New Systemic Inflammatory Indices as Predictors of Fulminant Myocarditis in Children
Abstract
1. Introduction
2. Materials and Methods
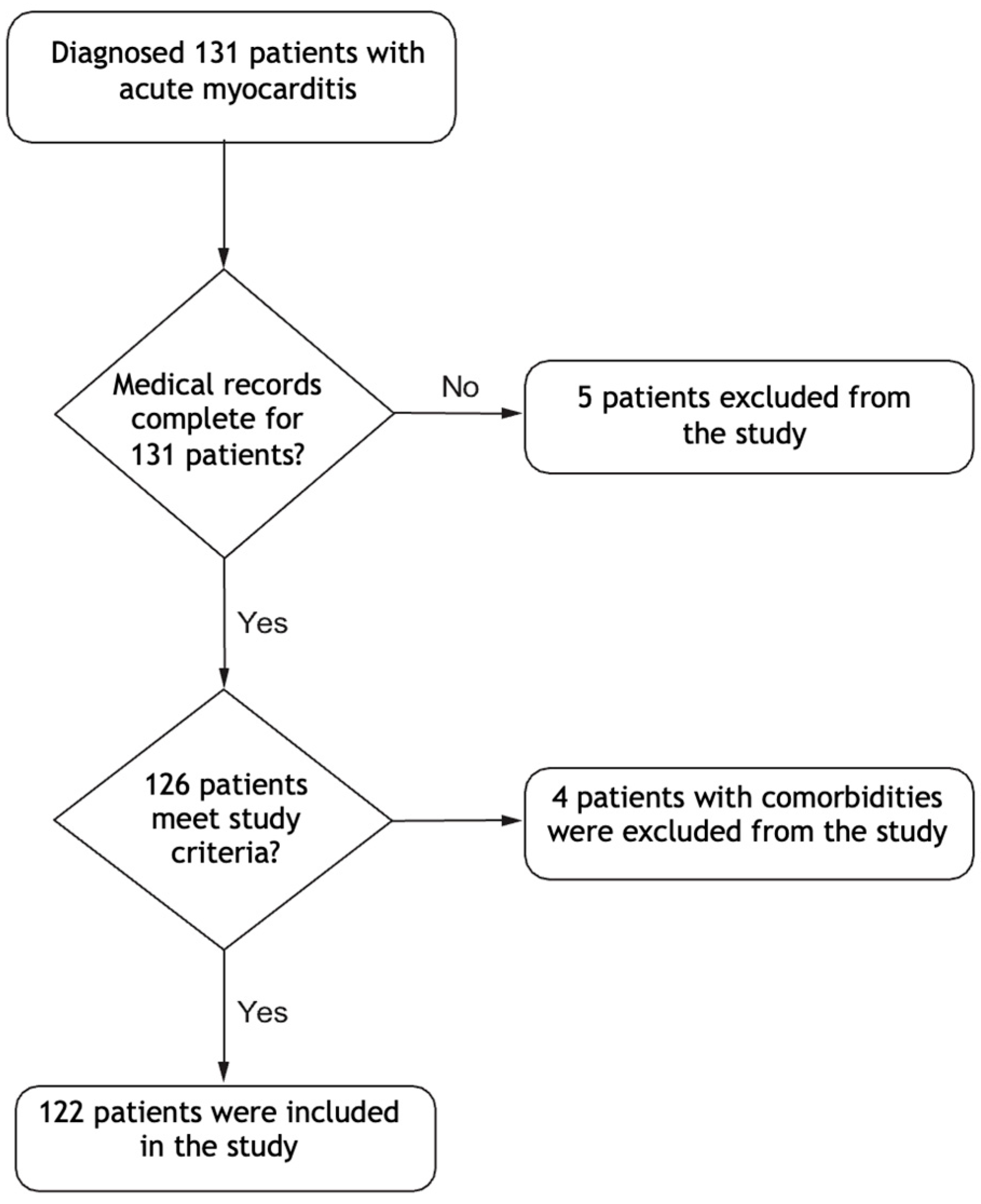
2.1. Study Design and Patient Selection
2.2. Definitions
2.3. Data Collection
2.4. Statistical Analysis
2.5. Outcome
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, J.; Cho, M.-J. Acute myocarditis in children: A 10-year nationwide study (2007–2016) based on the Health Insurance Review and Assessment Service Database in Korea. Korean Circ. J. 2020, 50, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Vasudeva, R.; Bhatt, P.; Lilje, C.; Desai, P.; Amponsah, J.; Umscheid, J.; Parmar, N.; Bhatt, N.; Adupa, R.; Pagad, S. Trends in acute myocarditis related pediatric hospitalizations in the United States, 2007–2016. Am. J. Cardiol. 2021, 149, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Drazner, M.H.; Bozkurt, B.; Cooper, L.T.; Aggarwal, N.R.; Basso, C.; Bhave, N.M.; Caforio, A.L.; Ferreira, V.M.; Heidecker, B. 2024 ACC Expert Consensus Decision Pathway on Strategies and Criteria for the Diagnosis and Management of Myocarditis: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2025, 85, 391–431. [Google Scholar] [CrossRef]
- Dincer, A.; Sezer, S. Systemic immune inflammation index as a reliable disease activity marker in psoriatic arthritis. JCPSP-J. Coll. Physicians Surg. Pak. 2022, 32, 773–778. [Google Scholar]
- Zhang, Y.; Xing, Z.; Zhou, K.; Jiang, S. The predictive role of systemic inflammation response index (SIRI) in the prognosis of stroke patients. Clin. Interv. Aging 2021, 16, 1997–2007. [Google Scholar] [CrossRef]
- Guven, D.C.; Sahin, T.K.; Erul, E.; Kilickap, S.; Gambichler, T.; Aksoy, S. The association between the pan-immune-inflammation value and cancer prognosis: A systematic review and meta-analysis. Cancers 2022, 14, 2675. [Google Scholar] [CrossRef]
- Yükcü, B.; Arslan, H.F. New systemic inflammatory indices as predictors of ascending aortic dilation in children with bicuspid aortic valve: A retrospective cross-sectional study. Medicine 2024, 103, e40904. [Google Scholar] [CrossRef]
- Yaradilmiş, R.M.; Güneylioğlu, M.M.; Öztürk, B.; Göktuğ, A.; Aydın, O.; Güngör, A.; Bodur, İ.; Kaya, Ö.; Örün, U.A.; Karacan, C.D. A novel marker for predicting fulminant myocarditis: Systemic immune–inflammation index. Pediatr. Cardiol. 2023, 44, 647–655. [Google Scholar] [CrossRef]
- Caforio, A.L.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef]
- Hu, B.; Yang, X.-R.; Xu, Y.; Sun, Y.-F.; Sun, C.; Guo, W.; Zhang, X.; Wang, W.-M.; Qiu, S.-J.; Zhou, J. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res. 2014, 20, 6212–6222. [Google Scholar] [CrossRef]
- Qi, Q.; Zhuang, L.; Shen, Y.; Geng, Y.; Yu, S.; Chen, H.; Liu, L.; Meng, Z.; Wang, P.; Chen, Z. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer 2016, 122, 2158–2167. [Google Scholar] [CrossRef] [PubMed]
- Fucà, G.; Guarini, V.; Antoniotti, C.; Morano, F.; Moretto, R.; Corallo, S.; Marmorino, F.; Lonardi, S.; Rimassa, L.; Sartore-Bianchi, A. The Pan-Immune-Inflammation Value is a new prognostic biomarker in metastatic colorectal cancer: Results from a pooled-analysis of the Valentino and TRIBE first-line trials. Br. J. Cancer 2020, 123, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Erbay, I.; Kokturk, U.; Eris Gudul, N.; Avci, A. Prognostic role of systemic immune-inflammation index versus other cardiac markers in acute myocarditis in young adults. Biomark. Med. 2024, 18, 889–897. [Google Scholar] [CrossRef]
- Tang, Y.; Zeng, X.; Feng, Y.; Chen, Q.; Liu, Z.; Luo, H.; Zha, L.; Yu, Z. Association of systemic immune-inflammation index with short-term mortality of congestive heart failure: A retrospective cohort study. Front. Cardiovasc. Med. 2021, 8, 753133. [Google Scholar] [CrossRef]
- Agus, H.Z.; Kahraman, S.; Arslan, C.; Yildirim, C.; Erturk, M.; Kalkan, A.K.; Yildiz, M. Systemic immune-inflammation index predicts mortality in infective endocarditis. J. Saudi Heart Assoc. 2020, 32, 58. [Google Scholar] [CrossRef]
- Wang, X.; Ni, Q.; Wang, J.; Wu, S.; Chen, P.; Xing, D. Systemic inflammation response index is a promising prognostic marker in elderly patients with heart failure: A retrospective cohort study. Front. Cardiovasc. Med. 2022, 9, 871031. [Google Scholar] [CrossRef]
- Xu, T.; Song, S.; Zhu, K.; Yang, Y.; Wu, C.; Wang, N.; Lu, S. Systemic inflammatory response index improves prognostic predictive value in intensive care unit patients with sepsis. Sci. Rep. 2025, 15, 1908. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Xia, C.; Wu, L.; Li, Z.; Li, H.; Zhang, J. Systemic immune inflammation index (SII), system inflammation response index (SIRI) and risk of all-cause mortality and cardiovascular mortality: A 20-year follow-up cohort study of 42,875 US adults. J. Clin. Med. 2023, 12, 1128. [Google Scholar] [CrossRef]
- Söğütlü, Y.; Altaş, U. Predictive Value of Neutrophil–Lymphocyte Ratio and Other Inflammation Indices in Febrile Seizures in Children. J. Clin. Med. 2024, 13, 5330. [Google Scholar] [CrossRef]
- Xu, H.-B.; Xu, Y.-H.; He, Y.; Lin, X.-H.; Suo, Z.; Shu, H.; Zhang, H. Association between admission pan-immune-inflammation value and short-term mortality in septic patients: A retrospective cohort study. Sci. Rep. 2024, 14, 15205. [Google Scholar] [CrossRef]
- Soongswang, J.; Durongpisitkul, K.; Nana, A.; Laohaprasittiporn, D.; Kangkagate, C.; Punlee, K.; Limpimwong, N. Cardiac troponin T: A marker in the diagnosis of acute myocarditis in children. Pediatr. Cardiol. 2005, 26, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Butto, A.; Rossano, J.W.; Nandi, D.; Ravishankar, C.; Lin, K.Y.; O’Connor, M.J.; Shaddy, R.E.; Shamszad, P. Elevated troponin in the first 72 h of hospitalization for pediatric viral myocarditis is associated with ECMO: An analysis of the PHIS+ database. Pediatr. Cardiol. 2018, 39, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Da, M.; Yang, X.; Xu, Y.; Qi, J. A retrospective analysis of clinical characteristics and outcomes of pediatric fulminant myocarditis. BMC Pediatr. 2024, 24, 553. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Kobayashi, T.; Oshikiri, Y.; Arakawa, Y.; Satoh, M.; Morino, Y. Clinical and electrocardiographic characteristics in patients with fulminant myocarditis. J. Arrhythmia 2022, 38, 763–771. [Google Scholar] [CrossRef]

| Variables | Fulminant (n = 26) | Non-Fulminant (n = 96) | p-Value |
|---|---|---|---|
| Demographic data and symptoms | |||
| Age (years) [median (IQR)] | 5 (3–7) | 10 (8–12) | <0.001 |
| Gender [n (%)] | |||
| Male | 17 (65) | 63 (66) | 0.981 |
| Female | 9 (35) | 33 (34) | |
| Symptoms [n (%)] | |||
| Chest pain | 2 (7.7) | 70 (72.9) | <0.001 |
| Fever | 3 (11.5) | 19 (19.8) | 0.403 |
| Palpitation | 3 (11.5) | 12 (12.5) | 0.981 |
| Shortness of breath | 15 (57.7) | 17 (17.5) | <0.001 |
| Abdominal pain | 13 (50) | 13 (13.5) | <0.001 |
| URTI | 7 (27) | 34 (35) | 0.488 |
| Laboratory factors (median [IQR]) | |||
| Basal troponin I (ng/mL) | 138 (90–240) | 188 (150–320) | 0.035 |
| Peak troponin I (ng/mL) | 184 (150–250) | 274 (220–350) | 0.100 |
| NT-proBNP (pg/mL) | 32,000 (25,000–42,000) | 2100 (1000–5600) | <0.001 |
| CRP (mg/L) | 14 (7–21) | 20 (10–40) | 0.020 |
| Procalcitonin (ng/mL) | 0.5 (0.3–0.9) | 0.7 (0.5–1.2) | 0.080 |
| WBC (×109/L) | 12.7 (10–14.4) | 9.4 (8–11) | <0.001 |
| ANC (×109/L) | 7.2 (6–8.5) | 4.8 (4–6.2) | <0.001 |
| ALC (×109/L) | 4.7 (3–6) | 2.7 (2.2–4) | 0.390 |
| AMC (×109/L) | 0.9 (0.6–1.2) | 0.7 (0.5–1) | 0.010 |
| Platelet (×109/L) | 320 (280–360) | 280 (2 50–330) | 0.470 |
| NLR | 2.3 (2–2.7) | 1.7 (1.5–1.9) | <0.001 |
| PLR | 70 (50–90) | 115 (100–130) | 0.250 |
| MLR | 0.14 | 0.27 | 0.060 |
| SII | 1300 (1000–1600) | 500 (350–650) | <0.001 |
| SIRI | 2.9 (2.5–3.2) | 1.5 (1.2–1.8) | <0.001 |
| PIV | 400 (380–420) | 380 (360–400) | 0.080 |
| Pathogens identified in patients [n (%)] | |||
| Adenovirus | 3 (11.5) | 5 (5.2) | |
| Enterovirus/rhinovirus | 3 (11.5) | 8 (8.3) | |
| Parainfluenza viruses | 2 (7.5) | 4 (4.2) | |
| SARS-Cov-2 | 2 (7.5) | 3 (3.1) | |
| Other coronaviruses | 1 (3.8) | 2 (2.1) | |
| Influenza A-B | - | 6 (6.2) | |
| Others | 2 (7.5) | 4 (4.2) | |
| Clinical data [n (%)] | <0.001 | ||
| ECMO | 7 (26.9) | 0 | |
| In-hospital mortality | 4 (15) | 0 | |
| Echocardiographic variables | |||
| LVEF (%) | 30 (24–35) | 45 (40–55) | <0.001 |
| Mitral regurgitation [n (%)] | <0.001 | ||
| Mild | 2 (7.5) | 8 (8.3) | |
| Mild to moderate | 4 (15) | 2 (2.1) | |
| Moderate | 6 (22.5) | 1 (1.1) | |
| Moderate to severe | 8 (30) | - | |
| Severe | 6 (22.5) | - | |
| Pericardial effusion [n (%)] | 4 (15.3) | 12 (12.5) | 0.875 |
| ECG variables [n (%)] | <0.001 | ||
| No findings | - | 24 (25) | |
| Sinus tachycardia | 13 (50) | 16 (16.6) | |
| ST elevation | 8 (30) | 12 (12.5) | |
| ST depression | 3 (11.5) | 8 (8.3) | |
| Ventricular extrasystoles | 8 (30) | 2 (2.1) | |
| CMR parameters (median [IQR]) | |||
| LGE (%) | 4 (2–6) | 3 (2–4) | 0.650 |
| T1 relaxation time (ms) | 1200 (1000–1400) | 1000 (800–1100) | 0.003 |
| T2 relaxation time (ms) | 68 (60–76) | 48 (40–56) | 0.001 |
| ECV fraction (%) | 15 (10–25) | 18 (10–30) | 0.890 |
| Pericardial effusion [n (%)] | 5 (19.2) | 14 (14.5) | 0.420 |
| Parameters | AUC | Cut-Off | Sensitivity % | Specificity % | 95% CI | p-Value |
|---|---|---|---|---|---|---|
| SII | 0.760 | 1050 | 80 | 90 | 0.620–0.860 | 0.001 |
| SIRI | 0.640 | 1.9 | 75 | 80 | 0.500–0.770 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kangel, D.; Ozyılmaz, İ.; Ozkok, S.; Özcanoğlu, H.D.; Güzelbağ, A.N.; Çevlik, B.; Tanıdır, İ.C.; Hatemi, A.C.; Öztürk, E. New Systemic Inflammatory Indices as Predictors of Fulminant Myocarditis in Children. Diagnostics 2025, 15, 961. https://doi.org/10.3390/diagnostics15080961
Kangel D, Ozyılmaz İ, Ozkok S, Özcanoğlu HD, Güzelbağ AN, Çevlik B, Tanıdır İC, Hatemi AC, Öztürk E. New Systemic Inflammatory Indices as Predictors of Fulminant Myocarditis in Children. Diagnostics. 2025; 15(8):961. https://doi.org/10.3390/diagnostics15080961
Chicago/Turabian StyleKangel, Demet, İsa Ozyılmaz, Sercin Ozkok, Hatice Dilek Özcanoğlu, Ali Nazım Güzelbağ, Burcu Çevlik, İbrahim Cansaran Tanıdır, Ali Can Hatemi, and Erkut Öztürk. 2025. "New Systemic Inflammatory Indices as Predictors of Fulminant Myocarditis in Children" Diagnostics 15, no. 8: 961. https://doi.org/10.3390/diagnostics15080961
APA StyleKangel, D., Ozyılmaz, İ., Ozkok, S., Özcanoğlu, H. D., Güzelbağ, A. N., Çevlik, B., Tanıdır, İ. C., Hatemi, A. C., & Öztürk, E. (2025). New Systemic Inflammatory Indices as Predictors of Fulminant Myocarditis in Children. Diagnostics, 15(8), 961. https://doi.org/10.3390/diagnostics15080961






