Prediction of Treatment Response by Thyroid Bed Uptake on Post-Ablative Whole-Body Scan in Intermediate-Risk Patients with Papillary Thyroid Cancer
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics
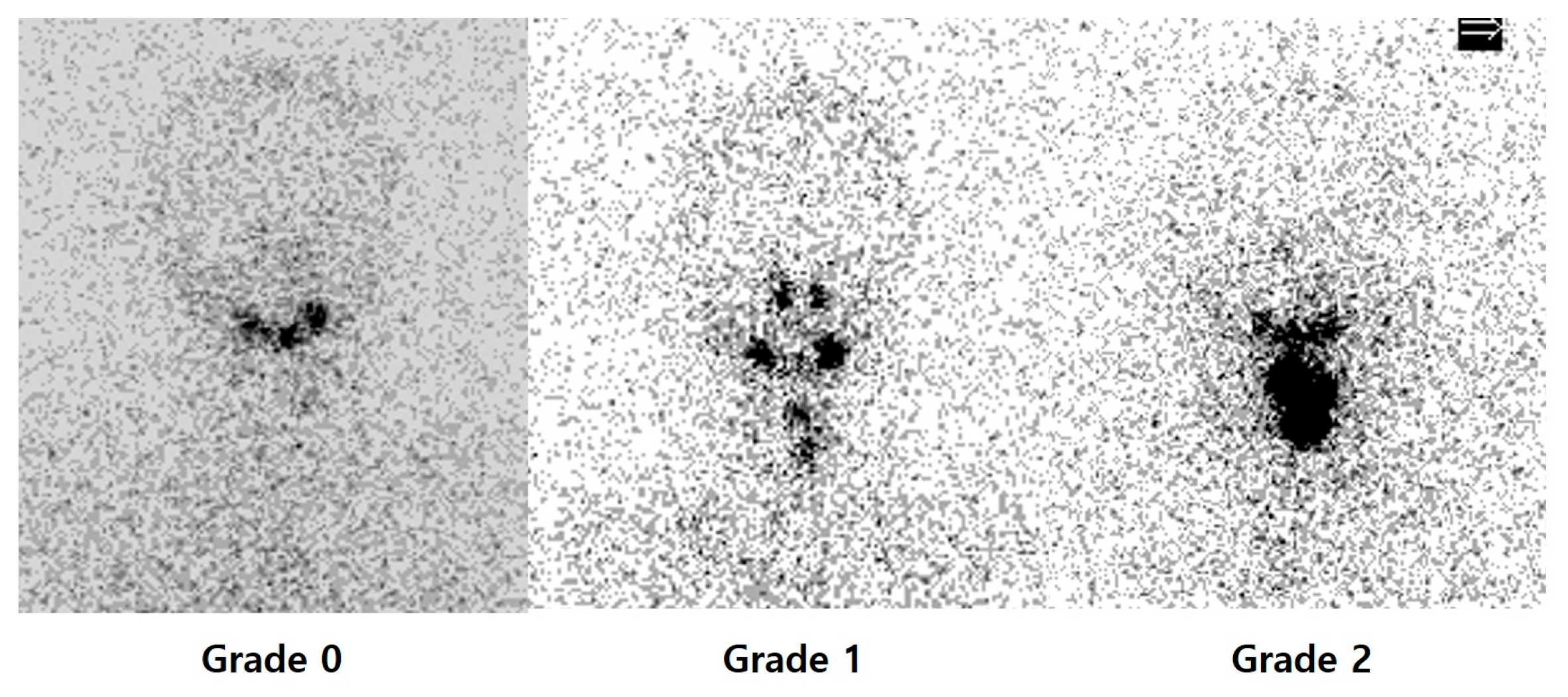
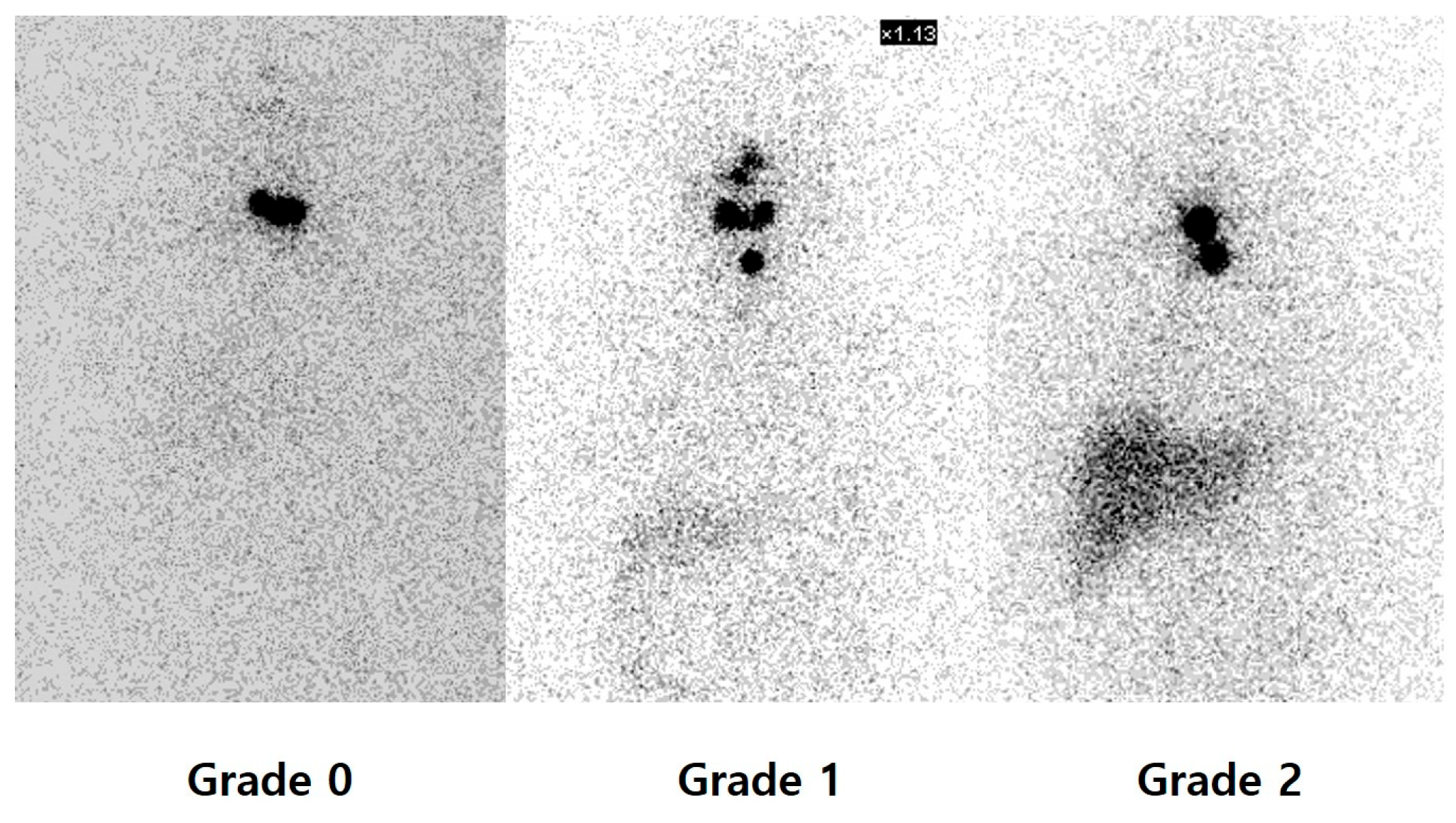
3.2. Relationship Between Uptake on PAWBS and Therapy Response
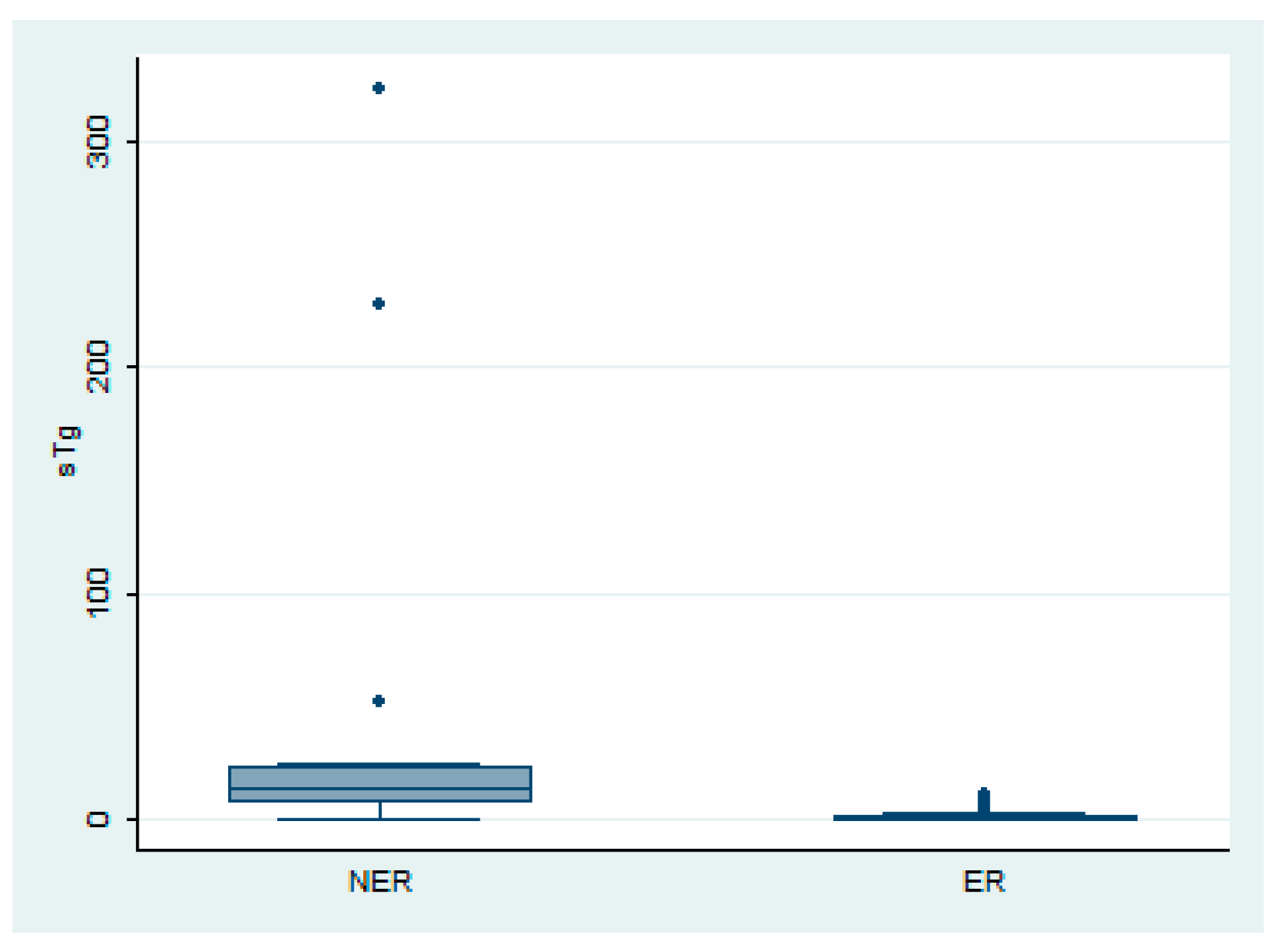
3.3. Relationship Between sTg and Thyroid Bed Uptake on PAWBS
3.4. Uni- and Multivariate Analyses
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PTC | Papillary thyroid carcinoma |
| RAI | Radioactive iodine |
| ATA | American Thyroid Association |
| PAWBS | Post-ablative whole-body scan |
| rh-TSH | Recombinant human thyroid-stimulating hormone |
| sTg | Stimulated Tg |
| ER | Excellent response |
| NER | Non-excellent response |
| NCCN | National Comprehensive Cancer Network |
| SPECT/CT | Single-photon emission computed tomography/computed tomography |
References
- Hundahl, S.A.; Fleming, I.D.; Fremgen, A.M.; Menck, H.R. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995. Cancer 1998, 83, 2638–2648. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Luster, M.; Clarke, S.E.; Dietlein, M.; Lassmann, M.; Lind, P.; Oyen, W.J.; Tennvall, J.; Bombardieri, E. Guidelines for radioiodine therapy of differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1941–1959. [Google Scholar] [CrossRef] [PubMed]
- Iwano, S.; Kato, K.; Nihashi, T.; Ito, S.; Tachi, Y.; Naganawa, S. Comparisons of I-123 diagnostic and I-131 post-treatment scans for detecting residual thyroid tissue and metastases of differentiated thyroid cancer. Ann. Nucl. Med. 2009, 23, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.; Lee, J.J.; Park, S.H.; Kim, T.Y.; Kim, W.B.; Shong, Y.K.; Ryu, J.S. Prediction of treatment response to 131I therapy by diffuse hepatic uptake intensity on post-therapy whole-body scan in patients with distant metastases of differentiated thyroid cancer. Ann. Nucl. Med. 2015, 29, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, S.J.; Kim, I.J.; Kim, Y.K.; Kim, B.S.; Pak, K. Clinical significance of diffuse hepatic visualization and thyroid bed uptake on post-ablative iodine-131 whole body scan in differentiated thyroid cancer. Onkologie 2012, 35, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Torlontano, M.; Crocetti, U.; Augello, G.; D’Aloiso, L.; Bonfitto, N.; Varraso, A.; Dicembrino, F.; Modoni, S.; Frusciante, V.; Di Giorgio, A.; et al. Comparative evaluation of recombinant human thyrotropin-stimulated thyroglobulin levels, 131I whole-body scintigraphy, and neck ultrasonography in the follow-up of patients with papillary thyroid microcarcinoma who have not undergone radioiodine therapy. J. Clin. Endocrinol. Metab. 2006, 91, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Haddad, R.I.; Bischoff, L.; Ball, D.; Bernet, V.; Blomain, E.; Busaidy, N.L.; Campbell, M.; Dickson, P.; Duh, Q.Y.; Ehya, H.; et al. Thyroid Carcinoma, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 2022, 20, 925–951. [Google Scholar] [CrossRef] [PubMed]
- Giannoula, E.; Melidis, C.; Papadopoulos, N.; Bamidis, P.; Raftopoulos, V.; Iakovou, I. Dynamic Risk Stratification for Predicting Treatment Response in Differentiated Thyroid Cancer. J. Clin. Med. 2020, 9, 2708. [Google Scholar] [CrossRef] [PubMed]
- Chandekar, K.R.; Satapathy, S.; Bal, C. Impact of radioiodine therapy on recurrence and survival outcomes in intermediate-risk papillary thyroid carcinoma—A systematic review and meta-analysis. Clin. Endocrinol. 2024, 100, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mao, Y.; Li, L.; Liang, J.; Huang, H.; Lin, W.; Chen, G.; Wen, J. Survival benefit of postoperative radioiodine therapy among patients with intermediate-risk differentiated thyroid carcinoma. Endocrine 2024, 86, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Lin, R.; Wang, Y.; Yu, J. Correlation analysis between the quantitative parameters of iodine-131 single-photon emission computed tomography-computed tomography thyroid bed uptake and the efficacy of radioactive iodine adjuvant therapy for papillary thyroid cancer. Quant. Imaging Med. Surg. 2024, 14, 3665–3675. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.; Kim, M.H.; Jo, K.; Lim, Y.; Bae, J.S.; Lee, S.; Kang, M.I.; Cha, B.Y.; Lim, D.J. Recombinant human TSH stimulated thyroglobulin levels at remnant ablation predict structural incomplete response to treatment in patients with differentiated thyroid cancer. Medicine 2017, 96, e7512. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.; Pak, K.; Seok, J.W.; Kim, I.J. Prognostic Value of Extranodal Extension in Thyroid Cancer: A Meta-Analysis. Yonsei Med. J. 2016, 57, 1324–1328. [Google Scholar] [CrossRef] [PubMed]
- Genpeng, L.; Pan, Z.; Tao, W.; Rixiang, G.; Jingqiang, Z.; Zhihui, L.; Jianyong, L. Prognostic implications of extranodal extension in papillary thyroid carcinomas: A propensity score matching analysis and proposal for incorporation into current tumor, lymph node, metastasis staging. Surgery 2022, 171, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Palmer, F.L.; Nixon, I.J.; Tuttle, R.M.; Shah, J.P.; Patel, S.G.; Shaha, A.R.; Ganly, I. Lateral Neck Lymph Node Characteristics Prognostic of Outcome in Patients with Clinically Evident N1b Papillary Thyroid Cancer. Ann. Surg. Oncol. 2015, 22, 3530–3536. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, M.; Ito, Y.; Hirokawa, M.; Miya, A.; Shimizu, K.; Miyauchi, A. Prognostic impact of extrathyroid extension and clinical lymph node metastasis in papillary thyroid carcinoma depend on carcinoma size. World J. Surg. 2010, 34, 3007–3014. [Google Scholar] [CrossRef] [PubMed]
- Oppenheimer, J.H. Thyroid hormones in liver. Mayo Clin. Proc. 1972, 47, 854–863. [Google Scholar] [PubMed]
- Ringel, M.D.; Sosa, J.A.; Baloch, Z.; Bischoff, L.; Bloom, G.; Brent, G.A.; Brock, P.L.; Chou, R.; Flavell, R.R.; Goldner, W.; et al. 2025 American Thyroid Association Management Guidelines for Adult Patients with Differentiated Thyroid Cancer. Thyroid 2025, 35, 841–985. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Excellent Response (n = 126) | Non-Excellent Response (n = 22) | p Values |
|---|---|---|---|
| Sex | |||
| Female | 99 (78.6%) | 14 (63.6%) | 0.212 |
| Male | 27 (21.4%) | 8 (36.4%) | |
| Age (mean ± SD) | 45.6 ± 13.1 | 49.9 ± 14.3 | 0.159 |
| Thyroid bed uptake on PAWBS | p < 0.001 | ||
| Grade 0 | 10 (7.9%) | 0 | |
| Grade 1 | 80 (63.5%) | 6 (27.3%) | |
| Grade 2 | 36 (28.6%) | 16 (72.7%) | |
| Liver uptake on PAWBS | 0.755 | ||
| Grade 0 | 4 (3.2%) | 1 (4.6%) | |
| Grade 1 | 38 (30.1%) | 5 (22.7%) | |
| Grade 2 | 84 (66.7%) | 16 (72.7%) | |
| Initial sTg (ng/mL, median, range) | 0.34 (0.01–10.5) | 13.64 (0.3–323.2) | p < 0.001 |
| Primary tumor size | 1.2 (0.3–6.3) | 1.9 (0.3–4.5) | 0.105 |
| Presence of extrathyroid extension | 97 (77%) | 17 (77.3%) | 1.0 |
| Multifocality | 55 (43.7%) | 9 (40.9%) | 0.995 |
| Extranodal spread | 18 (14.3%) | 9 (40.9%) | 0.007 |
| Central lymph node metastasis | 104 (82.5%) | 18 (81.8%) | 1.0 |
| Lateral lymph node metastasis | 18 (14.3%) | 7 (31.8%) | 0.086 |
| Coefficient | Standard Error | Odds Ratio (95% Confidence Interval) | p Value | |
|---|---|---|---|---|
| Thyroid bed uptake on PAWBS | −0.1499 | 0.0453 | 0.861 (0.787–0.941) | p < 0.001 |
| Stimulated thyroglobulin | −0.0035 | 0.0007 | 0.996 | p < 0.001 |
| Lateral lymph node metastasis | −0.128 | 0.0691 | 0.88 (0.768–1.009) | 0.065 |
| Extranodal spread | −0.0427 | 0.0611 | 0.958 (0.849–1.081) | 0.486 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Choi, E.; Chung, Y.-A.; Kwon, S.J.; Oh, J. Prediction of Treatment Response by Thyroid Bed Uptake on Post-Ablative Whole-Body Scan in Intermediate-Risk Patients with Papillary Thyroid Cancer. Diagnostics 2026, 16, 19. https://doi.org/10.3390/diagnostics16010019
Choi E, Chung Y-A, Kwon SJ, Oh J. Prediction of Treatment Response by Thyroid Bed Uptake on Post-Ablative Whole-Body Scan in Intermediate-Risk Patients with Papillary Thyroid Cancer. Diagnostics. 2026; 16(1):19. https://doi.org/10.3390/diagnostics16010019
Chicago/Turabian StyleChoi, Eunkyoung, Yong-An Chung, Soo Jin Kwon, and Jinkyoung Oh. 2026. "Prediction of Treatment Response by Thyroid Bed Uptake on Post-Ablative Whole-Body Scan in Intermediate-Risk Patients with Papillary Thyroid Cancer" Diagnostics 16, no. 1: 19. https://doi.org/10.3390/diagnostics16010019
APA StyleChoi, E., Chung, Y.-A., Kwon, S. J., & Oh, J. (2026). Prediction of Treatment Response by Thyroid Bed Uptake on Post-Ablative Whole-Body Scan in Intermediate-Risk Patients with Papillary Thyroid Cancer. Diagnostics, 16(1), 19. https://doi.org/10.3390/diagnostics16010019





