Diagnostic Performance of PET or PET/CT Using 18F-FDG Labeled White Blood Cells in Infectious Diseases: A Systematic Review and a Bivariate Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
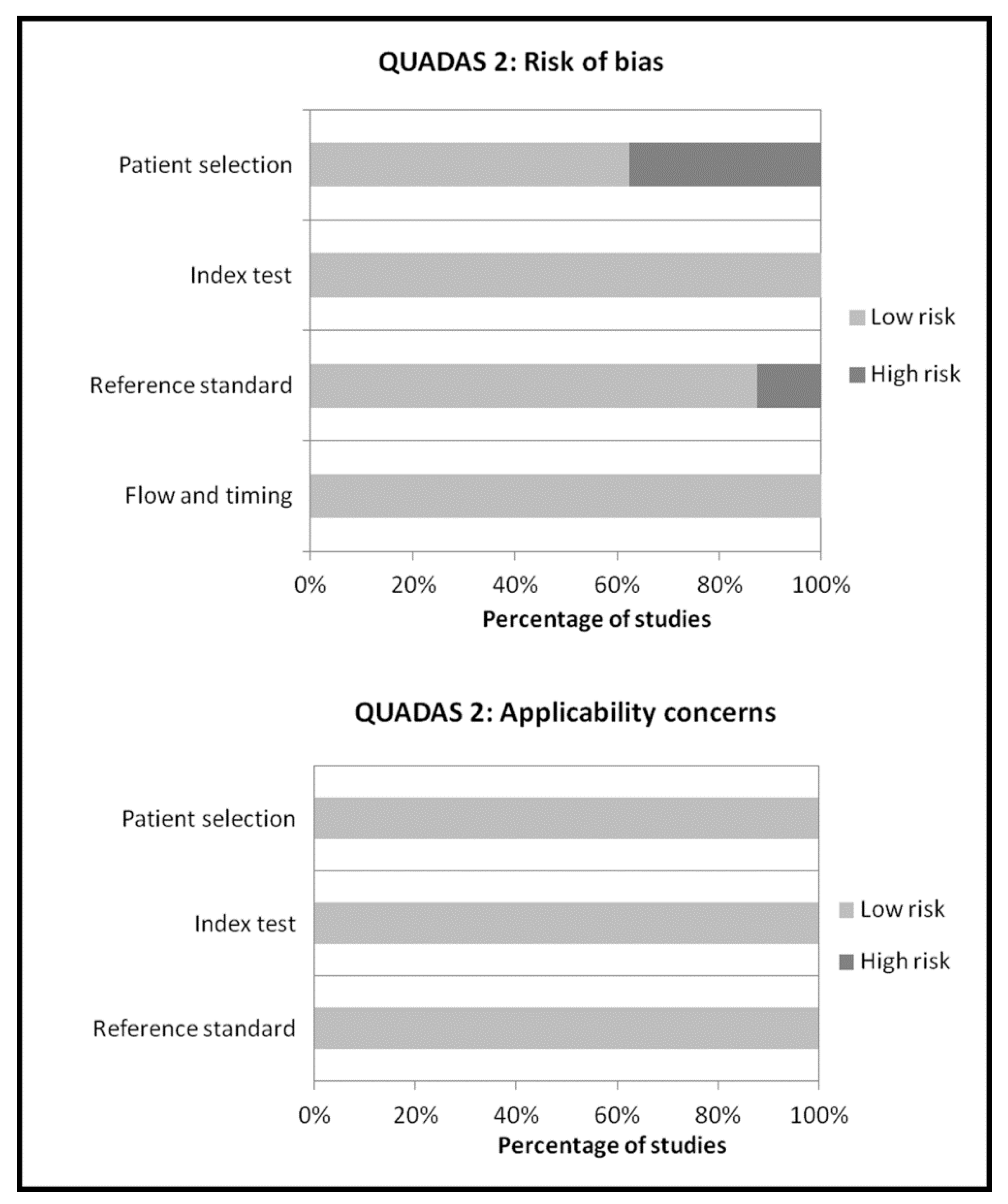
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
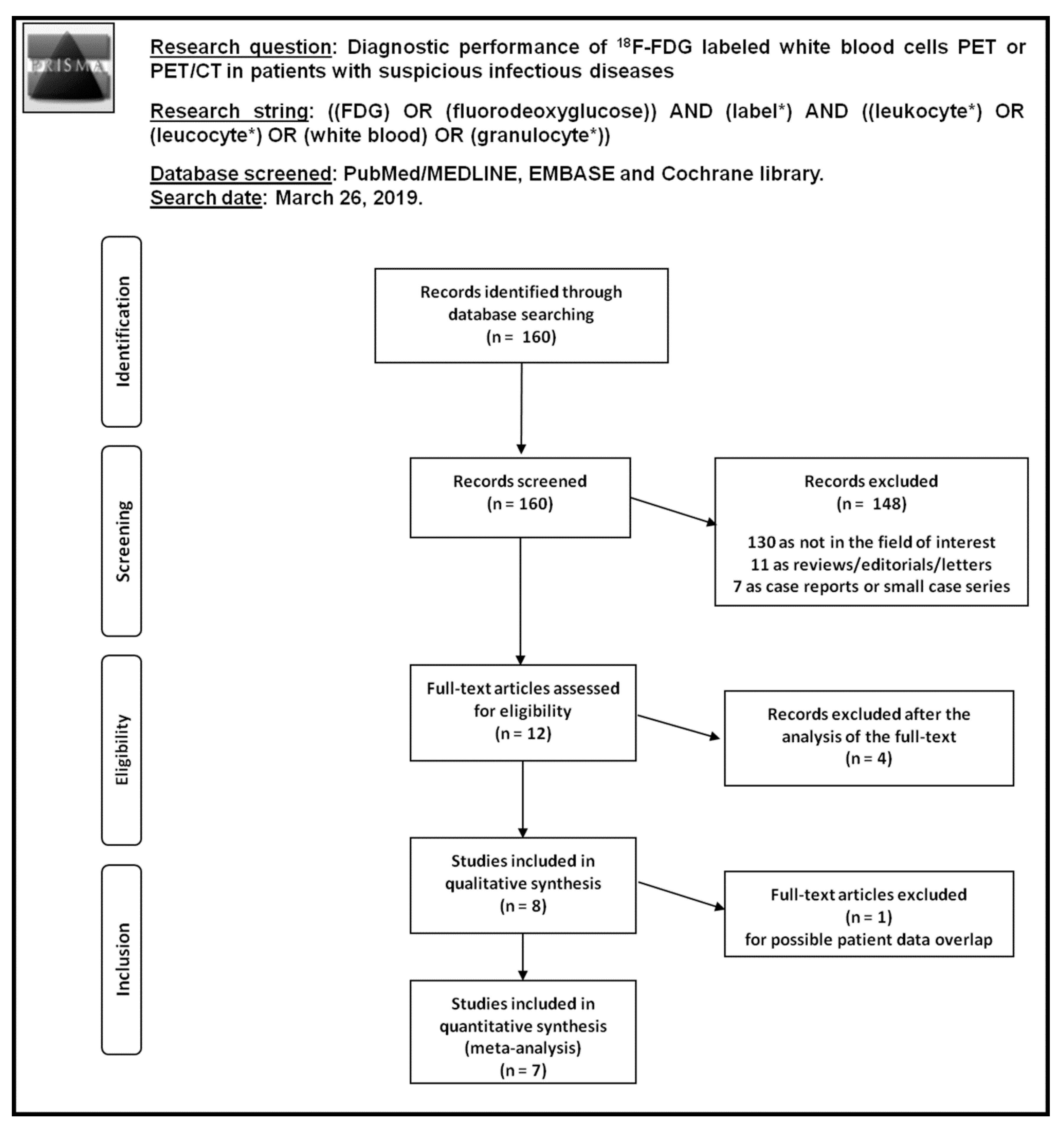
3.1. Literature Search
3.2. Qualitative Analysis (Systematic Review)
3.2.1. Basic Study and Patient Characteristics
3.2.2. Technical Aspects
3.2.3. Main Findings
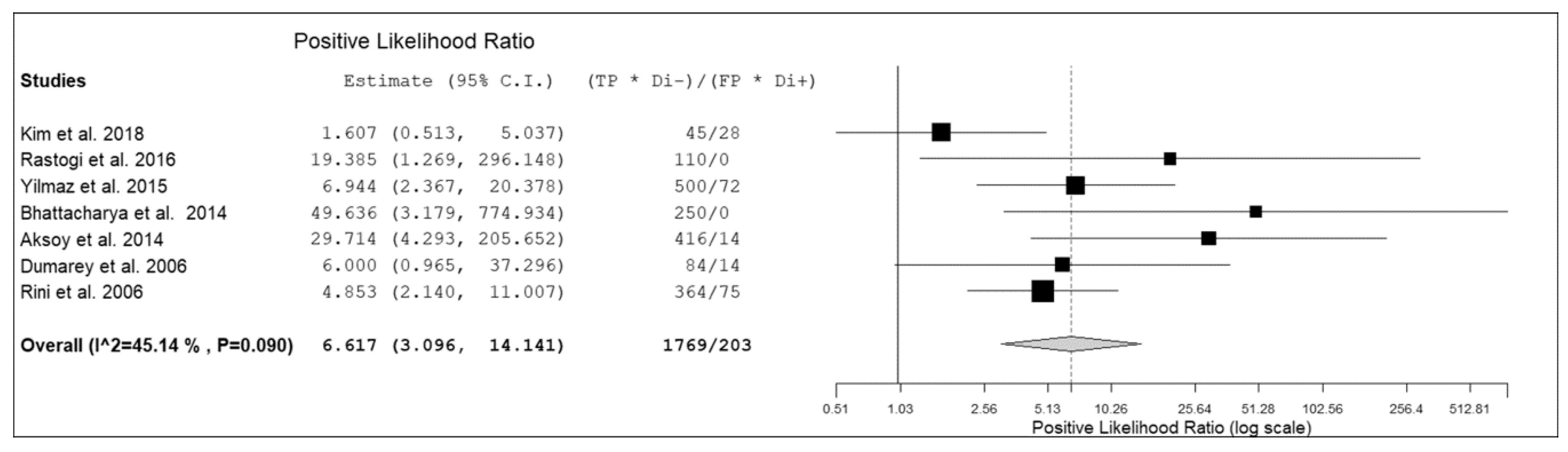
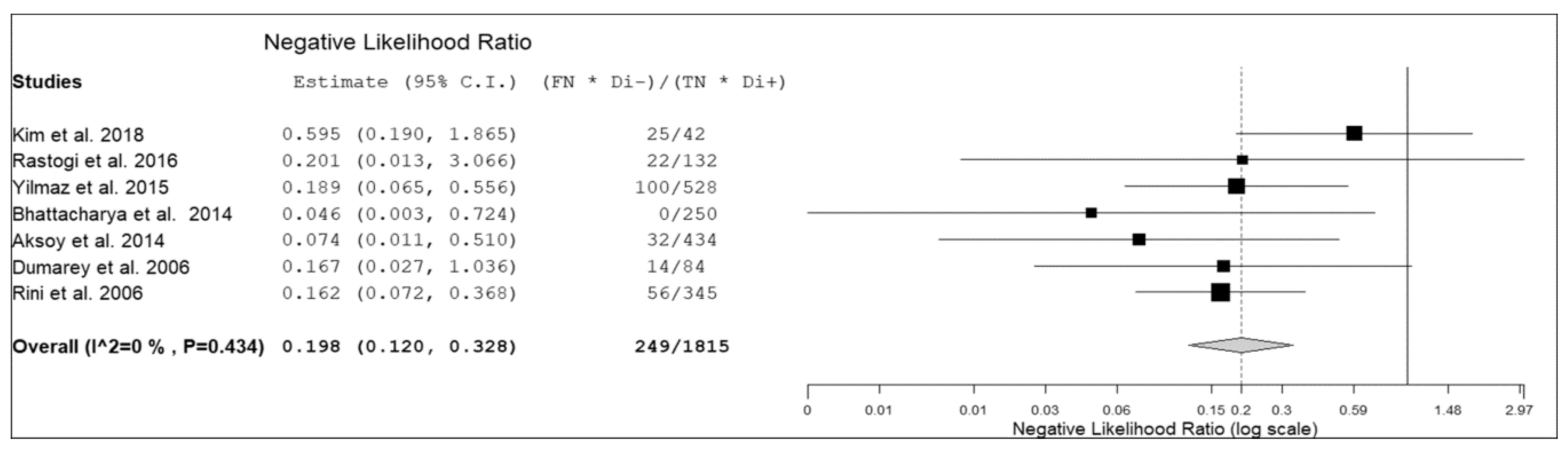
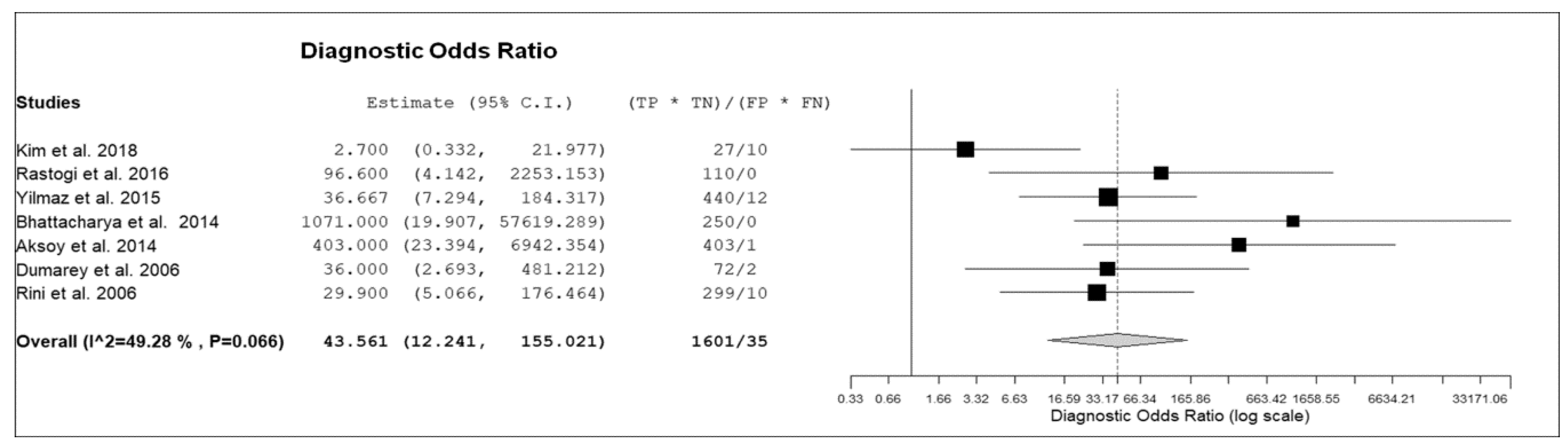
3.3. Quantitative Analysis (Meta-Analysis)
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Torres-Sangiao, E.; Holban, A.M.; Gestal, M.C. Advanced Nanobiomaterials: Vaccines, Diagnosis and Treatment of Infectious Diseases. Molecules 2016, 21, 867. [Google Scholar] [CrossRef] [PubMed]
- Salmanoglu, E.; Kim, S.; Thakur, M.L. Currently Available Radiopharmaceuticals for Imaging Infection and the Holy Grail. Semin. Nucl. Med. 2018, 48, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K. The Promise of Molecular Imaging in the Study and Treatment of Infectious Diseases. Mol. Imaging Biol. 2017, 19, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Sethi, I.; Baum, Y.S.; Grady, E.E. Current Status of Molecular Imaging of Infection: A Primer. AJR Am. J. Roentgenol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Lawal, I.; Zeevaart, J.; Ebenhan, T.; Ankrah, A.; Vorster, M.; Kruger, H.G.; Govender, T.; Sathekge, M. Metabolic Imaging of Infection. J. Nucl. Med. 2017, 58, 1727–1732. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, S.; Patel, C.N.; Scarsbrook, A.F.; Chowdhury, F.U. FDG PET/CT in infection and inflammation–current and emerging clinical applications. Clin. Radiol. 2015, 70, 787–800. [Google Scholar] [CrossRef]
- Treglia, G.; Sadeghi, R. Meta-analyses and systematic reviews on PET and PET/CT in oncology: The state of the art. Clin. Transl. Imaging 2013, 1, 73–75. [Google Scholar] [CrossRef][Green Version]
- McInnes, M.D.F.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; the PRISMA-DTA Group; Clifford, T.; Cohen, J.F.; Deeks, J.J.; Gatsonis, C.; et al. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA 2018, 319, 388–396. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Int. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Sadeghi, R.; Treglia, G. Systematic reviews and meta-analyses of diagnostic studies: A practical guideline. Clin. Transl. Imaging 2017, 5, 83–87. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Harbord, R.M.; Egger, M.; Sterne, J.A. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat. Med. 2006, 25, 3443–3457. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Oh, Y.K.; Park, H.C.; Park, S.; Lee, S.; Lee, H.Y.; Hwang, Y.H.; Ahn, C. Clinical experience with white blood cell-PET/CT in autosomal dominant polycystic kidney disease patients with suspected cyst infection: A prospective case series. Nephrology 2018, 23, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Bhattacharya, A.; Prakash, M.; Sharma, S.; Mittal, B.R.; Khandelwal, N.; Bhansali, A. Utility of PET/CT with fluorine-18-fluorodeoxyglucose-labeled autologous leukocytes for diagnosing diabetic foot osteomyelitis in patients with Charcot’s neuroarthropathy. Nucl. Med. Commun. 2016, 37, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.W.; Lee, H.Y.; Hwang, Y.H.; Park, H.C.; Ahn, C.; Kang, K.W. Diagnostic performance of 18F-FDG-labeled white blood cell PET/CT for cyst infection in patients with autosomal dominant polycystic kidney disease: A prospective study. Nucl. Med. Commun. 2016, 37, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, S.; Aliyev, A.; Ekmekcioglu, O.; Ozhan, M.; Uslu, L.; Vatankulu, B.; Sager, S.; Halaç, M.; Sönmezoğlu, K. Comparison of FDG and FDG-labeled leukocytes PET/CT in diagnosis of infection. Nuklearmedizin 2015, 54, 262–271. [Google Scholar] [PubMed]
- Bhattacharya, A.; Kochhar, R.; Sharma, S.; Ray, P.; Kalra, N.; Khandelwal, N.; Mittal, B.R. PET/CT with 18F-FDG-labeled autologous leukocytes for the diagnosis of infected fluid collections in acute pancreatitis. J. Nucl. Med. 2014, 55, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, S.Y.; Asa, S.; Ozhan, M.; Ocak, M.; Sager, M.S.; Erkan, M.E.; Halac, M.; Kabasakal, L.; Sönmezoglu, K.; Kanmaz, B. FDG and FDG-labelled leucocyte PET/CT in the imaging of prosthetic joint infection. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Dumarey, N.; Egrise, D.; Blocklet, D.; Stallenberg, B.; Remmelink, M.; del Marmol, V.; Van Simaeys, G.; Jacobs, F.; Goldman, S. Imaging infection with 18F-FDG-labeled leukocyte PET/CT: Initial experience in 21 patients. J. Nucl. Med. 2006, 47, 625–632. [Google Scholar] [PubMed]
- Rini, J.N.; Bhargava, K.K.; Tronco, G.G.; Singer, C.; Caprioli, R.; Marwin, S.E.; Richardson, H.L.; Nichols, K.J.; Pugliese, P.V.; Palestro, C.J. PET with FDG-labeled leukocytes versus scintigraphy with 111In-oxine-labeled leukocytes for detection of infection. Radiology 2006, 238, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Osman, S.; Danpure, H.J. The use of 2-[18F]fluoro-2-deoxy-d-glucose as a potential in vitro agent for labelling human granulocytes for clinical studies by positron emission tomography. Int. J. Rad. Appl. Instrum. B 1992, 19, 183–190. [Google Scholar] [CrossRef]
- Pellegrino, D.; Bonab, A.A.; Dragotakes, S.C.; Pitman, J.T.; Mariani, G.; Carter, E.A. Inflammation and infection: Imaging properties of 18F-FDG-labeled white blood cells versus 18F-FDG. J. Nucl. Med. 2005, 46, 1522–1530. [Google Scholar] [PubMed]
- Forstrom, L.A.; Dunn, W.L.; Mullan, B.P.; Hung, J.C.; Lowe, V.J.; Thorson, L.M. Biodistribution and dosimetry of [(18)F]fluorodeoxyglucose labelled leukocytes in normal human subjects. Nucl. Med. Commun. 2002, 23, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Miñana, E.; Roldán, M.; Chivato, T.; Martínez, T.; Fuente, T. Quantification of the chromosomal radiation damage induced by labelling of leukocytes with [18F]FDG. Nucl. Med. Biol. 2015, 42, 720–723. [Google Scholar] [CrossRef] [PubMed]
| Authors | Year | Country | Study Design | Type of Patients Evaluated | No. of Patients Performing 18F-FDG-WBC PET or PET/CT | Mean Age (Years) | % Male |
|---|---|---|---|---|---|---|---|
| Kim et al. [14] | 2018 | South Korea | prospective single center | Patients with autosomal dominant polycystic kidney disease and suspicious cyst infection | 19 | 54 (38–73) | 37% |
| Rastogi et al. [15] | 2016 | India | prospective single center | Patients with Charcot’s neuroarthropathy and suspicious diabetic foot osteomyelitis | 23 | 57.9 ± 7.8 | 96% |
| Kwon et al. [16] | 2016 | South Korea | prospective single center | Patients with autosomal dominant polycystic kidney disease and suspicious cyst infection | 17 | 53 (38–73) | 35% |
| Yilmaz et al. [17] | 2015 | Turkey | prospective single center | Patients with suspicious musculoskeletal, vascular or soft-tissue infection | 49 | 55.7 (16–89) | 55% |
| Bhattacharya et al. [18] | 2014 | India | prospective single center | Patients with suspicious infected fluid collections in acute pancreatitis | 41 | 41 ± 11.5 | 68% |
| Aksoy et al. [19] | 2014 | Turkey | prospective single center | Patients with suspicious prosthetic joint infection | 46 | 61 ± 12.2 | 22% |
| Dumarey et al. [20] | 2006 | Belgium | prospective single center | Patients with suspicious infectious diseases | 21 | 56 (24–84) | 62% |
| Rini et al. [21] | 2006 | USA | prospective single center | Patients with suspicious infectious diseases | 43 | 59 (32–86) | 47% |
| Authors | Hybrid Imaging Modality | Activity Used for WBC Labeling | Mean WBC Labeling Efficiency (%) | Injected Activity | Image Analysis | Other Imaging Modalities Performed for Comparison |
|---|---|---|---|---|---|---|
| Kim et al. [14] | PET/CT (low-dose CT) | 740 MBq | NR | NR | visual | MRI or CT |
| Rastogi et al. [15] | PET/CT (low-dose CT) | NR | 73 ± 28 | NR | visual and semi-quantitative (SUVmax) | 18F-fluoride PET/CT and MRI |
| Kwon et al. [16] | PET/CT (low-dose CT) | 740 MBq | NR | 189 ± 107 MBq | visual | MRI or CT |
| Yilmaz et al. [17] | PET/CT (low-dose CT) | 740–925 MBq | 70 ± 13 | 70-433 MBq | visual and semi-quantitative (SUVmax) | 18F-FDG PET/CT |
| Bhattacharya et al. [18] | PET/CT (low-dose or contrast enhanced CT) | 314.5–555 MBq | 81 ± 17 | 262.7 ± 70.3 MBq | visual and semi-quantitative (SUVmax) | - |
| Aksoy et al. [19] | PET/CT (low-dose CT) | 740–925 MBq | 75 ± 17 | 296–703 MBq | visual and semi-quantitative (SUVmax) | 18F-FDG PET/CT |
| Dumarey et al. [20] | PET/CT (low-dose CT) | 740 MBq | 75 ± 21 | 303 ± 111 MBq | visual and semi-quantitative (SUVmax) | - |
| Rini et al. [21] | No (PET only) | 660–740 MBq | 72 ± 8 | 196–315 MBq | visual | 111In-WBC scintigraphy |
| Authors | True Positive | False Negative | False Positive | True Negative | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|---|---|---|---|
| Kim et al. [14] | 9 | 5 | 2 | 3 | 64.3% | 60% | 81.8% | 37.5% | 63.2% |
| Rastogi et al. [15] | 10 | 2 | 0 | 11 | 83.3% | 100% | 100% | 84.6% | 91.3% |
| Kwon et al. [16] * | NA | NA | NA | NA | 85.7% | 87.5% | 85.7% | 87.5% | NA |
| Yilmaz et al. [17] | 20 | 4 | 3 | 22 | 83.3% | 88% | 87% | 84.6% | 85.7% |
| Bhattacharya et al. [18] | 10 | 0 | 0 | 25 | 100% | 100% | 100% | 100% | 100% |
| Aksoy et al. [19] | 13 | 1 | 1 | 31 | 92.9% | 96.9% | 92.9% | 96.9% | 95.7% |
| Dumarey et al. [20] | 12 | 2 | 1 | 6 | 85.7% | 85.7% | 92.3% | 75% | 85.7% |
| Rini et al. [21] | 13 | 2 | 5 | 23 | 86.7% | 82.1% | 72.2% | 92% | 83.7% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyer, M.; Testart, N.; Jreige, M.; Kamani, C.; Moshebah, M.; Muoio, B.; Nicod-Lalonde, M.; Schaefer, N.; Giovanella, L.; Prior, J.O.; et al. Diagnostic Performance of PET or PET/CT Using 18F-FDG Labeled White Blood Cells in Infectious Diseases: A Systematic Review and a Bivariate Meta-Analysis. Diagnostics 2019, 9, 60. https://doi.org/10.3390/diagnostics9020060
Meyer M, Testart N, Jreige M, Kamani C, Moshebah M, Muoio B, Nicod-Lalonde M, Schaefer N, Giovanella L, Prior JO, et al. Diagnostic Performance of PET or PET/CT Using 18F-FDG Labeled White Blood Cells in Infectious Diseases: A Systematic Review and a Bivariate Meta-Analysis. Diagnostics. 2019; 9(2):60. https://doi.org/10.3390/diagnostics9020060
Chicago/Turabian StyleMeyer, Marie, Nathalie Testart, Mario Jreige, Christel Kamani, Mohammed Moshebah, Barbara Muoio, Marie Nicod-Lalonde, Niklaus Schaefer, Luca Giovanella, John O. Prior, and et al. 2019. "Diagnostic Performance of PET or PET/CT Using 18F-FDG Labeled White Blood Cells in Infectious Diseases: A Systematic Review and a Bivariate Meta-Analysis" Diagnostics 9, no. 2: 60. https://doi.org/10.3390/diagnostics9020060
APA StyleMeyer, M., Testart, N., Jreige, M., Kamani, C., Moshebah, M., Muoio, B., Nicod-Lalonde, M., Schaefer, N., Giovanella, L., Prior, J. O., & Treglia, G. (2019). Diagnostic Performance of PET or PET/CT Using 18F-FDG Labeled White Blood Cells in Infectious Diseases: A Systematic Review and a Bivariate Meta-Analysis. Diagnostics, 9(2), 60. https://doi.org/10.3390/diagnostics9020060







