Abstract
Whole body magnetic resonance imaging (MRI) with diffusion-weighted imaging (WB-MRI-DWI) is currently emerging as a diagnostic technique in the evaluation of bone metastases from breast, prostate, lung, thyroid, and melanoma tumors. The most relevant articles regarding the detection of solid tumor bone metastases with MRI have been reviewed and cited. The imaging methods currently used in the detection of bone metastases are bone scintigraphy, computed tomography (CT), and positron emission tomography (PET/CT) with 2-deoxy-2-[fluorine-18] fluoro-d-glucose (18F-FDG PET/CT). WB-MRI-DWI allows qualitative and quantitative evaluation of focal lesions through signal intensity evaluation on DWI images and the reconstruction of the apparent diffusion coefficient (ADC) map. In prostate and breast cancer, WB-MRI-DWI is useful in assessing the response of bone lesions to therapy and to detecting early non-responders, while in lung cancer the method shows a similar sensitivity to 18F-FDG PET/CT in the detection of bone metastases. In bone metastases of thyroid tumors and melanoma, the WB-MRI-DWI shows a higher sensitivity when compared to 18F-FDG PET/CT. With a standardization of the WB-MRI-DWI protocol, this method seems to play an important role in the diagnosis of bone solid tumor metastases.
1. Introduction
Bone metastases cause a significant reduction in quality of life, producing pain and pathological fractures. Bone metastases are detectable in patients with advanced stages of breast, prostate, lung, and thyroid cancer, as well as melanoma. They are also minor entities in uterine and gastrointestinal tumors, as well as other types of neoplasms.
The most useful imaging techniques for the detection of bone marrow secondary lesions were bone scintigraphy (BS), contrast enhanced computed tomography (CE/CT), and positron emission tomography (PET/CT) with 2-deoxy-2-[fluorine-18] fluoro-d-glucose (18F-FDG PET/CT). A few decades ago, magnetic resonance imaging (MRI) began to be used in diagnostic practice; its use does not lead to ionization radiation exposure.
Diffusion-weighted imaging (DWI) is based on the evaluation of microscopic movements of water at the cellular level, providing quantitative (e.g., apparent diffusion coefficient (ADC)) and qualitative (e.g., signal intensity) information, which can be used to distinguish benign from malignant disorders. This pulse sequence is mandatory for oncologic imaging and in the specific field of distant metastases detection.
Recently, whole-body magnetic resonance arose as a new technique in assessing systemic disease and has been improved by the addition of DWI pulses (WB-MRI-DWI).
In our review, we concentrated on evaluating the role of WB-MRI-DWI and, particularly, the additional information provided by DWI sequences in the most common adult tumors which metastasize to bones.
2. Protocols and Pitfalls
Bone localizations, detected with WB-MRI-DWI, appear hypointense in T1-weighted images, hyperintense in fat-suppression sequences, and hyperintense in DWI with a low ADC value, due to the restriction of water and enhancement after contrast administration.
To view the appearance of bone metastases, a WB-MRI-DWI protocol should include these sequences: T1W, T2 with or without fat suppression, and DWI [1].
However, it is not always as easy as it seems. Metastases characteristics are not the same because the primary tumor and its secondary lesions have different microstructures. All secondary bone lesions could be divided into two main groups: osteoblastic and osteolytic. These do not always have the same appearance on DWI sequences which may be a problem, as shown in the study by Bauret et al. where sclerotic metastases treated with chemotherapy had a hypointense appearance on DWI due to low water content [2,3]. The solution seems to be a protocol which includes morphologic and functional sequences, including DIXON sequences that offer faster acquisition and a more homogenous suppression of fat [4].
The protocol might also include “whole spine” acquisition, as reported in previous literature, to evaluate all the vertebrae in the sagittal plane. In this way, it is possible to improve the accuracy of lesion localizations thanks to a higher spatial resolution, instead of a solely coronal plane. On the other hand, pathological fractures, vertebra collapses, and involvement of the medullary channel may be discovered in the sagittal plane. Sometimes a differential diagnosis between malignant lesions and other conditions could be very difficult, despite the application of DWI sequences. On T2-weighted sequences and on DWI, the hypersignal of bone marrow may induce false positive interpretations, such as for edema related to fractures, degenerative diseases, bone infarctions, infections, hemangiomas, isolated red bone marrow islands, and treated inactive lesions (T2-shine through). An ADC map can lead to a correct diagnosis. False negative results could be found in the evaluation of the skull due to the high signal of the adjacent brain [1,5].
3. Diffusion-Weighted Imaging (DWI) Sequences in Bone Metastases Detection
After the introduction of DWI sequences into the WB-MRI standard protocol, it has become easier to identify and differentiate benign bone marrow lesions from skeletal metastases [6].
3.1. Prostate Cancer
Prostate cancer (PC) is a common genitourinary malignancy which affects males and is the second most common cause of cancer mortality. Its incidence continues to rise in western countries, with the most frequent bone metastases located in the pelvis, spine, ribs, and femurs [7,8].
The diagnosis of PC is based on rise of prostate-specific antigen (PSA) serum values. Radiological imaging is necessary to distinguish local and systemic recurrence.
The Prostate Cancer Consensus Conference of 2017 reported the use of CT and scintigraphy for the detection of metastatic bone disease, although these methods of image analysis have low sensitivity and specificity. They mentioned the WB-MRI-DWI as a new method to improve the detection of bone metastases; it has an intrinsic bias in that there is an absence of a histopathologic control for each lesion and the absence of a gold standard to verify the real presence of tumoral cells [9].
Bone scintigraphy (BS) is considered a commonly used technique for suspected bone lesions. Nowadays, comparative studies of WB-MRI and PET/CT (with different radio tracers) have shown that their metastasis detection in advanced cancer is more accurate than that of BS.
PET/CT has been used with different tracers for the detection of prostate metastases, such as 11C-choline, 18F-fluorocholine, and 18F-Na fluoride. In particular, 18F-NaF PET/CT is a highly sensitive technique but, with regards to specificity, it is necessary to consider the tracer accumulation in inflammatory and degenerative bone diseases [10].
WB-MRI can identify skeletal involvement with higher sensitivity than BS and the same sensitivity as PET/CT, as Shen et al. demonstrated in his meta-analysis where the three techniques were compared [11].
Hengqing et al., in his region-based analysis, highlighted the importance of WB-MRI at the level of the spine and pelvis, which are the most frequent sites of bone metastases for prostate cancer; on the other hand, they demonstrated the weakness of the technique in some skeletal segments, such as its low sensitivity in the ribs, clavicles, and scapulae [12].
Moreover, the most important aspect is that quantitative DWI can provide clear categorization of the treatment response of bone metastases due to calculation of the ADC value. BS and PET/CT are able to identify only disease progression, as was demonstrated in two recent studies by Padhani et al. and Lecouvet et al. [13,14].
Some studies evaluated the advantages of using DWI in determining the treatment response of advanced prostate cancer. An example is that of Barchetti at al. who assessed the accuracy of DWI with different morphological sequences in detecting skeletal metastases in 152 patients who had undergone DWI for restaging after treatment. The MRI protocol, including sequences T1W, T2W, STIR, and DWI, showed a sensitivity of 99%, a specificity of 98%, a positive predictive value of 98%, a negative predictive value of 96%, and an accuracy of 98% for the identification of bone metastatic lesions [15].
The real limit of DWI is the absence of studies which report a standardized “gold standard” to verify the real presence of bone metastases. In literature, several studies used other diagnostic exams to verify the real presence of bone metastases, like CT, PET/CT, X-ray, or follow-up. However, no study used bone biopsy as a control [16,17,18].
The question must be asked: can WB-MRI with DWI replace BS for single-step detection of metastases in patients with high-risk prostate cancer? Lecouvet F. et al. asked this in his work in 2012. They answered “yes”, like Shen at al. in their 2014 metanalysis and Phadani et al. in their works [11,19].
Despite the fact that there are numerous works that support the importance of WB-MRI-DWI for the identification of prostatic skeletal metastases in literature, this technique is not yet universally recognized, as evidenced in a recent work by Wieder et al. showing the superiority of 11C-choline PET/CT in detecting bone lesions (p = 0.02). In this work, however, no difference was reported in the detection of lymph node metastasis (p = 0.65) [20] (Figure 1).
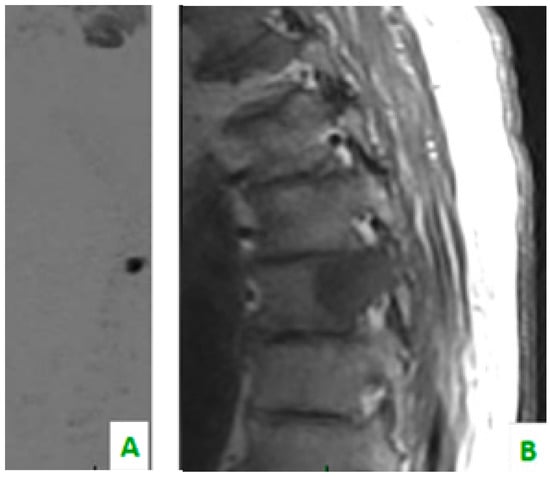
Figure 1.
(A) Diffusion-weighted imaging (DWI) sequence in sagittal plane shows focal alteration of diffusivity. (B) The sagittal plane of TSE T1 shows, in the same level, a hypointense region of the dorsal soma related to the secondary localization of prostate cancer.
3.2. Breast Cancer
Breast cancer (BC) is the most common tumor affecting women worldwide. Despite local and adjuvant therapy, bone metastases appear in 70% of cases, impacting negatively on patient survival and quality of life [21].
The most widely used imaging modality to detect bone metastasis in breast cancer is BS and PET/CT with (18)F-NaF, but several studies demonstrate the limited sensitivity and specificity of these techniques [22].
WB-MRI with and without DWI seems to be effective in the assessment of bone marrow metastases in patients with advanced BC, but it is important to determine the most useful protocol in these patients.
Grankvist et al. demonstrated that the T1W sequence had the best sensitivity (98%) but low specificity (77%) in the detection of breast metastases. However, with the addition of STIR and DWI, the specificity increased to 95%. The sensitivity of DWI was 70% [23]. The authors concluded that MRI with T1, STIR, and DWI is useful for the evaluation of secondary bone lesions from BC and compares well to PET/CT.
In the study by Heusner et al., who compared DWI alone with PET/CT, DWI demonstrated a lower sensitivity (86%) in bone metastases detection than PET/CT (100%), which could be explained by DWI’s low spatial resolution and the susceptibility of DWI to artifacts [24].
Moreover, in a study by Pearce et al. who compared bone metastases detection from BC, PC, and myeloma, it was reported that DWI conspicuity is similar to that of the STIR sequence in BC. However, it is higher in the other two tumors. The authors concluded that this difference can be determined by the different cellular microstructure of the neoplasm (metastases from the breast are mixed, i.e., sclerotic/lytic) [25].
Jambor et al., in their region-based analysis, found that the WB-MRI protocol, including T1W, STIR, and DWI sequences, has a sensitivity of 91%, a specificity of 99%, and an accuracy of 97%. They also reported that DWI associated with STIR and T1W images can reduce false positive lesions [22].
Moreover, like in PC bone metastases, quantitative evaluation using DWI can be useful for monitoring therapy response and for the early detection of non-responders, in order to change their therapy [22] (Figure 2).
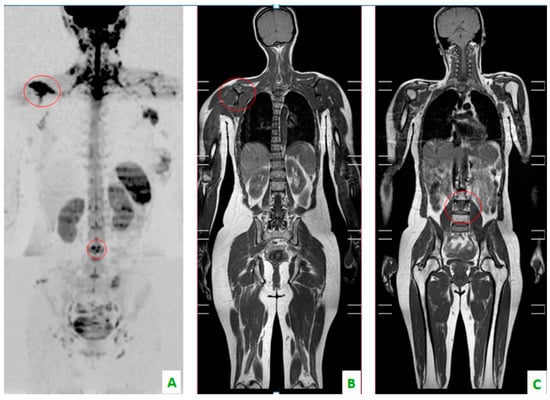
Figure 2.
Bone localizations of breast cancer in the coronal plane: scapula and vertebral metastases (in the red circles). (A) DWI with a “positron emission tomography (PET)-like” view. The right scapula and one lumbar vertebra present an alteration of diffusivity. (B) T1 DIXON showing the hypointensity of the scapula and (C) lumbar soma.
3.3. Lung Cancer
In non-small cell lung cancer (LC), secondary lesions commonly localize in bone. Bone metastasis detection is not only important in determining the stage of the disease but also in reducing the comorbidity associated with a worse quality of life [26].
Skeletal metastases, which are predominantly osteolytic, localize in the spine in 50% of patients. This the most common area of localization, followed by the ribs (27.1%), ilium (10%), sacrum (7.1%), femur (5.7%), and the humerus, scapula, and sternum (2.9%). This is according to a retrospective study by Tsuya et al. [27] (Figure 3, Figure 4 and Figure 5).
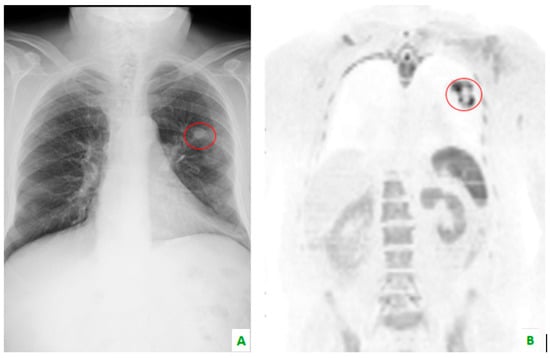
Figure 3.
(A) This chest X-ray reveals, in the red circle, a lung tumor which is also visible in (B) whole body magnetic resonance imaging with diffusion-weighted imaging (WB-MRI-DWI) in the coronal plane, in the red circle.
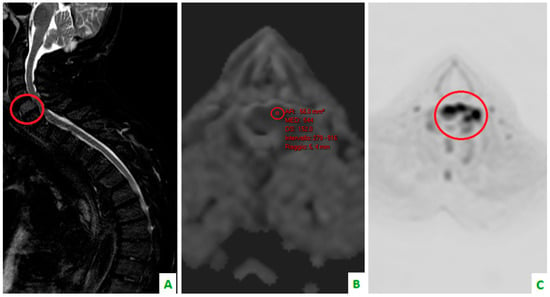
Figure 4.
Focus on the red circles: (A) DIXON T2 weighted in the sagittal plane showing bone cervical metastasis (C5) (B) a low apparent diffusion coefficient (ADC) value in the same level, and (C) a restriction of diffusivity.
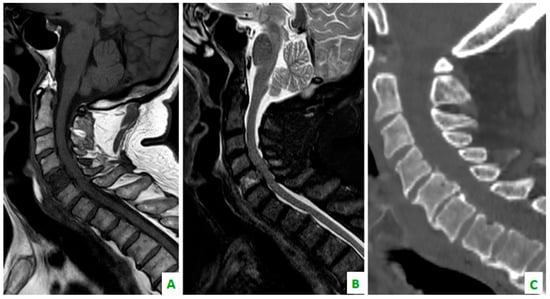
Figure 5.
(A) A T1 sequence and (B) STIR sequence, performed with a dedicated spinal MRI, highlight the lung metastases. (C) Cervical spine on a sagittal reconstruction of a computed tomography (CT). The bone metastasis is not so clear.
BS and PET/CT, used to detect the neoplasia cited above, remain the most commonly employed techniques for suspected bone metastases caused by LC. Unfortunately, comparative studies of WB-MRI-DWI with other techniques are very few. Most of the studies focused on the comparison of WB-MRI standard protocol with PET/CT and BS [28,29].
A study performed by Takenaka et al. assessed the utility of DWI by analyzing 115 patients with non-small cell lung carcinoma, demonstrating that WB-MRI detected bone localization with more sensitivity than BS or BS integrated with PET/CT and more specificity than DWI alone. Association with DWI sequences and WB-MRI improves the specificity and accuracy of WB-MRI [30].
A study by Ohno et al. concluded that the ability of DWI alone in the detection of metastases, including bone localizations, was lower than WB-MRI with DWI imaging and integrated with PET/CT. The same results were obtained with regards to specificity and accuracy [31].
In conclusion, nowadays, WB-MRI-DWI and PET/CT are the most sensible techniques to identify bone metastasis in LC as well.
3.4. Thyroid Cancer and Melanoma
Bone metastases are complications of thyroid carcinoma, especially of the follicular type, which determine a significant reduction of quality of life by causing pain and pathological fractures. Patients with differentiated thyroid carcinoma (DTC) have a 10-year survival rate of 80–95%. However, when distant metastases are present, the overall 10-year survival rate reduced to 40% [32].
Unfortunately, in literature, reports about WB-MRI-DWI detection of bone metastases due to thyroid cancer are limited.
One of them, the study by Sakurai et al., examined 23 patients with differentiated thyroid carcinoma and total thyroidectomy who underwent WB-MRI with standard protocol, WB-MRI with DWI, and WB PET/CT. Authors showed that the sensitivity of WB-MRI with DWI in detecting bone metastases was higher (82%) compared to WB-MRI without DWI (64%) and PET/CT (79%). The techniques had an WB-MRI with DWI accuracy of 94%, 90%, and 94%, respectively. Authors concluded that DWI improves the sensitivity and accuracy of WB-MRI in the identification of secondary skeletal lesions in patients with DTC [33].
In another work by Nagamachi et al., the authors evaluated 70 postoperative DTC patients who underwent whole body scintigraphy, PET/CT, and WB-MRI-DWI. The study demonstrated that the detectability of the three techniques was 67.1%, 84.2%, and 57.6%, respectively. Authors concluded that DWI could be the method of choice for monitoring postoperative DTC for possible secondary lesions. In this study WB-MRI alone, without a DWI sequence, was not evaluated [34].
Bone is a frequent site for metastases in patients with malignant melanoma, detectable in about 23–49% of cases [35]. Some reports have tried to determine the role of WB-MRI-DWI, but this technique is used more often for the loco regional staging of melanoma.
Mosavi et al. found that the sensitivity of WB-MRI and DWI varies considerably in different regions of the body. At the level of the bone, WB-MRI and WB-MRI-DWI detected a significantly higher number of bone lesions compared to CT. WB-MRI and DWI equally detected 56 bone lesions in 12 patients, while CT showed 42 bone lesions in 8 patients [36].
Laurent et al. compared WB-MRI, including DWI, and PET/CT and demonstrated a higher sensitivity and specificity for WB-MRI in the detection of melanoma bone metastases. The sensitivity and specificity for WB-MRI were 82% and 97%, respectively, and 72.8% and 92.7% for PET/CT, respectively. DWI detected 20% more lesions compared with the standard MRI protocol [37].
Petraglia et al. compared WB-MRI-DWI with contrast-enhancement (CE MRI) in detecting bone metastases in patients with advanced melanoma. The authors concluded that WB-MRI-DWI without additional CE MRI sequences is promising for the detection of extracranial metastases in melanoma patients, but CE MRI is necessary for evaluating the brain [38].
DWI is able to detect bone metastases in patients with thyroid carcinoma and malignant melanoma with a higher sensitivity and specificity compared to WB-MRI standard protocol, PET/CT, and bone scintigraphy.
4. Conclusions
WB-MRI-DWI seems to be a promising method of imaging in the detection of bone metastases. Standardization of protocols and more studies are needed (Table 1).

Table 1.
Summary of some articles about nuclear medicine and WB-MRI-DWI cited in the text.
Author Contributions
All authors have approved the submitted version and agree to be personally accountable for the authors’ own contributions and for ensuring that questions related to the accuracy or integrity of any part of the work, even ones in which the authors were not personally involved, are appropriately investigated, resolved, and documented in the literature. A.S. and A.C. have led the project and reviewed each version of the manuscript, from the beginning to the end. A.T. has searched literature and written the section “Prostate Cancer”; she also has prepared all references. E.S. has searched literature and written the sections “Protocols and Pitfalls” and “Lung cancer”. A.T. and A.S. have searched all figures and tables and written related comments and the abstract. S.B. has searched literature and written the section “Breast Cancer”. L.S. has searched the literature and written the sections “Introduction” and “Thyroid cancer and melanoma”.
Conflicts of Interest
The authors declare no conflict of interest
References
- Wilhelm, T.; Stieltjes, B. Whole-body-MR-diffusion weighted imaging in oncology. Rofo 2013, 185, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Baur, A.; Dietrich, O.; Reise, M. Diffusion-weighted imaging of the spinal column. Neuroimaging Clin. 2002, 12, 147–160. [Google Scholar] [CrossRef]
- Baur, A.; Dietrich, O.; Reise, M. Diffusion-weighted imaging of bone marrow: Current status. Eur. Radiol. 2003, 13, 1699–1708. [Google Scholar] [CrossRef] [PubMed]
- Costelloe, C.M.; Kundra, V.; Ma, J.; Chasen, B.A.; Rohren, E.M.; Bassett, R.L.; Madewell, J.E. Fast dixon whole-body MRI for detecting distant cancer metastasis: A preliminary clinical study. J. Magn. Reson. Imaging 2012, 35, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Padhani, A.R. Whole body MRI—How I do it and wht it tells you. Proc. Intl. Soc. Mag. Reson. Med. 2012, 20, 1–6. [Google Scholar]
- Liu, L.P.; Cui, L.B.; Zhang, X.X.; Cao, J.; Chang, N.; Tang, X.; Qi, S.; Zhang, X.L.; Yin, H.; Zhang, J. Diagnostic performance of diffusion-weighted magnetic resonance imaging in bone malignancy. Medicine 2015, 94, E1998. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of world wide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Cooperberg, M.; Broering, J.; Carroll, P. Time trends and local variation in primary treatment of localized prostate cancer. J. Clin. Oncol. 2010, 28, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Gillessen, S.; Attard, G.; Beer, T.M.; Beltran, H.; Bossi, A.; Bristow, R.; Carver, B.; Castellano, D.; Chung, B.H.; Clarke, N.; et al. Management of patients with advanced prostate cancer: The report of the advanced prostate cancer consensus conference APCCC 2017. Eur. Urol. 2018, 73, 178–211. [Google Scholar] [CrossRef] [PubMed]
- Beheshti, M.; Rezaee, A. Evaluation of prostate cancer bone metastases with 18F-NaF and 18F-Fluorocholine PET/CT. J. Nucl. Med. 2016, 57, 55S–60S. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Deng, H. Comparison of choline-PET/CT, MRI, SPECT, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: A meta-analysis. Skeletal Radiol. 2014, 43, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Hengqing, A.; Ning, T. Detection of prostate cancer metastasis by whole body magnetic resonance imaging combined with bone scintigraphy and PSA levels. Cell. Physiol. Biochem. 2016, 40, 1052–1062. [Google Scholar] [CrossRef]
- Padhani, A.R.; Lecouvet, F.E. Rationale for modernizing imaging in advanced prostate cancer. Eur. Urol. Focus 2017, 3, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Lecouvet, F.E.; Talbot, J.N. Monitoring the response of bone metastases to treatment with magnetic resonance imaging and nuclear medicine techniques: A review and position statement by the European organisation for research and treatment of cancer imaging group. Eur. J. Cancer 2014, 50, 2519–2531. [Google Scholar] [CrossRef] [PubMed]
- Barchetti, F.; Stagnitti, A. Unenhancedwhole-body MRI versus PET-CT for the detection of prostate cancer metastase safter primary treatment. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3770–3776. [Google Scholar] [PubMed]
- Jacobs, M.A.; Macura, K.J.; Zaheer, A.; Antonarakis, E.S.; Stearns, V.; Wolff, A.C.; Feiweier, T.; Kamel, I.R.; Wahl, R.L.; Pan, L. Multiparametric whole-body MRI with diffusion-weighted imaging and ADC mapping for the identification of visceral and osseous metastases from solid tumors. Acad. Radiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Kosmin, M.; Makris, A.; Joshi, P.V.; Ah-See, M.L.; Woolf, D.; Padhani, A.R. The addition of whole-body magnetic resonance imaging to body computerised tomography alters treatment decisions in patients with metastatic breast cancer. Eur. J. Cancer 2017, 77, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Yachida, Y.; Yoshida, S.; Takeshita, H.; Sawamura, C.; Tanaka, H.; Satoh, S.; Uchida, Y.; Ishioka, J.; Matsuoka, Y.; Numao, N.; et al. Bone abnormal signal incidentally found in pre-biopsy diffusion-weighted MRI for suspected prostate cancer: What does it reflect? Urol. Int. 2014, 93, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Lecouvet, F.E.; El Mouedden, J. Can whole-body magnetic resonance imaging with diffusion-weighted imaging replace Tc 99m bone scanning and computed tomography for single-step detection of metastases in patients with high-risk prostate cancer? Eur. Urol. 2012, 62, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Wieder, H.; Beer, A.J. 11C-choline PET/CT and whole-body MRI including diffusion-weighted imaging for patients with recurrent prostate cancer. Oncotarget 2017, 8, 66516–66527. [Google Scholar] [CrossRef] [PubMed]
- Brook, N.; Brook, E. Breast cancer bone metastases: Pathogenesis and therapeutic targets. Int. J. Biochem. Cell Biol. 2018, 96, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Jambor, I.; Kuisma, A. Prospective evaluation of planar bone scintigraphy, SPECT, SPECT/CT, 18F-NaF PET/CT and whole body 1.5T MRI, including DWI, for the detection of bone metastases in high risk breast and prostate cancer patients: SKELETA clinical trial. Acta Oncol. 2016, 55, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Grankvist, J.; Fisker, R. MRI and PET/CT of patients with bone metastases from breast carcinoma. Eur. J. Radiol. 2012, 81, E13–E18. [Google Scholar] [CrossRef] [PubMed]
- Heusner, T.A.; Kuemmel, S. Diagnosticvalue of diffusion-weightedmagnetic resonance imaging (DWI) compared to FDGPET/CT for whole-bodybreast cancerstaging. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Pearce, T.; Philip, S. Bone metastases from prostate, breast and multiple myeloma: Differences in lesion conspicuity at short-tau inversion recovery and diffusion-weighted MRI. Br. J. Radiol. 2012, 85, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- D’Antonio, C.; Passaro, A. Bone and brain metastasis in lung cancer: Recent advances in therapeutic strategies. Ther. Adv. Med. Oncol. 2014, 6, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Tsuya, A.; Kurata, T. Skeletal metastases in non-small cell lung cancer: A retrospective study. Lung Cancer 2007, 57, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.A.; Shin, K.M.; Chung, M.J. Non-small cell lung cancer staging: Efficacy comparison of integrated PET/CT versus 3.0-T whole-body MR imaging. Radiology 2008, 248, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Q.; Yang, Y. Detection of rib metastases in patients with lung cancer: A comparative study of MRI, CT and bone scintigraphy. PLoS ONE 2012, 7, e52213. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, D.; Ohno, Y. Detection of bone metastases in non-small cell lung cancer patients: Comparison of whole-body diffusion-weighted imaging (DWI), whole-body MR imaging without and with DWI, whole-body FDG-PET/CT, and bone scintigraphy. J. Magn. Reson Imaging 2009, 30, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Koyama, H. Non–small cell lung cancer: Whole-body MR examination for M-stage assessment-utility for whole-body diffusion-weighted imaging compared with integrated FDG PET/CT. Radiographics 2012, 248, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Muresan, M.; Olivier, P. Bone metastases from differentiated thyroid carcinoma. Endocr.-Relat. Cancer 2008, 15, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, Y.; Kawai, H. Supplemental value of diffusion-weighted whole-body imaging with background body signal suppression (DWIBS) technique to whole-body magnetic resonance imaging in detection of bone metastases from thyroid cancer. J. Med. Imaging Radiat. Oncol. 2013, 57, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Nagamachi, S.; Wakamatsu, H.; Kiyohara, S.; Nishii, R.; Mizutani, Y.; Fujita, S.; Futami, S.; Arita, H.; Kuroki, M.; Nakada, H.; et al. Comparison of diagnostic and prognostic capabilities of ¹⁸F-FDG-PET/CT, ¹³¹I-scintigraphy, and diffusion-weighted magnetic resonance imaging for postoperative thyroid cancer. Jpn. J. Radiol. 2011, 29, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Akslen, L.A.; Hove, L.M. Metastatic distribution in malignant melanoma. A 30-year autopsy study. Invasion Metastasis 1987, 7, 253–263. [Google Scholar] [PubMed]
- Mosavi, F.; Ullenhag, G. Whole-body MRI including diffusion-weighted imaging compared to CT for staging of malignant melanoma. Upsala J. Med. Sci. 2013, 118, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Laurent, V.; Trausch, G. Comparative study of two whole-body imaging techniques in the case of melanoma metastases: Advantages of multi-contrast MRI examination including a diffusion-weighted sequence in comparison with PET-CT. Eur. J. Radiol. 2010, 75, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Petralia, G.; Padhani, A. Whole-body diffusion-weighted imaging: Is it all we need for detecting metastases in melanoma patients? Eur. Radiol. 2013, 23, 3466–3476. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).