Microcirculatory Dysfunction and Its Role in Diagnosing Acute Rejection in Pediatric Heart Transplantation: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Physiological Assessment and Microcirculatory Dysfunction Definition
2.3. Echocardiographic Assessment
2.4. Acute Rejection Definitions and Protocol for the Diagnosis
2.5. Patient Follow-Up
2.6. Endpoints
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics and Echocardiographic Assessment
3.2. Acute Celular and Acute Antibody-Mediated Rejection
3.3. Coronary Microcirculatory Dysfunction
3.4. Study Endpoints
3.4.1. Primary Endpoints
- A.
- IMR in Relation to Previous Acute Rejection
- B.
- IMR in Relation to Acute Rejection During Follow-up
3.4.2. Secondary Endpoints
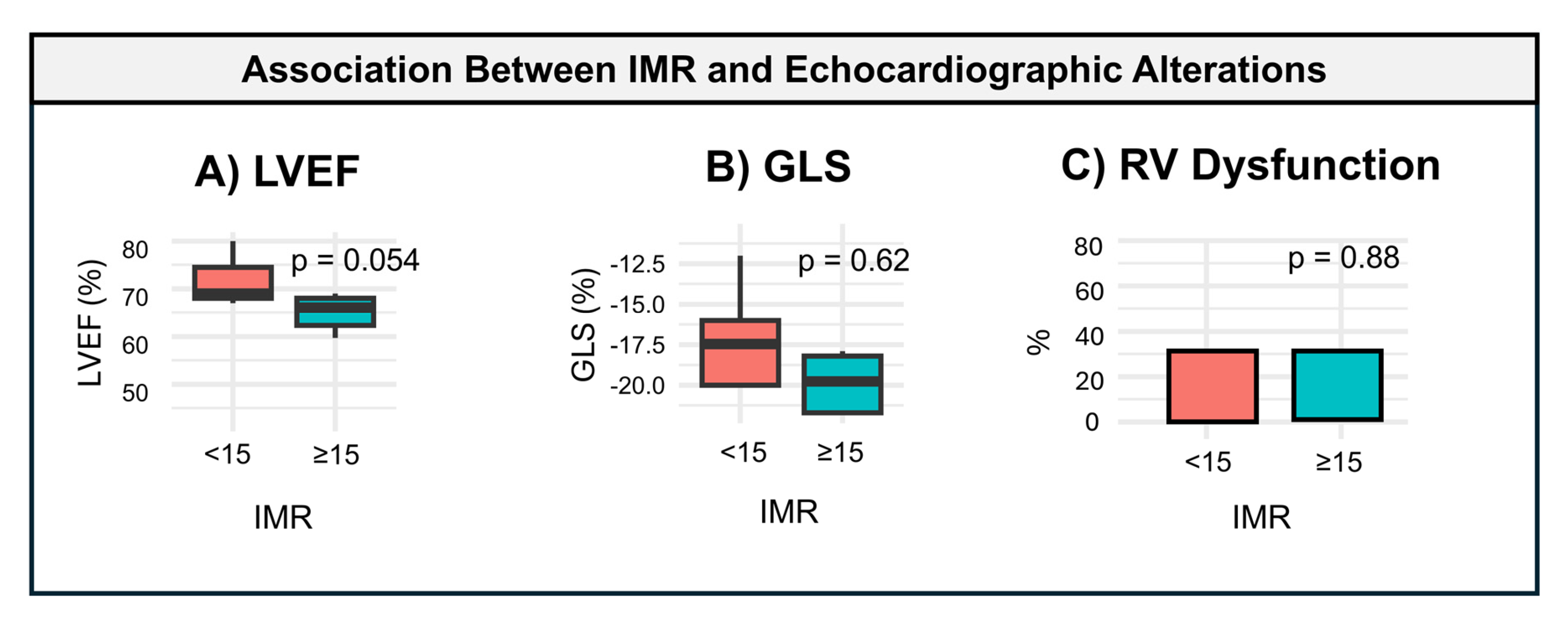
- A.
- IMR in Relation to Echocardiographic Assessment
- B.
- Safety Endpoint
4. Discussion
4.1. Challenges in Diagnosing Acute Rejection
4.2. Echocardiographic Alterations
4.3. Persistent Microvascular Dysfunction
4.4. Clinical Implications
4.5. Safety and Methodology
4.6. IMR vs. CFR in Assessing Microcirculatory Dysfunction
4.7. Implications for AMR
4.8. Future Directions
4.9. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AR | Acute rejection |
| ACR | Acute cellular rejection |
| AMR | Antibody-mediated rejection |
| CFR | Coronary flow reserve |
| GLS | Global longitudinal strain |
| HT | Heart transplantation |
| IMR | Index of microcirculatory resistance |
| LVEF | Left ventricular ejection fraction |
| RV | Right ventricle |
| TAPSE | Tricuspid annular plane systolic excursion |
References
- Dipchand, A.I.; Webber, S.A. Pediatric heart transplantation: Looking forward after five decades of learning. Pediatr. Transplant. 2024, 28, e14675. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, S.; Sparks, J.D.; Alsoufi, B. Pediatric heart transplantation: Year in review 2020. J. Thorac. Cardiovasc. Surg. 2021, 162, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Dipchand, A.I. Current state of pediatric cardiac transplantation. Ann. Cardiothorac. Surg. 2018, 7, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Everitt, M.D.; Pahl, E.; Koehl, D.A.; Cantor, R.S.; Kirklin, J.K.; Reed, A.C.; Thrush, P.; Zinn, M.; McCormick, A.D.; Yester, J.; et al. Clinical outcomes after a biopsy diagnosis of antibody-mediated rejection in pediatric heart transplant recipients. J. Heart Lung Transpl. 2025, 44, 82–91. [Google Scholar] [CrossRef]
- Tolani, D.; Butts, R.J.; Sutcliffe, D.L.; Power, A. Decreasing Endomyocardial Biopsy Frequency in Pediatric Heart Transplantation Using A Rejection Risk Prediction Score—A Single Center Study. Pediatr. Transplant. 2024, 28, e14894. [Google Scholar] [CrossRef]
- Ahn, J.-M.; Zimmermann, F.M.; Gullestad, L.; Angerås, O.; Karason, K.; Russell, K.; Lunde, K.; Okada, K.; Luikart, H.; Khush, K.K.; et al. Microcirculatory Resistance Predicts Allograft Rejection and Cardiac Events After Heart Transplantation. J. Am. Coll. Cardiol. 2021, 78, 2425–2435. [Google Scholar] [CrossRef]
- Iwańczyk, S.; Woźniak, P.; Smukowska-Gorynia, A.; Araszkiewicz, A.; Nowak, A.; Jankowski, M.; Konwerska, A.; Urbanowicz, T.; Lesiak, M. Microcirculatory Disease in Patients after Heart Transplantation. J. Clin. Med. 2023, 12, 3838. [Google Scholar] [CrossRef]
- Shahandeh, N.; Song, J.; Saito, K.; Honda, Y.; Zimmermann, F.M.; Ahn, J.-M.; Fearon, W.F.; Parikh, R.V. Invasive Coronary Physiology in Heart Transplant Recipients: State-of-the-Art Review. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 2, 100627. [Google Scholar] [CrossRef]
- Nardone, M.; McCarthy, M.; Ardern, C.I.; Nield, L.E.; Toleva, O.; Cantor, W.J.; Miner, S.E. Concurrently Low Coronary Flow Reserve and Low Index of Microvascular Resistance Are Associated With Elevated Resting Coronary Flow in Patients With Chest Pain and Nonobstructive Coronary Arteries. Circ. Cardiovasc. Interv. 2022, 15, e011323. [Google Scholar] [CrossRef]
- Lee, J.M.; Choi, K.H.; Choi, J.-O.; Shin, D.; Park, Y.; Kim, J.; Lee, S.H.; Kim, D.; Yang, J.H.; Cho, Y.H.; et al. Coronary Microcirculatory Dysfunction and Acute Cellular Rejection After Heart Transplantation. Circulation 2021, 144, 1459–1472. [Google Scholar] [CrossRef]
- Berry, G.J.; Burke, M.M.; Andersen, C.; Bruneval, P.; Fedrigo, M.; Fishbein, M.C.; Goddard, M.; Hammond, E.H.; Leone, O.; Marboe, C.; et al. The 2013 International Society for Heart and Lung Transplantation Working Formulation for the standardization of nomenclature in the pathologic diagnosis of antibody-mediated rejection in heart transplantation. J. Heart Lung Transpl. 2013, 32, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- Amdani, S.; Kirklin, J.K.; Cantor, R.; Koehl, D.; Lal, A.; Chau, P.; Curren, V.; Edelson, J.B.; Parent, J.J.; Victor, H.; et al. Prevalence and Impact of Recurrent Rejection on Pediatric Heart Transplant Recipients. J. Am. Coll. Cardiol. 2024, 84, 2170–2182. [Google Scholar] [CrossRef] [PubMed]
- Akabas, L.; Bravo, S.A.; Zhang, Y.; Simonelli, A.; Zuckerman, W.A.; Richmond, M.E.; Lytrivi, I.D. Progress in Noninvasive Surveillance for Acute Rejection in Pediatric Heart Transplant Recipients: A Real-World Analysis of Donor-Derived Cell-Free DNA-Based Surveillance Protocol. Clin. Transplant. 2024, 38, e15481. [Google Scholar] [CrossRef] [PubMed]
- Bansal, N.; Everitt, M.D.; Nandi, D.; Spinner, J.; Conway, J.; Magnetta, D.A.; Profita, E.L.; Townsend, M.; Alejos, J.C.; Deshpande, S.R. Clinical approach to acute cellular rejection from the pediatric heart transplant society. Pediatr. Transplant. 2022, 26, e14393. [Google Scholar] [CrossRef]
- Frandsen, E.; Gimferrer, I.; Rudzinski, E.; Ahmed, H.; Albers, E.; Friedland-Little, J.; Hong, B.; Kemna, M.; Newland, D.; Law, Y. Antibody Mediated Rejection in Pediatric Heart Transplant Recipients. J. Heart Lung Transpl. 2022, 41, S499. [Google Scholar] [CrossRef]
- Holzhauser, L.; DeFilippis, E.M.; Nikolova, A.; Byku, M.; Contreras, J.P.; De Marco, T.; Hall, S.; Khush, K.K.; Vest, A.R. The End of En-domyocardial Biopsy? JACC Heart Fail 2023, 11, 263–276. [Google Scholar] [CrossRef]
- Wu, G.W.; Kobashigawa, J.A.; Fishbein, M.C.; Patel, J.K.; Kittleson, M.M.; Reed, E.F.; Kiyosaki, K.K.; Ardehali, A. Asymptomatic Anti-body-mediated Rejection After Heart Transplantation Predicts Poor Outcomes. J. Heart Lung Transpl. 2009, 28, 417–422. [Google Scholar] [CrossRef]
- Konstantinou, K.; Keeble, T.R.; Davies, J.R.; Gamma, R.A.; Tang, K.H.; Alsanjari, O.; Kelly, P.A.; Clesham, G.J.; Karamasis, G.V. Discordance Between Coronary Flow Reserve and the Index of Microcirculatory Resistance Post-Revascularization for ST-Segment–Elevation Myocardial Infarction. Circ. Cardiovasc. Interv. 2021, 14, e010529. [Google Scholar] [CrossRef]
- Barten, M.J.; Schulz, U.; Beiras-Fernandez, A.; Berchtold-Herz, M.; Boeken, U.; Garbade, J.; Hirt, S.; Richter, M.; Ruhpawar, A.; Sandhaus, T.; et al. The clinical impact of donor-specific antibodies in heart transplantation. Transplant. Rev. 2018, 32, 207–217. [Google Scholar] [CrossRef]
- Goldberg, J.F.; Tian, X.; Bon, A.; Xu, Y.; Gerhard, E.; Brower, R.; Jang, M.K.; Kong, H.; Andargie, T.E.; Park, W.; et al. Redefining Cardiac Antibody-Mediated Rejection with Donor-Specific Antibodies and Graft Dysfunction. Circ. Heart Fail. 2024, 17, e011592. [Google Scholar] [CrossRef]
- Yerly, P.; Rotman, S.; Regamey, J.; Aubert, V.; Aur, S.; Kirsch, M.; Hullin, R.; Pascual, M. Complement blockade with eculizumab to treat acute symptomatic humoral rejection after heart transplantation. Xenotransplantation 2022, 29, e12726. [Google Scholar] [CrossRef]

| IMR < 15 (n = 4) | IMR ≥ 15 (n = 6) | Total (n = 10) | p Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 12.2 (2.1) | 13.2 (2.7) | 12.8 (2.4) | 0.584 |
| Female | 3 (75%) | 1 (16.7%) | 4 (4%) | 0.065 |
| HT indication | 0.076 | |||
| DCM | 1 (25%) | 3 (50%) | 4(40%) | |
| NCCM | N/A | 3 (5%) | 3 (30%) | |
| RCM | 2 (50%) | N/A | 2 (20%) | |
| Shone Complex | 1 (25%) | N/A | 1 (10%) | |
| BMI, kg/m2 | 21.2 (4.5) | 18.7 (5.7) | 19.7 (5.1) | 0.477 |
| Height, cm | 156.3 (16.2) | 153.4 (13.0) | 154.2 (13.1) | 0.763 |
| Weight, kg | 59.0 (0.9) | 46.8 (13.4) | 50.5 (12.5) | 0.165 |
| Post transplant, years | 3.9 [3.5; 4.9] | 6.6 [2.2; 8.7] | 4.2 [3.2; 8.4] | 0.402 |
| IMR < 15 (n = 4) | IMR ≥ 15 (n = 6) | Total (n = 10) | p Value | |
|---|---|---|---|---|
| Echocardiographic assessment | ||||
| LV | ||||
| LVEF, % | 76.3 (6.4) | 62.5 (9.6) | 66.7 (10.7) | 0.054 |
| GLS, % | −18.8 (3.9) | −17.3 (4.6) | −18.4 (3.9) | 0.622 |
| E/A | 2.8 (1.2) | 2.3 (0.7) | 2.5 (0.9) | 0.394 |
| E/e’ | 8.4 (3.1) | 6.9 (1.2) | 7.5 (2.2) | 0.307 |
| RV | ||||
| TAPSE, mm | 14.8 (3.2) | 13.1 (4.0) | 13.8 (3.6) | 0.508 |
| S wave, cm/s | 9.4 (2.8) | 9.3 (1.2) | 9.3 (1.8) | 0.888 |
| RV dysfunction | 1.0 (33.3%) | 2.0 (33.3%) | 3.0 (30.0%) | 0.880 |
| Physiological indices | ||||
| FFR | 0.96 (0.02) | 0.94 (0.03) | 0.95 (0.03) | 0.357 |
| CFR | 4.6 (2.3) | 1.8 (1.1) | 2.4 (1.7) | 0.022 |
| IMR | 10.2 (5.6) | 23.7 (5.2) | 18.3 (8.6) | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivero-Santana, B.; Balbacid-Domingo, E.; Abelleira-Pardeiro, C.; Labrandero de Lera, C.; Arreo del Val, V.; Jiménez-Valero, S.; Fernández-Velasco, M.; Moreno, R.; Gutiérrez-Larraya, F. Microcirculatory Dysfunction and Its Role in Diagnosing Acute Rejection in Pediatric Heart Transplantation: A Pilot Study. Diagnostics 2025, 15, 545. https://doi.org/10.3390/diagnostics15050545
Rivero-Santana B, Balbacid-Domingo E, Abelleira-Pardeiro C, Labrandero de Lera C, Arreo del Val V, Jiménez-Valero S, Fernández-Velasco M, Moreno R, Gutiérrez-Larraya F. Microcirculatory Dysfunction and Its Role in Diagnosing Acute Rejection in Pediatric Heart Transplantation: A Pilot Study. Diagnostics. 2025; 15(5):545. https://doi.org/10.3390/diagnostics15050545
Chicago/Turabian StyleRivero-Santana, Borja, Enrique Balbacid-Domingo, César Abelleira-Pardeiro, Carlos Labrandero de Lera, Viviana Arreo del Val, Santiago Jiménez-Valero, María Fernández-Velasco, Raúl Moreno, and Federico Gutiérrez-Larraya. 2025. "Microcirculatory Dysfunction and Its Role in Diagnosing Acute Rejection in Pediatric Heart Transplantation: A Pilot Study" Diagnostics 15, no. 5: 545. https://doi.org/10.3390/diagnostics15050545
APA StyleRivero-Santana, B., Balbacid-Domingo, E., Abelleira-Pardeiro, C., Labrandero de Lera, C., Arreo del Val, V., Jiménez-Valero, S., Fernández-Velasco, M., Moreno, R., & Gutiérrez-Larraya, F. (2025). Microcirculatory Dysfunction and Its Role in Diagnosing Acute Rejection in Pediatric Heart Transplantation: A Pilot Study. Diagnostics, 15(5), 545. https://doi.org/10.3390/diagnostics15050545






