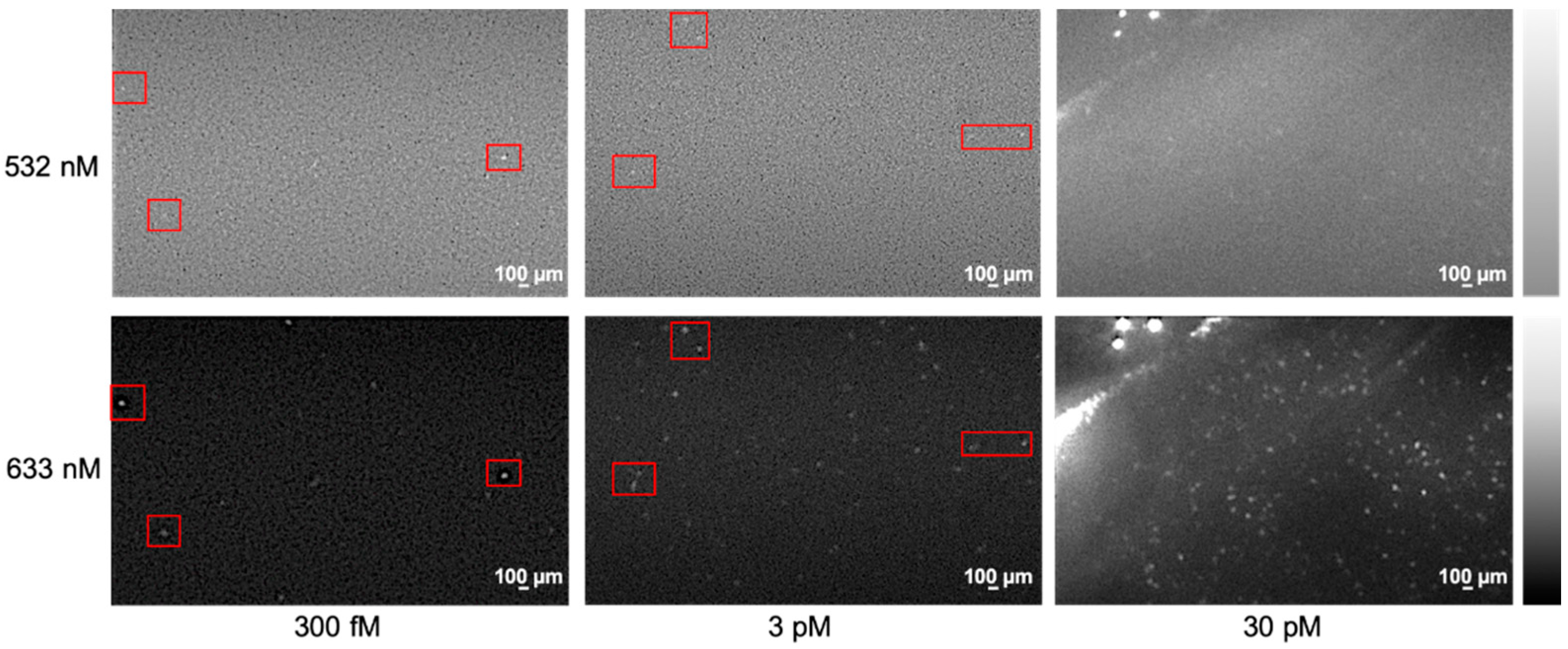
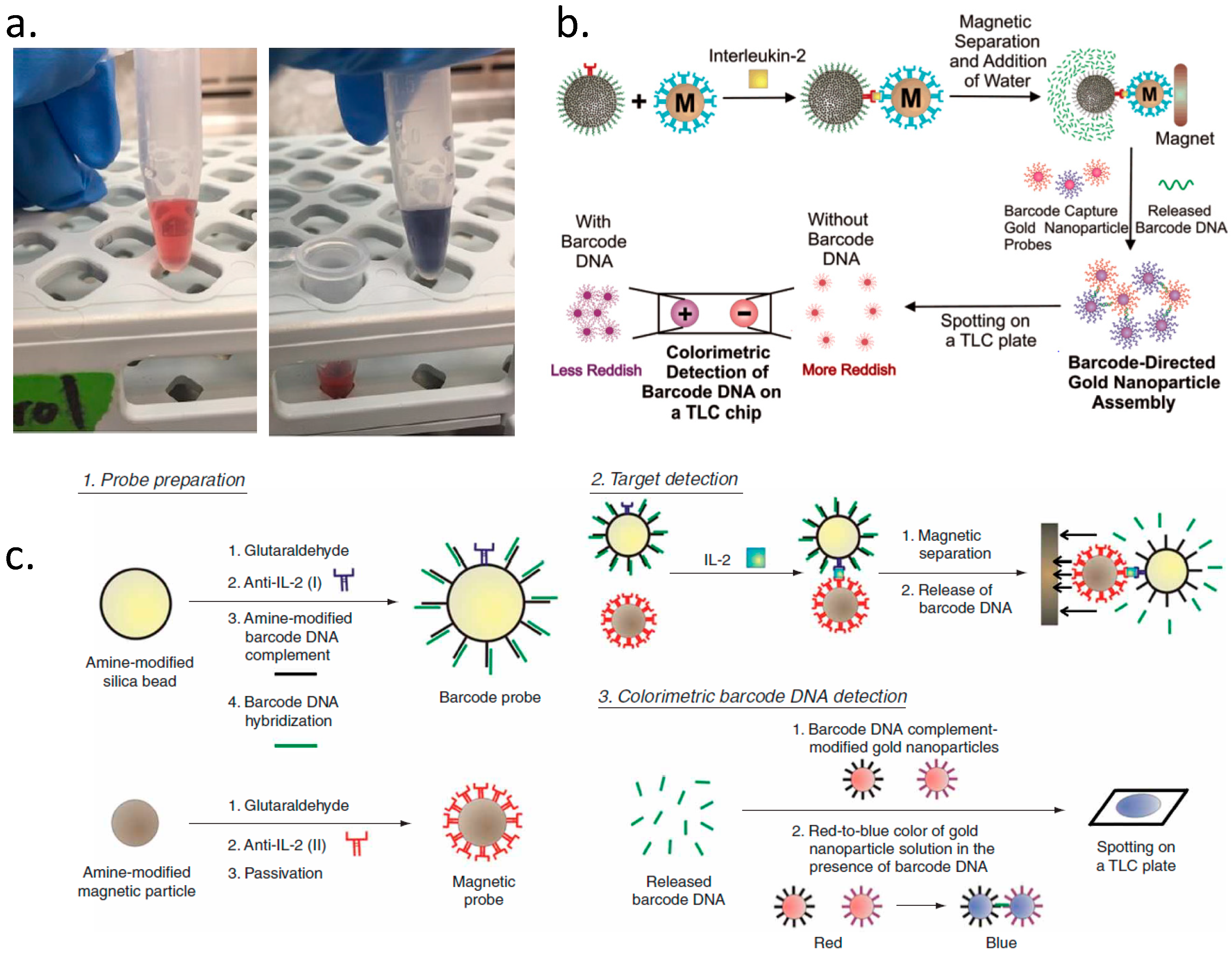
2. Classification of Barcodes for Bioassay
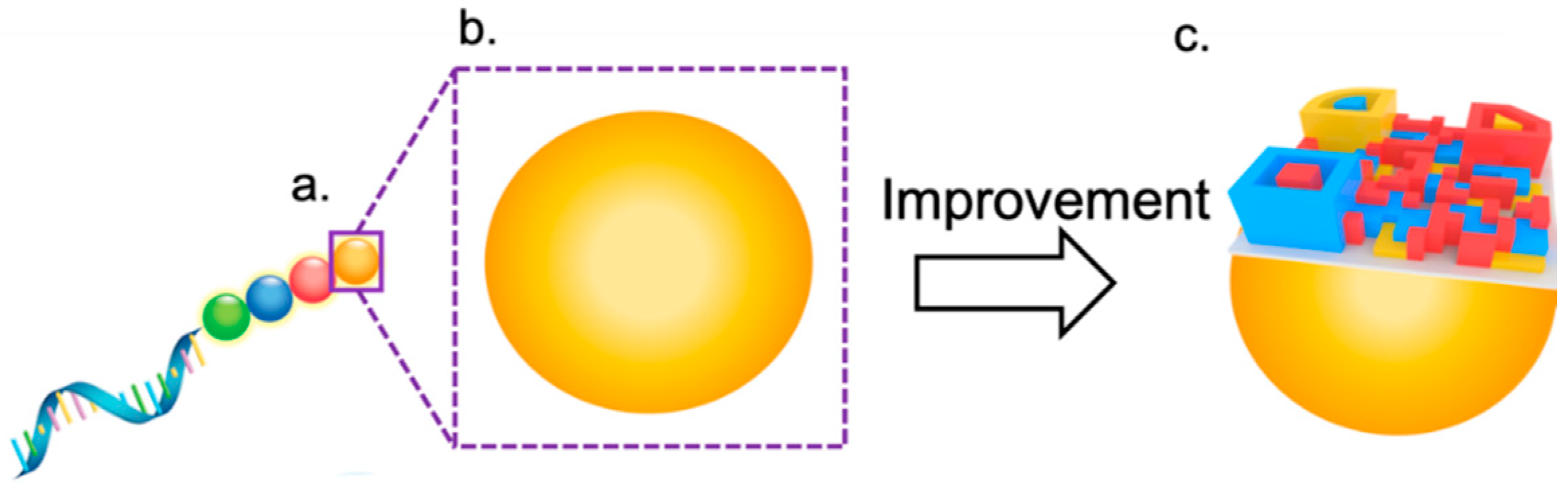
3. Perspective on Barcodes
Funding
Acknowledgments
Conflicts of Interest
References
- Hall, H.; Syed, A.; Zhang, J.X.J. Two-dimensional, error-corrected barcode readout for point-of-care colorimetrie assays. In Proceedings of the 2016 IEEE Healthcare Innovation Point-of-Care Technologies Conference, HI-POCT 2016, Cancun, Mexico, 9–11 November 2016. [Google Scholar]
- Strudwick, G.; Reisdorfer, E.; Warnock, C.; Kalia, K.; Sulkers, H.; Clark, C.; Booth, R. Factors Associated with Barcode Medication Administration Technology That Contribute to Patient Safety: An Integrative Review. J. Nurs. Care Qual. 2016, 33, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Lawson, L.S.; Rodriguez, J.D. Raman Barcode for Counterfeit Drug Product Detection. Anal. Chem. 2016, 88, 4706–4713. [Google Scholar] [CrossRef] [PubMed]
- Basker, E. Raising the barcode scanner: Technology and productivity in the retail sector. Am. Econ. J. Appl. Econ. 2012, 4, 1–27. [Google Scholar] [CrossRef]
- Hachesu, P.R.; Zyaei, L.; Hassankhani, H. Recommendations for using barcode in hospital process. Acta Inform. Medica. 2016, 24, 206. [Google Scholar] [CrossRef] [PubMed]
- Culver, H.R.; Wechsler, M.E.; Peppas, N.A. Label-Free Detection of Tear Biomarkers Using Hydrogel-Coated Gold Nanoshells in a Localized Surface Plasmon Resonance-Based Biosensor. ACS Nano 2018, 12, 9342–9354. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Shi, K.; Dou, B.; Xiang, Y.; Chai, Y.; Yuan, R. In situ DNA-templated synthesis of silver nanoclusters for ultrasensitive and label-free electrochemical detection of MicroRNA. ACS Appl. Mater. Interfaces 2015, 7, 1188–1193. [Google Scholar] [CrossRef] [PubMed]
- Avella-Oliver, M.; Carrascosa, J.; Puchades, R.; Maquieira, Á. Diffractive Protein Gratings as Optically Active Transducers for High-Throughput Label-free Immunosensing. Anal. Chem. 2017, 89, 9002–9008. [Google Scholar] [CrossRef]
- Henihan, G.; Schulze, H.; Corrigan, D.K.; Giraud, G.; Terry, J.G.; Hardie, A.; Campbell, C.J.; Walton, A.J.; Crain, J.; Pethig, R.; et al. Label-and amplification-free electrochemical detection of bacterial ribosomal RNA. Biosens. Bioelectron. 2016, 81, 487–494. [Google Scholar] [CrossRef]
- Wang, J.; Guo, J.; Zhang, J.; Zhang, W.; Zhang, Y. RNA aptamer-based electrochemical aptasensor for C-reactive protein detection using functionalized silica microspheres as immunoprobes. Biosens. Bioelectron. 2017, 95, 100–105. [Google Scholar] [CrossRef]
- Cai, S.; Chen, M.; Liu, M.; He, W.; Liu, Z.; Wu, D.; Xia, Y.; Yang, H.; Chen, J. A signal amplification electrochemical aptasensor for the detection of breast cancer cell via free-running DNA walker. Biosens. Bioelectron. 2016, 85, 184–189. [Google Scholar] [CrossRef]
- Yang, M.; Liu, Y.; Jiang, X. Barcoded point-of-care bioassays. Chem. Soc. Rev. 2019, 48, 850–884. [Google Scholar] [CrossRef] [PubMed]
- Poon, E.G.; Keohane, C.A.; Yoon, C.S.; Ditmore, M.; Bane, A.; Levtzion-Korach, O.; Moniz, T.; Rothschild, J.M.; Kachalia, A.B.; Hayes, J.; et al. Effect of bar-code technology on the safety of medication administration. Obstet. Gynecol. Surv. 2010, 18, 1698–1707. [Google Scholar]
- Han, S.; Lee, J.S.; Lee, J.B. Synthesis of a multi-functional DNA nanosphere barcode system for direct cell detection. Nanoscale 2017, 9, 14094–14102. [Google Scholar] [CrossRef] [PubMed]
- Pei, W.; Feyerabend, T.B.; Rössler, J.; Wang, X.; Postrach, D.; Busch, K.; Rode, I.; Klapproth, K.; Dietlein, N.; Quedenau, C.; et al. Polylox barcoding reveals haematopoietic stem cell fates realized in vivo. Nature 2017, 548, 456. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cu, Y.T.H.; Luo, D. Multiplexed detection of pathogen DNA with DNA-based fluorescence nanobarcodes. Nat. Biotechnol. 2005, 23, 885. [Google Scholar] [CrossRef] [PubMed]
- Geiss, G.K.; Bumgarner, R.E.; Birditt, B.; Dahl, T.; Dowidar, N.; Dunaway, D.L.; Fell, H.P.; Ferree, S.; George, R.D.; Grogan, T.; et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat. Biotechnol. 2008, 26, 37. [Google Scholar] [CrossRef] [PubMed]
- Danaher, P.; White, R.L.; Hanson, E.K.; Ballantyne, J. Facile semi-automated forensic body fluid identification by multiplex solution hybridization of NanoString® barcode probes to specific mRNA targets. Forensic Sci. Int. Genet. 2015, 14, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Parlak, M.; Kuscu, C.; Bandaria, J.; Mir, M.; Szlachta, K.; Singh, R.; Darzacq, X.; Yildiz, A.; Adli, M. Live cell imaging of low- and non-repetitive chromosome loci using CRISPR-Cas9. Nat. Commun. 2017, 8, 14725. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Park, M.; Griffiths, A.; Carrion, R.; Patterson, J.; Schmidt, H.; Mathies, R. Microfluidic System for Detection of Viral RNA in Blood Using a Barcode Fluorescence Reporter and a Photocleavable Capture Probe. Anal. Chem. 2017, 89, 12433–12440. [Google Scholar] [CrossRef] [PubMed]
- Bian, F.; Sun, L.; Cai, L.; Wang, Y.; Zhao, Y.; Wang, S.; Zhou, M. Molybdenum disulfide-integrated photonic barcodes for tumor markers screening. Biosens. Bioelectron. 2019, 133, 199–204. [Google Scholar] [CrossRef]
- Wu, X.; Degottardi, Q.; Wu, I.C.; Wu, L.; Yu, J.; Kwok, W.W.; Chiu, D.T. Ratiometric Barcoding for Mass Cytometry. Anal. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Oracz, J.; Adolfsson, K.; Westphal, V.; Radzewicz, C.; Borgström, M.T.; Sahl, S.J.; Prinz, C.N.; Hell, S.W. Ground State Depletion Nanoscopy Resolves Semiconductor Nanowire Barcode Segments at Room Temperature. Nano Lett. 2017, 17, 2652–2659. [Google Scholar] [CrossRef] [PubMed]
- Hudson, Z.M.; Lunn, D.J.; Winnik, M.A.; Manners, I. Colour-tunable fluorescent multiblock micelles. Nat. Commun. 2014, 5, 3372. [Google Scholar] [CrossRef] [PubMed]
- Giedt, R.J.; Pathania, D.; Carlson, J.C.T.; McFarland, P.J.; del Castillo, A.F.; Juric, D.; Weissleder, R. Single-cell barcode analysis provides a rapid readout of cellular signaling pathways in clinical specimens. Nat. Commun. 2018, 9, 4550. [Google Scholar] [CrossRef] [PubMed]
- Rajwani, A.; Restall, B.; Muller, N.J.; Roebuck, S.; Willerth, S.M. An affordable microsphere-based device for visual assessment of water quality. Biosensors 2017, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.M.; Wise, A.R.; Groves, J.T. Colorimetric bio-barcode amplification assay for cytokines. Anal. Chem. 2005, 77, 6985–6988. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.M.; Jang, K.J.; Groves, J.T. Detection of proteins using a colorimetric bio-barcode assay. Nat. Protoc. 2007, 2, 1438. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.; Kim, H.; Kim, J.; Kwon, S. Colour-barcoded magnetic microparticles for multiplexed bioassays. Nat. Mater. 2010, 9, 745. [Google Scholar] [CrossRef]
- Oh, B.K.; Nam, J.M.; Lee, S.W.; Mirkin, C.A. A fluorophore-based bio-barcode amplification assay for proteins. Small 2006, 2, 103–108. [Google Scholar] [CrossRef]
- Kretzschmar, K.; Watt, F.M.; Kretzschmar, K.; Watt, F.M. Lineage tracing. Cell 2012, 148, 33–45. [Google Scholar] [CrossRef]
- Kalhor, R.; Kalhor, K.; Mejia, L.; Leeper, K.; Graveline, A.; Mali, P.; Church, G.M. Developmental barcoding of whole mouse via homing CRISPR. Science 2018, 361, eaat9804. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, Z.; Deng, Y.; Li, Y.; Wei, W.; Shi, Q. Single-cell, multiplexed protein detection of rare tumor cells based on a beads-on-barcode antibody microarray. Anal. Chem. 2016, 88, 11077–11083. [Google Scholar] [CrossRef] [PubMed]
- Schlecht, U.; Liu, Z.; Blundell, J.R.; St Onge, R.P.; Levy, S.F. A scalable double-barcode sequencing platform for characterization of dynamic protein-protein interactions. Nat. Commun. 2017, 8, 15586. [Google Scholar] [CrossRef] [PubMed]
- Peikon, I.D.; Kebschull, J.M.; Vagin, V.V.; Ravens, D.I.; Sun, Y.C.; Brouzes, E.; Corrêa, I.R.; Bressan, D.; Zador, A.M. Using high-throughput barcode sequencing to efficiently map connectomes. Nucleic Acids Res 2017, 45, 115. [Google Scholar] [CrossRef] [PubMed]
- Tantama, M.; Martínez-François, J.R.; Mongeon, R.; Yellen, G. Imaging energy status in live cells with a fluorescent biosensor of the intracellular ATP-to-ADP ratio. Nat. Commun. 2013, 4, 2550. [Google Scholar] [CrossRef] [PubMed]
- Liggett, S.B. Phosphorylation barcoding as a mechanism of directing GPCR signaling. Sci. Signal 2011, 185, pe36. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yang, F.; Zhang, D.; Liu, Z.; Lin, A.; Liu, C.; Xiao, P.; Yu, X.; Sun, J.P. Phosphorylation of G Protein-Coupled Receptors: From the Barcode Hypothesis to the Flute Model. Mol. Pharmacol. 2017, 92, 201–210. [Google Scholar] [CrossRef]
- Nobles, K.N.; Xiao, K.; Ahn, S.; Shukla, A.K.; Lam, C.M.; Rajagopal, S.; Strachan, R.T.; Huang, T.Y.; Bressler, E.A.; Hara, M.R.; et al. Distinct phosphorylation sites on the β 2-adrenergic receptor establish a barcode that encodes differential functions of β-arrestin. Sci. Signal. 2011, 4, 51. [Google Scholar] [CrossRef]
- Flock, T.; Hauser, A.S.; Lund, N.; Gloriam, D.E.; Balaji, S.; Babu, M.M. Selectivity determinants of GPCR-G-protein binding. Nature 2017, 545, 317. [Google Scholar] [CrossRef]
- Matsunaga, Y.T.; Morimoto, Y.; Takeuchi, S. Molding cell beads for rapid construction of macroscopic 3D tissue architecture. Adv. Mater. 2011, 23, 90–94. [Google Scholar] [CrossRef]
- Kim, Y.B.; Lee, H.; Kim, G.H. Strategy to Achieve Highly Porous/Biocompatible Macroscale Cell Blocks, Using a Collagen/Genipin-bioink and an Optimal 3D Printing Process. ACS Appl. Mater. Interfaces. 2016, 8, 32230–32240. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Okajima, T.; Onoe, H.; Subagyo, A.; Sueoka, K.; Kuribayashi-Shigetomi, K. Origami-based self-folding of co-cultured NIH/3T3 and HepG2 cells into 3D microstructures. Sci. Rep. 2018, 8, 4556. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Q.; Bao, M.; Hass, K.; Lin, W.; Qin, P.; Du, K. Perspective of Molecular Diagnosis in Healthcare: From Barcode to Pattern Recognition. Diagnostics 2019, 9, 75. https://doi.org/10.3390/diagnostics9030075
He Q, Bao M, Hass K, Lin W, Qin P, Du K. Perspective of Molecular Diagnosis in Healthcare: From Barcode to Pattern Recognition. Diagnostics. 2019; 9(3):75. https://doi.org/10.3390/diagnostics9030075
Chicago/Turabian StyleHe, Qian, Mengdi Bao, Kenneth Hass, Wenxia Lin, Peiwu Qin, and Ke Du. 2019. "Perspective of Molecular Diagnosis in Healthcare: From Barcode to Pattern Recognition" Diagnostics 9, no. 3: 75. https://doi.org/10.3390/diagnostics9030075
APA StyleHe, Q., Bao, M., Hass, K., Lin, W., Qin, P., & Du, K. (2019). Perspective of Molecular Diagnosis in Healthcare: From Barcode to Pattern Recognition. Diagnostics, 9(3), 75. https://doi.org/10.3390/diagnostics9030075






